Abstract
A new xenograft model of multiple myeloma (MM), where growth is strongly regulated by interleukin-6 (IL-6), was established in severe combined immunodeficiency (SCID) mice. In this model, endogenous IL-6 from SCID mice was ineffective at eliciting growth of the established human MM cell line KPMM2; these cells achieved autonomous growth through their autocrine secretion of IL-6. The etiopathology in this disease model is consistent with that of human MM. When greater than 3 × 106 KPMM2 cells were injected intravenously (IV), tumors developed in all mice and were predominantly localized in their bone marrow. Tumors were also apparent in the lymph nodes, but absent from other organs. Immunostaining of cell surface antigen (CD38) showed that more than 40% of bone marrow cells in femur were of myeloma origin in the advanced stage of tumor progression (day 37). Histologic analysis of these mice show that bone marrow was largely occupied by plasmablastic cells and bones had developed osteolytic lesions at multiple sites. Concurrently, there was a decrease in bone density throughout the body and a significant increase in ionized plasma calcium. M-protein was detected in the serum within 10 days after transplantation, which correlated with the tumor progression. Between 30 and 40 days after the transplantation, mice presented with a rapid and severe loss of body weight, hind leg paralysis, and fatigue. Subsequently, the mice died within a week. A single IV injection of 0.2 mg humanized anti–IL-6 receptor antibody (hPM1) into mice on the day after tumor transplantation substantially suppressed the elevation of serum M-protein and development of the tumor-associated abnormalities and significantly increased in the life span of tumor-bearing mice. Our data show the usefulness of this model to analyze the pathologic role of IL-6 in MM and the efficacy of targeting the IL-6 receptor in IL-6–dependent KPMM2 cells.
MULTIPLE MYELOMA (MM) is plasma cell malignancy. Typical myeloma cells constitutively secrete high amounts of monoclonal Ig (M-protein) into blood. A malignant proliferation of myeloma cells in bone marrow causes many severe symptoms of morbid fracture, hypercalcemia, anemia, and recurrent infections in patients where the malignancy has reached an advanced stage.1-3 Chemotherapy in combination with steroids and/or interferon is most commonly used for treatment of this disease; nevertheless, prognosis of the patients is still poor.4 5
Interleukin-6 (IL-6) is a multifunctional cytokine. Its aberrant expression and that of its receptor are associated with various diseases.6,7 With regard to MM, IL-6 is implicated with the pathology of their malignancy.8 IL-6 is secreted by both myeloma cells and/or stromal cells of the bone marrow.9,10 Both freshly isolated and established myeloma cells express the IL-6 receptor and its signal transducer, gp130,9 and some of them show IL-6–dependent proliferation in vitro.11-13 Furthermore, IL-6 is also reported to prevent apoptosis of myeloma cells in vitro.14 15
Klein et al16 17 reported that mouse monoclonal antibodies against IL-6 showed some therapeutic efficacy in the patients with advanced MM. However, this response was transient because of the appearance of human antimouse antibody and the unexpected elevation of serum IL-6 by the formation of antigen-antibody complex. In their study, they suggested that reducing antigenicity of murine antibody was essential for clinical use. Furthermore, they proposed that the antibody against IL-6 had the added problem of functioning as carrier protein, which prolongs half-life of ligands.
To overcome these problems, we decided to target the IL-6 receptor by use of the humanized murine anti–hIL-6 receptor antibody PM1. Humanized PM1 (hPM1) was constructed by grafting the complementarity-determining regions (CDRs) of PM1 into human antibody. Recognition of the IL-6 receptor and inhibition of its IL-6 binding was achieved with equal activity for both hPM1 and PM1.18
To examine the efficacy of hPM1, an appropriate animal model was required. Although some xenograft models of human myeloma existed, they were inadequate for our purpose for reasons that follow. In the model of Feo-Zuppardi et al19 freshly isolated human myeloma cells, which had been transplanted into severe combined immunodeficiency (SCID) mice, survived for several months, but did not show malignancy. Furthermore, tumor progression was dependent on cell type. Huang et al20 reported that the intravenous (IV) transplantation of the human myeloma line, ARH-77, into SCID mice showed a high tumor incidence in several organs, including bone marrow and similar abnormalities to the human disease. This model was useful for analyzing characteristics of the disease, but the growth of ARH-77 cells was not dependent on IL-6.21 Generally, it is difficult to transplant IL-6–dependent human myeloma cells into mice. We have previously reported the xenograft model of MM using IL-6–dependent human myeloma cell line transfected with hIL-6 cDNA.22 However, in this model, the tumor growth was restricted in the subcutaneous (SC) region and did not develop in bone marrow; it was not characteristic of the disease.
The human myeloma cell line KPMM2 was originally established from the ascites of a patient with myeloma.23 KPMM2 cells were shown to constitutively secrete IL-6 and a high amount of human IgG (M-protein). The proliferation of KPMM2 cells is highly and specifically dependent on IL-6. In this study, we describe a newly established SCID mouse model of MM by transplanting KPMM2 cells, which shows the localized development of the tumors in bone marrow and induces several pathogenic features of MM. Importantly, hPM1 was therapeutically effective in this model.
MATERIALS AND METHODS
Animals.SCID mice (C.B.-17 scid/scid; male, 5 weeks old) were purchased from Japan Clea Co, Inc. (Tokyo, Japan) and used for experiments when between 6 and 9 weeks of age. The animals were kept in a specific pathogen-free facility.
Maintenance of tumor.The SC transplantation of 107 KPMM2 cells into SCID mice formed solid tumors. This tumor was passaged every month in SCID mice by SC transplantation of a 3-mm cube tumor block.
Proliferation assay.The in vivo dependency of passaged KPMM2 cells for IL-6 was checked by examining their ability to proliferate after treatment with anti–hIL-6 receptor antibody.18 24 Briefly, 104 cells were cultured in RPMI1640 (GIBCO, Grand Island, NY) supplemented with 20% fetal bovine serum (FBS; GIBCO) for 7 days in 96-well microplates (Becton Dickinson, Lincoln Park, NJ). Cell proliferation was estimated by the 3-[4,5-Dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT)-assay.
Antibody.Precise methods of preparation and biologic properties of hPM1 are described elsewhere.18 hPM1 was solubilized in phosphate-buffered saline (PBS) immediately before administration into mice.
Xenograft model.For the transplantation of KPMM2 cells, the tumor mass growing SC was resected, then minced well in the medium and disaggregated into a single cell suspension by means of a pipette. This suspension was passed through 70 μm nylon mesh (Becton Dickinson), then adjusted to the required concentration according to the particular experiment. A total of 0.1 mL or 0.2 mL of cell suspension was transplanted SC or IV to the SCID mice, respectively. Tumor growth after SC transplantation was estimated by volume, which was calculated by the formula: 1/2ab2 (mm3 ) where “a” is the longest, and “b” is the shortest axis of tumor. Antitumor activity of the antibody against hIL-6 receptor was evaluated as follows: mice were injected IV with 6 × 106 KPMM2 cells, then on the next day, by the same route with 0.2 mL of the antibody solution or PBS. Life span and serum hIgG levels of mice were used to determine the antitumor activity.
Morphologic and flow cytometric observations.Bones and bone marrow histology of mice after IV transplantation with KPMM2 cells was examined. Bone marrow cells were flushed out from the femur, adhered to slides by centrifugation and air dried, then stained with anti-CD38 antibody (Anti-Leu-17; Becton Dickinson) and Vectastain ABC kit (Vector, Burlingame, CA). Bones were fixed with 10% formalin solution, cut into thin sections of 8 μm, and stained with hematoxylin and eosin solution. Both staining procedures were evaluated under the optical microscope. The outline and density of bones were analyzed by x-ray photography using the TRS-M100C system (Sofron, Tokyo, Japan). Bone marrow cells stained with phycoerythrin (PE)-labeled anti-CD38 antibody were also analyzed by flow cytometry (FACScan; Becton Dickinson).
Blood parameters.The mice were bled by retroorbital puncture. Plasma ionized calcium levels were estimated by the 643 Ca++/pH Analyzer (Ciba-Corning, Halstead, UK). Serum human IgG (M-protein) concentration was estimated by sandwich enzyme-linked immunosorbent assay (ELISA) using goat polyclonal antihuman IgG antibody (Tago, Burlingame, CA) and alkaline phosphatase-conjugated goat polyclonal antihuman IgG antibody (Tago). The detection limit was 1.56 ng/mL. The number of peripheral blood cells was counted by microcell counter F-800 (Toa Electric, Kobe, Japan).
Statistical analysis.All of the data, except for life span of mice, were analyzed by Wilcoxon's rank sum test; the latter data were analyzed by generalized Wilcoxon's test.
RESULTS
Engraftment of KPMM2 cells in SCID mice.To examine the tumorigenicity of KPMM2 cells, 107 cells in culture were injected SC into the flank of SCID mice. The mice developed solid tumors within 2 weeks. These tumors were transplantable and could be passaged a least 30 times. The IL-6 dependency of these in vivo passaged KPMM2 cells was not changed. Tumorigenicity was also reexamined using KPMM2 cells recovered from these tumors. As shown in Table 1, all the mice injected with more than 3 × 106 cells developed solid tumor or hind leg paralysis after SC transplantation or IV transplantation, respectively. The bone marrows of mice with hind leg paralysis were occupied by tumor cells, as will be described in detail later in this section. For both routes, the minimum number of the cells required for tumor engraftment was 105. Indications of tumor growth was not observed in the mice injected with 104 cells. The time of tumor appearance or onset of paralysis was shortened by increasing the cell number for transplantation.
Tumorigenicity of KPMM2 in SCID Mice
| No. of Inoculated Cells . | Route of Inoculation . | |
|---|---|---|
| . | SC . | IV . |
| . | Appearance (d)* . | Lifespan (d) . |
| 107 | 8, 8, 8 | 38, 40, 43 |
| 3 × 106 | 14, 14, 22 | 41, 42, 43, 51 |
| 106 | 14, 14, 14, N† | 48, 50, 56, >100 |
| 105 | 30, 30, 44, 44 | 49, 67, 76, >100 |
| 104 | N, N, N, N | >100, >100, >100 |
| No. of Inoculated Cells . | Route of Inoculation . | |
|---|---|---|
| . | SC . | IV . |
| . | Appearance (d)* . | Lifespan (d) . |
| 107 | 8, 8, 8 | 38, 40, 43 |
| 3 × 106 | 14, 14, 22 | 41, 42, 43, 51 |
| 106 | 14, 14, 14, N† | 48, 50, 56, >100 |
| 105 | 30, 30, 44, 44 | 49, 67, 76, >100 |
| 104 | N, N, N, N | >100, >100, >100 |
Days required to appear pulpuble tumor. Mice were observed every 3 or 4 days until day 100.
No tumor growth was observed on day 100.
Tumor growth in SC transplanted mice was localized at the site of injection with no obvious signs of invasion and metastasis. Although these tumors showed exponential growth with a rapid doubling time (2.6 days; when transplanted with 3 × 106 cells), the mice survived more than 40 days.
Mice injected IV with KPMM2 cells developed behavioral abnormalities such as hind leg paralysis, loss of balance, and severe fatigue between 30 to 40 days. Concurrently, the mice also suffered from prominent body weight loss and subsequently died within a week. Survival time of the mice transplanted with more than 3 × 106 KPMM2 cells was approximately 40 days. Life span was prolonged when the number of transplanted cells was decreased.
These results suggested the malignant growth of engrafted KPMM2 cells in mice.
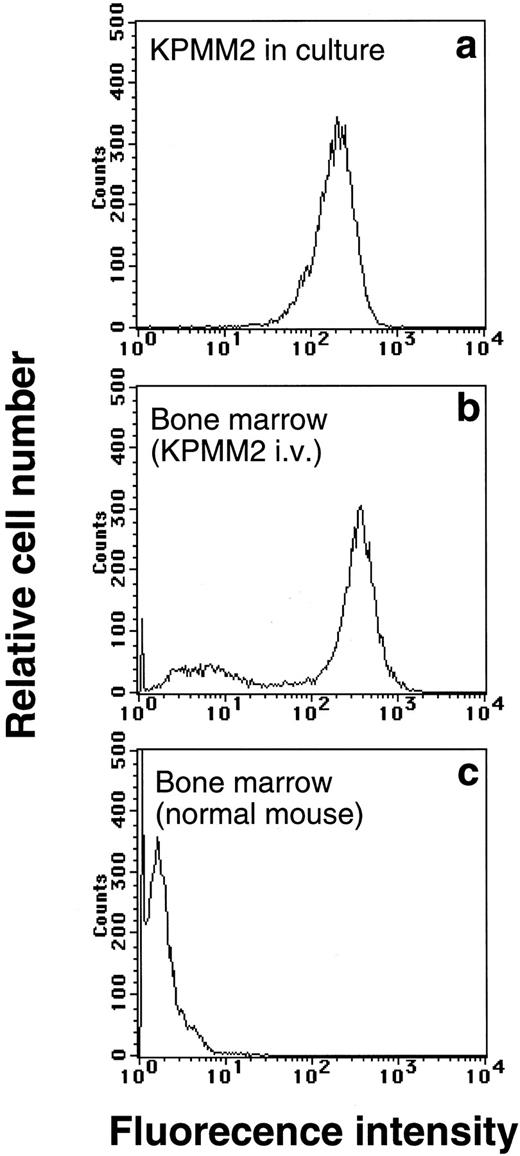
Tumor development in bone marrow.The abnormalities of mice transplanted IV with KPMM2 cells were examined in more detail as follows: 37 days after transplantation, myeloma cells in bone marrow were estimated by flow cytometric analysis of CD38 and accounted for more than 60% of the marrow cell volume (Fig 1). This was also confirmed immunohistochemically (Fig 2). CD38 positive cells in bone marrow were large and rich in cytoplasm. The tumor growth was highly localized in bone marrow, but also observed in abdominal lymph nodes (4 of 5 mice) and kidney (1 of 5 mice; Table 2). No clear evidence of the tumor development was found in liver, spleen, lung, stomach, and intestine.
Flow cytometric analysis of bone marrow cells in mice IV inoculated with 107 cells of KPMM2. Bone marrow cells were flushed out from femur of the mice on day 37, then stained with PE-labeled anti-CD38 antibody. As shown by (b), bone marrow was occupied by tumor cells (CD38+), wheras no CD38+ cells was observed in normal mice (c). (a) Fluorescence pattern of cultured KPMM2 cells.
Flow cytometric analysis of bone marrow cells in mice IV inoculated with 107 cells of KPMM2. Bone marrow cells were flushed out from femur of the mice on day 37, then stained with PE-labeled anti-CD38 antibody. As shown by (b), bone marrow was occupied by tumor cells (CD38+), wheras no CD38+ cells was observed in normal mice (c). (a) Fluorescence pattern of cultured KPMM2 cells.
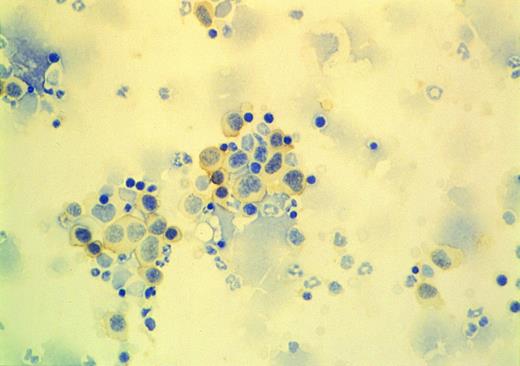
Immunostaining of bone marrow cells from the tumor bearing mice (see Fig 1). Cells were adhered to slides by centrifugation, then stained with anti-CD38 antibody. CD38 expressing cells (stained to brown) showed large, cytoplasm-rich and plasmablastic morphology.
Immunostaining of bone marrow cells from the tumor bearing mice (see Fig 1). Cells were adhered to slides by centrifugation, then stained with anti-CD38 antibody. CD38 expressing cells (stained to brown) showed large, cytoplasm-rich and plasmablastic morphology.
Tumor Incidence in Various Tissues
| Tissue . | Tumor Development/Total Mice . |
|---|---|
| Bone marrow | 5/5 |
| Lymph node | 4/5 |
| Liver | 0/5 |
| Spleen | 0/5 |
| Kidney | 1/5 |
| Lung | 0/5 |
| Tissue . | Tumor Development/Total Mice . |
|---|---|
| Bone marrow | 5/5 |
| Lymph node | 4/5 |
| Liver | 0/5 |
| Spleen | 0/5 |
| Kidney | 1/5 |
| Lung | 0/5 |
Mice were IV transplanted with 107 KPMM2 cells, and tumor incidence was observed on day 37.
Bone resorption.Because bone resorption and extramedullary invasion of tumor cells are often induced in advanced MM, the appearance of hind leg paralysis in the mice was thought to be caused by compression of spinal cord by the tumor mass. X-ray photography showed that bone density of mice with advanced tumor growth was decreased throughout their bodies (Fig 3). Some of these mice showed obvious fractures in the femur (data not shown).
X-ray photographic observation of bones in mice 37 days after IV inoculation with KPMM2 cells. Decrease in bone density was observed in the tumor bearing mouse (right) in comparison with normal mouse (left).
X-ray photographic observation of bones in mice 37 days after IV inoculation with KPMM2 cells. Decrease in bone density was observed in the tumor bearing mouse (right) in comparison with normal mouse (left).
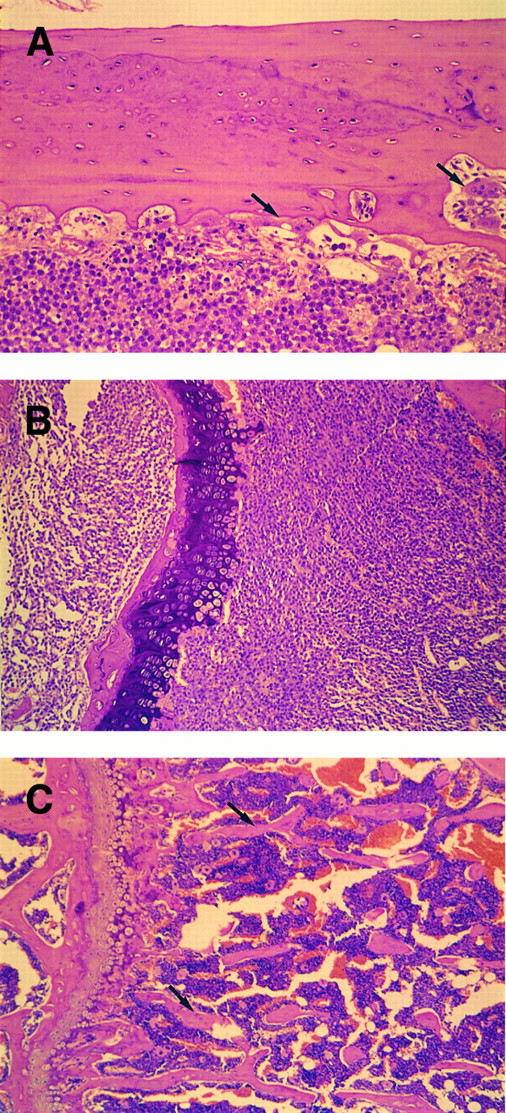
Morphologic changes of the bones were examined by the thin sections in which the bone marrow of femur was largely occupied by plasmablastic cells (Fig 4). Typical osteolytic lesions were formed in cortical bone, where polynuclear osteoclastic cells were located (Fig 4A). Moreover, spongy bone in the end of femur was completely resorbed (Fig 4B).
Histologic observation of bone resorption in the mice IV inoculated with KPMM2 cells. (A, B) Tumor-bearing mouse. (C) Normal mouse. (A) Osteolytic lesions and osteoclastic cells (arrow) were observed in cortical bone surface. Bone marrow was occupied by tumor cells. (B) The end of femur. Spongy bone completely disappeared in IV transplanted mouse. (C) Spongy bone (arrow) was observed in normal mouse.
Histologic observation of bone resorption in the mice IV inoculated with KPMM2 cells. (A, B) Tumor-bearing mouse. (C) Normal mouse. (A) Osteolytic lesions and osteoclastic cells (arrow) were observed in cortical bone surface. Bone marrow was occupied by tumor cells. (B) The end of femur. Spongy bone completely disappeared in IV transplanted mouse. (C) Spongy bone (arrow) was observed in normal mouse.
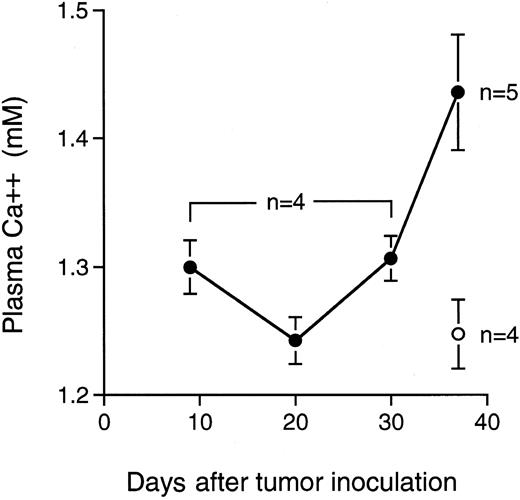
In accordance with these morphologic changes, a significant increase of plasma ionized calcium was found in the tumor-bearing mice 37 days after transplantation (Fig 5).
Level of ionized calcium in plasma of the mice after IV transplantation with KPMM2 cells. Values are given as the mean ± SE. (○), Normal mice; (•), tumor bearing mice.
Level of ionized calcium in plasma of the mice after IV transplantation with KPMM2 cells. Values are given as the mean ± SE. (○), Normal mice; (•), tumor bearing mice.
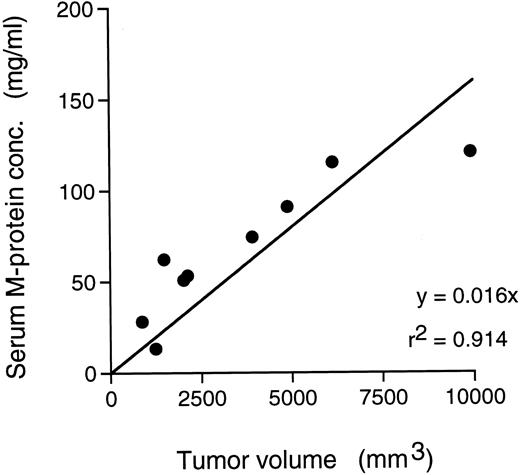
Elevation of serum M-protein.A significant amount of M-protein was detected as early as 9 days after the SC transplantation of the tumor cells (0.87 ± 0.15 mg/mL), which rapidly increased with the tumor growth; approximately 100 mg/mL M-protein was detected in these mice on day 31 (data not shown). A strong correlation was shown between the amount of M-protein and the tumor volume (Fig 6).
Relationship between tumor volume and serum M-protein in SC transplanted mice with KPMM2.
Relationship between tumor volume and serum M-protein in SC transplanted mice with KPMM2.
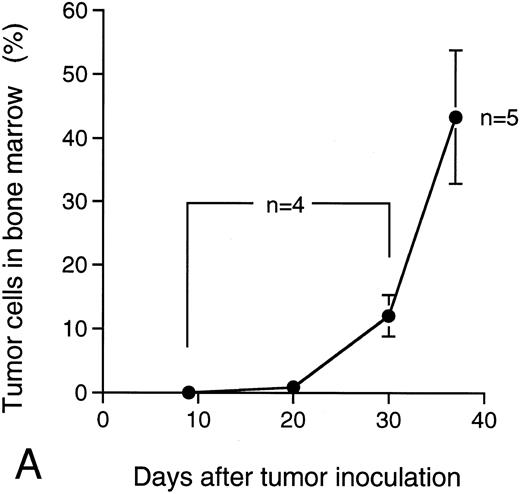
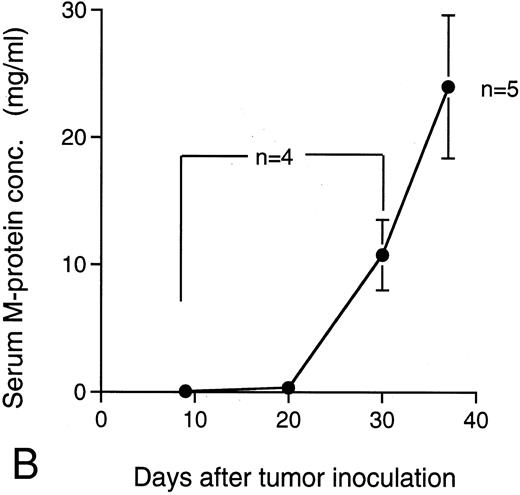
M-protein was also detected in sera of the mice 9 days after the IV transplantation of KPMM2 cells (0.075 ± 0.025 mg/mL; Fig 7B). In these mice, M-protein rapidly increased on day 30 and reached levels that were greater than 20 mg/mL on day 37. The ratio of the tumor cells in bone marrow was concomitantly increased with the accumulation of M-protein (Fig 7A); until day 20, less than 2% of bone marrow cells were of the tumor origin, whereas this ratio was approximately 50% at day 37.
Ratio of tumor cells in bone marrow and serum M-protein level in the mice IV transplanted with KPMM2 cells. The elevation of serum M-protein level closely correlated with the increase of tumor cells was observed. (A) Ratio of tumor cells in bone marrow. (B) Serum M-protein level.
Ratio of tumor cells in bone marrow and serum M-protein level in the mice IV transplanted with KPMM2 cells. The elevation of serum M-protein level closely correlated with the increase of tumor cells was observed. (A) Ratio of tumor cells in bone marrow. (B) Serum M-protein level.
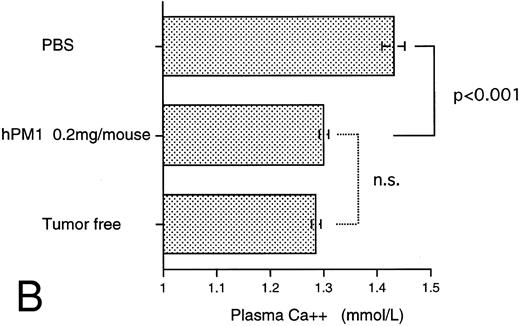
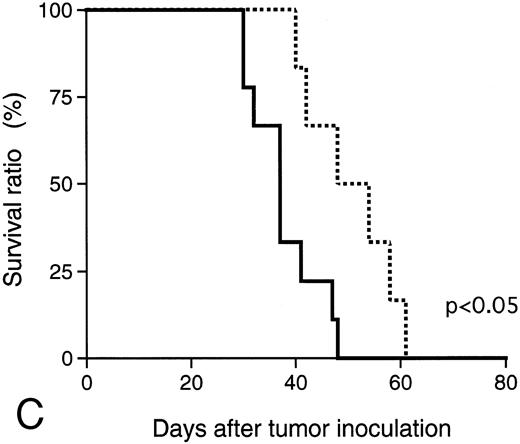
Antitumor effect of hPM1.The consistency for IL-6 dependency of KPMM2 cells in vivo was examined in cells resected from the tumor of mice. The in vitro growth dependency on IL-6 of recovered KPMM2 cells was similar to pretransplanted cells and inhibited by hPM1 (data not shown). The in vivo antitumor activity of hPM1 was examined in mice transplanted IV with KPMM2 cells. A total of 200 μg hPM1 was administered IV into mice on the day after tumor transplantation. Although significant loss of body weight was induced in the control mice 30 days after the tumor transplantation, no remarkable change was observed in the mice treated with hPM1 (data not shown). To estimate the tumor growth in these mice, the amount of M-protein was examined on day 34. The serum M-protein level in control mice was greater than 25 mg/mL, but was decreased by approximately 90% in those treated with hPM1 (Fig 8A). The treatment with hPM1 also suppressed the increased plasma ionized calcium (Fig 8B). This result suggested suppression of bone absorption. In addition, hPM1 increased the survival time of the mice by 30% (Fig 8C), although all these mice eventually died within 60 days.
Effect of anti–IL-6 receptor antibody (hPM1) in mice IV transplanted with KPMM2 cells. hPM1 was injected at 0.2 mg/mouse on day 1. (A) Serum M-protein level. (B) Plasma ionized calcium level on day 34. (Control; n = 12, hPM1; n = 10) (C) Life span. Control ( — ; n = 9); hPM1 (⋅⋅⋅; n = 6).
Effect of anti–IL-6 receptor antibody (hPM1) in mice IV transplanted with KPMM2 cells. hPM1 was injected at 0.2 mg/mouse on day 1. (A) Serum M-protein level. (B) Plasma ionized calcium level on day 34. (Control; n = 12, hPM1; n = 10) (C) Life span. Control ( — ; n = 9); hPM1 (⋅⋅⋅; n = 6).
DISCUSSION
IL-6 plays a central role for the proliferation and/or survival of some human myeloma cells in vitro,8,9,14,15 however, its control over the progression of tumor growth and the associated disease remains enigmatic. Therefore, a well-characterized animal model that closely mimics the disease is important to elucidate on this life-threatening condition. In this study, we established a new xenograft model of MM by transplanting IL-6–dependent human myeloma cells (KPMM2) into SCID mice. This cell line was originally established from ascites of a patient and shown to maintain characters such as secretion of M-protein and IL-6–dependent growth through autocrine mechanism.23 Kawano et al25 have reported that myeloma cells are found as very late antigen 5 (VLA-5) positive mature and VLA-5 negative immature cells; the latter shows higher IL-6 dependency. The characters of our KPMM2 cells were very similar to those of the immature type cells, and IL-6 was essential for their growth.
In comparison with ARH-77 cells, which are not dependent on IL-6, dissemination of KPMM2 cells was restricted mainly to the bone marrow and was not profound in other organs; ARH-77 cells disseminate into many organs other than bone marrow.20 Although the mechanisms for the localized growth of KPMM2 cells in bone marrow are unclear, it is thought that specific binding of myeloma cells to the stromal cells and/or humoral factors in this microenvironment might be involved. Several adhesion molecules are reportedly expressed on myeloma cells and thought to be important for localization and proliferation of myeloma cells in vivo.26-29 KPMM2 cells also express adhesion molecules, such as intercellular adhesion molecule-1 (ICAM-1), neural cell adhesion molecule (NCAM), lymphocyte function associated antigen-1 (LFA-1), VLA-4, and CD44.23 Therefore, it is possible that some mechanisms mediated by the adhesion molecules enable KPMM2 to localize in bone marrow.
Although KPMM2 cells require IL-6 supplementation for stable proliferation in vitro, these cells do not need addition of IL-6 for aggressive growth in mice. Uchiyama et al30 reported that adhesion to bone marrow stromal cells enhanced IL-6 production in myeloma cells. Therefore, we examined whether IL-6 production in KPMM2 cells was influenced by interaction with bone marrow stromal cells. However, upregulation of IL-6 production was not observed in coculture with murine bone marrow stromal cell line ST2. KPMM2 cells freshly isolated from bone marrow were not accelerated in IL-6 production (data no shown). More studies should be required to address the mechanisms of aggressive growth of KPMM2 cells in vivo.
At the advanced stage of tumor progression, most mice developed hind legs paralysis, bone resorption, hypercalcemia, and cachexia-like symptoms (anorexia, severe weight loss, and wasting) and all of these mice died within the week after. Interestingly, these abnormalities were not induced in mice that were SC transplanted with KPMM2 cells; these mice survived much longer than those that were IV transplanted. The development of tumor in bone marrow may be closely related to the induction of these symptoms.
X-ray photographic and histologic observation showed a decrease of bone density in the whole body and osteolytic lesions around osteoclastic polynuclear cells. Several cytokines including IL-6 are known to stimulate osteolysis through direct or indirect activation of osteoclasts.31 32 Therefore, IL-6, secreted by KPMM2 cells, may play an important role for bone absorption in this model.
hPM1 showed a significant antitumor activity against the growth of KPMM2 in bone marrow. A single injection of 200 μg hPM1 into the mice suppressed the increase of serum M-protein and plasma ionized calcium and significantly increased the survival time of the mice. Furthermore, the treatment with hPM1 delayed the development of several abnormalities such as hind leg paralysis, body weight loss, and bone resorption in the tumor-bearing mice (data not shown). Adverse effects were not observed in these mice after treatment with this antibody. Serum M-protein is clinically used as a marker for tumor progression of MM. In our model, the amount of serum M-protein correlated well with the ratio of KPMM2 cells in bone marrow, and the secretion of M-protein in KPMM2 cells is independent on IL-6.23 Therefore, the suppression of M-protein was thought to reflect on the inhibition of tumor growth by hPM1. However, the suppression of M-protein by hPM1 was temporary in this model. M-protein eventually increased in all the mice treated with hPM1 and these mice subsequently died. Higher doses of hPM1 in this model did not show more intense antitumor activity (data not shown). A more study-like combination therapy with chemotherapeutic agents would be required to fully evaluate the therapeutic potential of this antibody.
In summary, we have established a new xenograft model of MM with IL-6–dependent KPMM2 cells, which mimics the pathologic characteristics of the disease. Furthermore, antitumor activity of hPM1 was shown. This model is useful to elucidate on the roles of IL-6 and the therapeutic potential for its antagonists in MM.
ACKNOWLEDGMENT
The authors thank Dr P.K. Saunders for the critical reading of this manuscript.
Address reprint requests to Ken-ichi Akamatsu, PhD, Fuji Gotemba Research Laboratories, Chugai Pharmaceutical Co, Ltd, 1-135, Komakado, Gotemba, Shizuoka 412, Japan.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal