Abstract
Previous studies have shown that in non-Hodgkin's lymphomas and others neoplasms, tumoral progression, treatment response, and outcome are related to the expression of different oncogenic and tumor suppressor proteins. This study aimed to determine the prognostic significance of the expression of p53, bcl2, retinoblastoma protein (Rb), Ki67, CD15, and latent membrane protein 1-Epstein–Barr Virus (LMP1-EBV) proteins in Hodgkin's disease. A retrospective study was performed on 140 patients collected at the 11 participating centers belonging to the Spanish Collaborative Group for the Study of Hodgkin's Disease. A highly sensitive immunohistochemical method with previous microwave-induced antigen retrieval technique was used for the demonstration of the above-mentioned proteins. A Cox's multivariate analysis was performed to evaluate the impact of the variables in the overall survival, together with a logistic regression model for the achievement of complete remission. Univariate statistical analysis confirmed the prognostic significance of the alredy known clinical parameters: stage, age over 60 years, and B symptoms. High proliferation index (Ki67) and loss of Rb expression were also found to be adverse prognostic factors influencing respectively lower overall survival and failure to achieve complete remission. Multivariate analysis confirmed the independent significance of these two parameters and additionally identifies LMP1-EBV expression as a favorable prognostic marker, in relation with overall survival. Histopathological type, p53, bcl2, and CD15 expression lack significant influence on the outcome of this series. The progression of the disease or the response to treatment in HD patients is the consequence of the interrelationship of different factors, among which LMP1 expression, loss of Rb, and high growth fraction seems to play a more relevant role.
DESPITE ITS RELATIVELY indolent clinical course, Hodgkin's disease (HD) appears heterogeneous in terms of histology, immunophenotype, genotype, association with viruses, clinical presentation, and treatment response. Although some clinical parameters (age, stage, histologic subtype, tumor burden, etc) may allow survival time and treatment response to be predicted,1-4 the genetic and molecular basis of the progression of the disease have yet to be elucidated. Nevertheless, the possible involvement of different cell cycle regulators in the pathogenesis of HD has recently been suggested on the basis of immunohistochemical and genetic studies. These include apoptosis inhibitor such as bcl-2,5,6 tumor suppressor genes such as p537-10 and retinoblastoma protein (Rb),8,11 and viral oncogenic proteins, such as EBV-associated latent membrane protein 1-Epstein–Barr Virus (LMP1-EBV).12,13
The level of these proteins in different types of non-Hodgkin's lymphoma (NHL) have been found to predict overall survival or probability of achieving a complete remission in different studies. Thus, both bcl2 and p53 protein expression in large B-cell NHL have been found to be associated with a shorter survival time.14-16 p53 expression, in cases of mantle cell lymphoma, was associated as well with aggressive behavior.17 Although in other tumors a defective Rb expression constitutes an adverse prognostic factor,18 its value in NHL has yet to be established. EBV detection, was not found to be a significant factor in prognosis until recently, when it was found to be associated with a shorter survival time in T-cell NHL.19 Finally, high Ki67 expression, a protein detected in G1 , S, G2 , and M phases of the cell cycle, but not in G0 ,20 is a widely accepted proliferation marker which, in different studies in NHL, appears associated with a shorter survival.21 22
The aim of this study was to determine whether the presence of detectable levels of the above mentioned proteins might be a predictor for the treatment response and overall survival in patients with HD. To accomplish this, we performed a retrospective multicenter study of a large series of cases, which permitted a statistical multivariate study with a high level of confidence. Clinical data from a large series of patients with HD were collected by a cooperative group, and diagnostic tissue from each patient was analyzed immunohistochemically for the presence of p53, Rb, bcl2, LMP1-EBV, CD15, and Ki67 proteins. In 140 eligible patients, statistical analysis was performed to ascertain which variables may influence the outcome in terms of complete remission and survival.
MATERIALS AND METHODS
Patients and Samples
A total of 210 patients were retrospectively collected from 11 participating centers (see Appendix). Patients were eligible for this study if they fulfilled the following criteria: (1) initial diagnosis must be made in a lymph node biopsy before treatment; (2) paraffin-embedded formalin-fixed tissue blocks from the diagnostic lymph node should be available for immunohistochemical studies; (3) a minimum follow-up of 2 years was required; and (4) human immunodeficiency virus (HIV)-infected patients were excluded.
Patients were treated following the conventional therapeutics approches, ie, chemotherapy (MOPP [mechlorethamine, vincristine, procarbazine, and prednisone], ABVD [doxorubicin, bleomycin, vinblastine, and dacarbazine], or the combination of both) for stages III and IV and B symptoms, and radiotherapy for stages I and II without B symptoms. Treatment decisions were not based on molecular and/or immunohistochemical features.
The records of the patients were reviewed and clinical, analytical, therapeutic, and follow-up data were inserted in the data base including age, sex, Ann Arbor staging (I to IV), B symptoms (weight loss, fever, drenching sweat), response to therapy (complete remission v treatment failure), overall survival from histopathological diagnosis, and for the complete responders, relapse-free survival time from the end of therapy. Complete remission was defined as the resolution of clinical and radiologic evidence of disease for a minimun of 4 weeks. Other degrees of remission and/or disease progression were considered as treatment failure. Patients who died during treatment or due to consequences of treatment were considered as deaths due to HD. Patients known to have died after finishing the treatment due to causes unrelated to HD were considered as censored for the survival analysis. All data were checked for validity and coherence. Errors and missing values were reported to the contributors and investigators for correction or confirmation.
In 70 cases, immunohistochemical stains of one or more markers were considered to be unsatisfactory due to the lack of positive internal or external control, and these cases were excluded. Thus, only 140 of the 210 cases initially collected were considered eligible for the final statistical analysis.
Histopathologic Review
To give rise to a uniform series, all cases were reviewed independently by three hematopathologists (A.A., B.A., and H.O.) according to agreed criteria. Diagnostic confirmation and histologic subclassification were performed using hematoxylin and eosin (H&E), Giemsa, and Reticulin stains into the four main types of the Rye classification: lymphocyte predominance (LP), nodular sclerosis (NS), mixed cellularity (MC), and lymphocytic depletion (LD). Cases that did not fit specific criteria for LP, NS, or LD types were included as MC, according to the initial criteria.23 Discrepancies with the initial diagnosis were resolved by consensus obtained in a joint session. Controversial cases were excluded. Priority in the diagnosis was conferred to the morphologic study of the case. Immunohistochemical data, including CD20, CD3, CD30, CD15, and epithelial membrane antigen (EMA) were used as assistance for the differential diagnosis in a subsidiary way, when necessary.
Immunohistochemical Staining of Tissue Sections
All paraffin-embedded tissue sections were performed in a single laboratory. All immunohistochemical stainings for each marker were made in a single laboratory to reduce the heterogeneity of results. Those laboratories selected for performing the different stainings have previously proven their capacity for obtaining high-sensitivity reproducible results.
Paraffin sections cut at 4-μm intervals were stained with the above mentioned antibodies using microwave-mediated antigen retrieval and a standard immunohistochemical procedure by using streptavidin-biotin-complex labelled with alkaline phosphatase. Levamisole was used to inhibit endogenous alkaline phosphatase. Antivimentin stain was performed to confirm the antigenic preservation of tissues for cytoplasmic antigens24 and Ki67 was used as an internal control for the efficiency of the nuclear antigen heat-induced epitope retrieval technique. Internal controls of reactivity were used for Rb, Ki67, bcl2, and CD15. For all of the others antibodies, simultaneous staining of known positive cases was used as a positive control. The incubation of parallel slides omitting the specific antibody was performed as a negative control.
Microwave oven processing.Paraffin sections were dewaxed in xylene and rehydrated in alcohol. Rehydrated slides were placed in plastic Coplin jars filled with a 0.01 mol/L tri-sodium citrate solution and incubated twice for 7 minutes at 700 W in a microwave oven. During microwave processing, the section was always covered by the solution. After microwave heating, slides were allowed to cool down to room temperature for 15 minutes. They were then quickly washed in Tris-buffered–saline (TBS) pH 7.4, and incubated with the specific antibody.25
CD15.Primary antibody was monoclonal mouse antihuman granulocyte-associated antigen CD15, clone C3D-1 (Dako, Glostrup, Denmark, M733). Internal control was granulocyte staining. CD15 was scored as positive if any Hodgkin-Reed–Sternberg cell (HRSC) were labeled.
EBV-LMP.Primary antibody was monoclonal mouse anti-EBV–LMP, clone CS 1-4 (Dako, M897). The use of LMP1-EBV expression as a marker of EBV presence was based on previous studies showing that in HD the presence of EBV encoded RNA and LMP1-EBV expression are roughly equivalent.26 For evaluation of LMP1-EBV reactivity, an external positive control was used. LMP1-EBV staining was scored as positive if any HRSC was stained.
bcl2 oncoprotein.Primary antibody was mouse antihuman bcl-2 oncoprotein monoclonal antibody, clone 124 (Dako, M887). An internal control provided by the reactivity of small lymphocytes was used. Bcl2 staining was scored as positive if a significant fraction (>20%) of HRSC was stained as determined by semiquantitative analysis.
Rb and p53.Primary antibodies were (a) mouse antihuman nuclear Rb protein monoclonal antibody, clone PMG3-245 (14001A, PharMingen, San Diego, CA), which recognizes an epitope between amino acids 300-380 of the human Rb protein. This antibody binds both phosphorylated and unphosphorylated forms of Rb protein.18 Rb staining, which did not show positivity for endothelial cells or some reactive lymphocytes, was considered as unsatisfactory. (b) Rabbit antihuman nuclear p53 protein polyclonal antibody (Novocastra, NCL-p53-CM1, Newcastle Upon Tyne, UK), which recognizes mutant and wild-type p53 protein, was used. For evaluation of the reactivity, a known positive case was used as external control.
Quantitative studiesQuantitative immunohistochemical investigation with the Quantitative Nuclear Antigen application of the Computerized Analyzer System (CAS 200; Becton Dickinson, Mountain View, CA) was used to score individual nuclei for the presence of Rb and p53 nuclear protein. This program measured the percentage of total optical density of positive cell nuclei in tissue sections, ie, the intensity of nuclear staining in comparison with total nuclear area.8 27 The nuclear boundary optical density and antibody threshold was adjusted for each case examined to allow results to be compared. Representative fields down to a minimum of 30.000 μm2 were selected (approximately five fields with a 45× objective and 10× ocular lenses). Sectional analysis was performed by one of the authors (M.S.B.), focusing on tumoral areas.
Growth fraction.Growth fraction was tested by immunostaining with MIB1 monoclonal antibody (Immunotech, Coulter Corp, Miami, FL). This antibody is an equivalent to Ki67 for paraffin-embedded microwave-processed sections.28 An internal positive control was provided by the staining of reactive lymphocytes.
Quantitative studies.Growth fraction was quantified with the Quantitative Proliferation Index application of the CAS 200. This program measures the percentage of proliferating cells in a tissue section.8 27 Representative fields down to a minimum of 30.000 μm2 were selected (approximately five fields with a 45× objective and 10× ocular lenses). Sectional analysis was performed by one of the authors (M.S.B.), focusing on tumoral areas.
Statistical Analysis
Cox proportional model29 and a logistic regression model30 were used to identify factors that might significantly influence survival and initial response to the treatment, respectively. A backward procedure was followed as the modeling strategy. Only cases with all of the required data were selected for statistical analysis; analysis was therefore performed in 140 patients from the initial group of 210 patients. Before the final analysis, these cases were compared with the rejected series to investigate differences. For this initial analysis Student's t test was used to compare the means of the continuous variables between patients and the results were expressed as mean ± standard deviation (SD). The comparison between categorical variables was evaluated by the χ2 method.
Univariate analysis.Survival curves were calculated according to the Kaplan-Meier method31 and differences between curves were evaluated with the log-rank test.32 A Cox's proportional hazard univariate analysis33 was also performed independently for each variable, calculating the relative risk (RR) of each variable for the survival and confidence intervals (CI). A logistic regression analysis was performed independently for each variable to obtain the odds ratio (OR) for failure to achieve a complete response.30 Differences were considered significant if the P value was less than .05. A complementary survival study by using the Kaplan-Meier method was performed for p53, Rb, and Ki67 expression as categorized variables (cutoff = 20%).
Multivariate analysis.To identify the factors that might be of independent significance in influencing survival and the failure to achieve a complete response, a Cox stepwise proportional hazard model29 and a logistic regression model30 were respectively fitted. Variables including in the maximun models were: age (over or under 60 years), Ann Arbor stage (I-II, III, IV), B symptoms (present or absent), gender, histopathologic type (LP, NS, MC, LD), CD15 (positive or negative), bcl-2 (positive or negative), LMP1-EBV (positive or negative), Rb (continuous variable), p53 (continuous variable), growth fraction (continuous variable). Histopathologic type and stage were classified as dummy variable using the lowest level as reference. The four stages (I, II, III, IV) could not be separated due colineality because only one patient in stage I died; therefore stages I and II were analyzed in a joint group. Colineality study was undertaken for the logistic regression model by using the Beisley's criteria34 and for the Cox's proportional model, by the coeficients stability evaluation in the successive iterations. The log-likelihood ratio was the statistic used for models in terms of comparison and proper fit. All P values were two-sided and values of .05 or less were considered to indicate statistical significance. As a multiple significance test had been performed, it is possible that chance associations may have occurred; thus, when under .05, the exact P value is provided. The PRESTA software package was used for all statistical analysis.35
RESULTS
Patient Characteristics
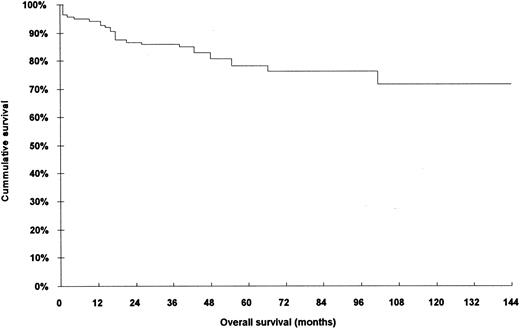
The characteristics of the 140 patients who were included in this analysis are shown in Table 1. When compared with the rejected series of patients (70 cases), no bias and/or statistically significant differences were found in relation to any of parametres studied: age, response to treatment, histopathologic type, stage, and overall survival (Table 2). The overall survival of the 140 patients is shown in Fig 1.
Clinical and Pathological Features of the 140 Analyzed Cases With HD
| Gender | ||
| Male | 93 | (66.43%) |
| Female | 47 | (33.57%) |
| Age (yr) | ||
| Range | 5-83 | |
| Mean ± SD | 37.2 ± 17.5 | |
| <61 | 115 | (83.67%) |
| >60 | 24 | (16.43%) |
| B symptoms | ||
| Present | 76 | (54.29%) |
| Absent | 64 | (45.71%) |
| Stage (Ann Arbor) | ||
| I | 24 | (17.14%) |
| II | 49 | (35.00%) |
| III | 39 | (27.83%) |
| IV | 28 | (20.00%) |
| Response to therapy and relapses | ||
| Complete remission | 111 | (79.29%) |
| Relapses | 20 | (18.02%) |
| Relapse free survival (mo) | ||
| Range | 5-75 | |
| Mean | 28 | |
| Relapses >2 yr from diagnosis | 12 | (60.00%) |
| Relapse free survival (mo) | ||
| Range | 5-24 | |
| Mean | 16 | |
| Treatment failure | 29 | (20.71%) |
| Actuarial status and overall survival | ||
| Alive | ||
| Cases | 98 | (70.00%) |
| Overall survival (mo) | ||
| Range | 24-144 | |
| Mean | 65 | |
| Deaths | ||
| Cases | 28 | (20.00%) |
| Overall survival (mo) | ||
| Range | 0-102 | |
| Mean | 25 | |
| Missing cases | 14 | (10.00%) |
| All | ||
| Cases | 140 | (20.00%) |
| Overall survival (mo) | ||
| Range | 0-144 | |
| Mean | 54 | |
| Histopathological type | ||
| Lymphocytic predominance | 13 | (09.29%) |
| Nodular sclerosis | 74 | (52.86%) |
| Mixed cellularity | 49 | (35.00%) |
| Lymphocytic depletion | 4 | (02.86%) |
| Gender | ||
| Male | 93 | (66.43%) |
| Female | 47 | (33.57%) |
| Age (yr) | ||
| Range | 5-83 | |
| Mean ± SD | 37.2 ± 17.5 | |
| <61 | 115 | (83.67%) |
| >60 | 24 | (16.43%) |
| B symptoms | ||
| Present | 76 | (54.29%) |
| Absent | 64 | (45.71%) |
| Stage (Ann Arbor) | ||
| I | 24 | (17.14%) |
| II | 49 | (35.00%) |
| III | 39 | (27.83%) |
| IV | 28 | (20.00%) |
| Response to therapy and relapses | ||
| Complete remission | 111 | (79.29%) |
| Relapses | 20 | (18.02%) |
| Relapse free survival (mo) | ||
| Range | 5-75 | |
| Mean | 28 | |
| Relapses >2 yr from diagnosis | 12 | (60.00%) |
| Relapse free survival (mo) | ||
| Range | 5-24 | |
| Mean | 16 | |
| Treatment failure | 29 | (20.71%) |
| Actuarial status and overall survival | ||
| Alive | ||
| Cases | 98 | (70.00%) |
| Overall survival (mo) | ||
| Range | 24-144 | |
| Mean | 65 | |
| Deaths | ||
| Cases | 28 | (20.00%) |
| Overall survival (mo) | ||
| Range | 0-102 | |
| Mean | 25 | |
| Missing cases | 14 | (10.00%) |
| All | ||
| Cases | 140 | (20.00%) |
| Overall survival (mo) | ||
| Range | 0-144 | |
| Mean | 54 | |
| Histopathological type | ||
| Lymphocytic predominance | 13 | (09.29%) |
| Nodular sclerosis | 74 | (52.86%) |
| Mixed cellularity | 49 | (35.00%) |
| Lymphocytic depletion | 4 | (02.86%) |
Comparation Between Included and Rejected Series for the Final Statistical Analysis
| . | Excluded (%) . | Included (%) . | P . |
|---|---|---|---|
| Cases | 70 | 140 | |
| Age (yr) (mean ± SD) | 38.6 ± 18.3 | 37.2 ± 17.5 | .5820* |
| Gender | |||
| Male | 37 (52.85) | 93 (66.42) | .0563† |
| Female | 33 (47.14) | 47 (33.57) | .0563† |
| Response to initial treatment | |||
| Complete remission | 55 (78.57) | 111 (79.28) | .8283† |
| Treatment failure | 15 (21.42) | 28 (20.00) | .8283† |
| Overall survivall | |||
| Mean ± SD (mo) | 60.6 ± 45.3 | 53.7 ± 34.0 | .2314‡ |
| Actuarial status | |||
| Alive | 46 (65.71) | 98 (70.00) | .5283† |
| Deaths | 18 (25.71) | 28 (20.00) | .3453† |
| Missing cases | 6 (8.57) | 14 (10.00) | .7395† |
| Histopathological type | |||
| Lymphocytic predominance | 6 (8.57) | 13 (9.28) | .8649† |
| Nodular sclerosis | 37 (52.85) | 74 (52.85) | 1.0000† |
| Mixed cellularity | 22 (31.42) | 49 (35.00) | .6060† |
| Lymphocytic depletion | 5 (7.14) | 4 (2.85) | .1483† |
| Stage | |||
| I | 5 (7.14) | 24 (17.14) | .0510† |
| II | 32 (45.71) | 49 (35.00) | .1327† |
| III | 20 (28.57) | 39 (27.85) | .9135† |
| IV | 13 (18.57) | 28 (20.00) | .8055† |
| . | Excluded (%) . | Included (%) . | P . |
|---|---|---|---|
| Cases | 70 | 140 | |
| Age (yr) (mean ± SD) | 38.6 ± 18.3 | 37.2 ± 17.5 | .5820* |
| Gender | |||
| Male | 37 (52.85) | 93 (66.42) | .0563† |
| Female | 33 (47.14) | 47 (33.57) | .0563† |
| Response to initial treatment | |||
| Complete remission | 55 (78.57) | 111 (79.28) | .8283† |
| Treatment failure | 15 (21.42) | 28 (20.00) | .8283† |
| Overall survivall | |||
| Mean ± SD (mo) | 60.6 ± 45.3 | 53.7 ± 34.0 | .2314‡ |
| Actuarial status | |||
| Alive | 46 (65.71) | 98 (70.00) | .5283† |
| Deaths | 18 (25.71) | 28 (20.00) | .3453† |
| Missing cases | 6 (8.57) | 14 (10.00) | .7395† |
| Histopathological type | |||
| Lymphocytic predominance | 6 (8.57) | 13 (9.28) | .8649† |
| Nodular sclerosis | 37 (52.85) | 74 (52.85) | 1.0000† |
| Mixed cellularity | 22 (31.42) | 49 (35.00) | .6060† |
| Lymphocytic depletion | 5 (7.14) | 4 (2.85) | .1483† |
| Stage | |||
| I | 5 (7.14) | 24 (17.14) | .0510† |
| II | 32 (45.71) | 49 (35.00) | .1327† |
| III | 20 (28.57) | 39 (27.85) | .9135† |
| IV | 13 (18.57) | 28 (20.00) | .8055† |
Student's t test.
χ2.
Kaplan Meier.
Immunohistochemical Features of the 140 Analyzed Cases
| CD15 | ||
| Positive | 104 | (74.29%) |
| Negative | 36 | (25.71%) |
| LMP1 | ||
| Positive | 72 | (51.43%) |
| Negative | 68 | (48.57%) |
| bcl-2 | ||
| Positive | 86 | (61.43%) |
| Negative | 54 | (38.57%) |
| Rb | ||
| Positive | 137 | (97.85%) |
| Range | 1.3-40.5 | |
| Mean ± SD | 11.93 ± 8.70 | |
| p53 | ||
| Positive | 130 | (92.85%) |
| Range | 0.1-30.1 | |
| Mean ± SD | 4.06 ± 4.50 | |
| Growth fraction (Ki67) | ||
| Range | 0.3-41.0 | |
| Mean ± SD | 15.45 ± 9.40 |
| CD15 | ||
| Positive | 104 | (74.29%) |
| Negative | 36 | (25.71%) |
| LMP1 | ||
| Positive | 72 | (51.43%) |
| Negative | 68 | (48.57%) |
| bcl-2 | ||
| Positive | 86 | (61.43%) |
| Negative | 54 | (38.57%) |
| Rb | ||
| Positive | 137 | (97.85%) |
| Range | 1.3-40.5 | |
| Mean ± SD | 11.93 ± 8.70 | |
| p53 | ||
| Positive | 130 | (92.85%) |
| Range | 0.1-30.1 | |
| Mean ± SD | 4.06 ± 4.50 | |
| Growth fraction (Ki67) | ||
| Range | 0.3-41.0 | |
| Mean ± SD | 15.45 ± 9.40 |
Immunohistochemical Study
p53 nuclear protein expression, was observed in 130 of the 140 cases (Table 3). The levels of expression ranged between 0.10% and 30.10% of protein staining (mean, 4.06). p53 reactivity detection in cell types other than HRSC was negligible in all cases. The amount of HRS positive cells and the intensity of staining varied from rare positive cells to a strong staining of most of the large mononuclear cells.
Although Rb nuclear protein expression varied from case to case, it was more generalized than that corresponding to p53. There were 137 of 140 cases with a detectable signal (ranging between 1.30 and 40.50; mean, 11.93). Most of the signals measured in all cases derived from HRSC, which usually showed a stronger staining than in small lymphocytes, endothelial, and epithelioid cells. The signal was confined to the nuclei with the exception of mitotic cells.
Ki67 expression was observed in all cases with staining area values of 0.3% and 41.0% (median, 15.45). In addition to the frequent HRSC staining, it was possible to recognize a distinct staining of some small lymphocytes, epithelioid cells, and endothelial cell nuclei intermingled with the supposed neoplastic population.
CD15 and bcl2 immunostaining in HRSC were found in 74% and 61% of cases, respectively. LMP1-EBV cytoplasmic expression was observed in HRSC in 51% of cases, mainly associated with MC type (χ2 = 8.66; P = .0034).
Statistical Analysis
Univariate analysis.The results of the univariate Cox's test is shown in Table 4. Advanced age (RR: 2.596; P = .0226), B symptoms (RR: 4.854; P = .0016), stage III (RR: 6.643; P = .0011), and IV (RR: 8.547; P = .0003) were significantly associated with a shorter survival. There were no differences in the overall survival of patients with different histopathologic types. The only immunohistochemical variables statistically associated with a shorter survival were the proliferation index (Ki67) and Rb. For Ki67, this was observed in both analysis, when studied as a continuous variable (RR: 1.045; P = .0206) and also as categorized variable (cutoff: 20%; r = 5.80; P = .0161). For Rb, a statistically significant relation was identified between loss of Rb expression and shorter survival time, when using a cutoff of 20% (r = 4.30; P = .0380) (Fig 2).
Overall Survival (Univariate Cox's Regression Model)
| Variable . | RL . | RR . | 95% CI . | P . |
|---|---|---|---|---|
| Clinical | ||||
| Age | <60 | 2.59 | 1.13-5.93 | .0226 |
| Gender | Female | 1.35 | 0.59-3.09 | .5266 |
| B-symptoms | Negative | 4.85 | 1.83-12.84 | .0016 |
| Stage III | I + II | 6.64 | 2.16-20.38 | .0011 |
| Stage IV | I + II | 8.54 | 2.71-26.87 | .0003 |
| Type | ||||
| NS | LP | 1.83 | 0.23-14.24 | .5678 |
| MC | NS | 4.02 | 0.52-30.58 | .1753 |
| LD | MC | 6.33 | 0.57-70.09 | .1277 |
| Immunohistochemistry | ||||
| CD 15 | Negative | 0.89 | 0.38-2.11 | .7990 |
| bcl.2 | Negative | 0.69 | 0.32-1.45 | .6655 |
| LMP1 | Negative | 0.85 | 0.40-1.80 | .6881 |
| Rb4-150 | 0 | 0.96 | 0.91-1.01 | .1540 |
| p534-150 | 0 | 0.95 | 0.85-1.06 | .6178 |
| Ki674-150 | 0 | 1.04 | 1.00-1.08 | .0206 |
| Variable . | RL . | RR . | 95% CI . | P . |
|---|---|---|---|---|
| Clinical | ||||
| Age | <60 | 2.59 | 1.13-5.93 | .0226 |
| Gender | Female | 1.35 | 0.59-3.09 | .5266 |
| B-symptoms | Negative | 4.85 | 1.83-12.84 | .0016 |
| Stage III | I + II | 6.64 | 2.16-20.38 | .0011 |
| Stage IV | I + II | 8.54 | 2.71-26.87 | .0003 |
| Type | ||||
| NS | LP | 1.83 | 0.23-14.24 | .5678 |
| MC | NS | 4.02 | 0.52-30.58 | .1753 |
| LD | MC | 6.33 | 0.57-70.09 | .1277 |
| Immunohistochemistry | ||||
| CD 15 | Negative | 0.89 | 0.38-2.11 | .7990 |
| bcl.2 | Negative | 0.69 | 0.32-1.45 | .6655 |
| LMP1 | Negative | 0.85 | 0.40-1.80 | .6881 |
| Rb4-150 | 0 | 0.96 | 0.91-1.01 | .1540 |
| p534-150 | 0 | 0.95 | 0.85-1.06 | .6178 |
| Ki674-150 | 0 | 1.04 | 1.00-1.08 | .0206 |
Abbreviations: RL, reference level; RR, risk ratio in specific disease mortality; CI, confidence intervals; LP, lymphocytic predominance; NS, nodular sclerosis; MC, mixed cellularity; LD, lymphocytic depletion.
Rb, p53, and Ki67 expression was analyzed as continuous variables.
Survival of the 140 evaluated patients according to Ki67 proliferation index and Rb expression.
Survival of the 140 evaluated patients according to Ki67 proliferation index and Rb expression.
The results of the univariate logistic regression analysis are shown in Table 5. B symptoms (OR: 5.219; P = .0020) and stage III (OR: 6.277; P = .0017) and IV (OR: 8.800; P = .0004) were also significantly associated with the probability of failure to achieve a complete remission. For the immunohistochemical variables, Rb expression (OR: 0.934; P = .0316) and proliferation index (OR: 1.047; P = .0316) were also significantly associated with the probability of failure to achieve a complete remission.
Failure to Achieve a Complete Remission (Univariate Logistic Regression)
| Variable . | RL . | OR . | 95% CI . | P . |
|---|---|---|---|---|
| Clinical | ||||
| Age | <60 | 1.97 | 0.72-.451 | .1800 |
| Gender | Female | 1.09 | 0.45-2.26 | .8291 |
| B-symptoms | Negative | 5.21 | 1.85-14.71 | .0020 |
| Stage III | I + II | 6.27 | 2.01-19.56 | .0017 |
| Stage IV | I + II | 8.80 | 2.69-28.73 | .0004 |
| Type | ||||
| NS | LP | 1.17 | 0.23-5.93 | .8423 |
| MC | NS | 1.83 | 0.35-9.47 | .5237 |
| LD | MC | 1.83 | 0.12-27.79 | .6662 |
| Immunohistochemistry | ||||
| CD 15 | Negative | 0.80 | 0.31-2.03 | .6490 |
| bcl.2 | Negative | 0.46 | 0.20-1.08 | .0734 |
| LMP1 | Negative | 0.63 | 0.27-1.47 | .2917 |
| Rb5-150 | 0 | 0.93 | 0.87-0.99 | .0316 |
| p535-150 | 0 | 0.92 | 0.81-1.05 | .2328 |
| Ki675-150 | 0 | 1.04 | 1.00-1.09 | .0316 |
| Variable . | RL . | OR . | 95% CI . | P . |
|---|---|---|---|---|
| Clinical | ||||
| Age | <60 | 1.97 | 0.72-.451 | .1800 |
| Gender | Female | 1.09 | 0.45-2.26 | .8291 |
| B-symptoms | Negative | 5.21 | 1.85-14.71 | .0020 |
| Stage III | I + II | 6.27 | 2.01-19.56 | .0017 |
| Stage IV | I + II | 8.80 | 2.69-28.73 | .0004 |
| Type | ||||
| NS | LP | 1.17 | 0.23-5.93 | .8423 |
| MC | NS | 1.83 | 0.35-9.47 | .5237 |
| LD | MC | 1.83 | 0.12-27.79 | .6662 |
| Immunohistochemistry | ||||
| CD 15 | Negative | 0.80 | 0.31-2.03 | .6490 |
| bcl.2 | Negative | 0.46 | 0.20-1.08 | .0734 |
| LMP1 | Negative | 0.63 | 0.27-1.47 | .2917 |
| Rb5-150 | 0 | 0.93 | 0.87-0.99 | .0316 |
| p535-150 | 0 | 0.92 | 0.81-1.05 | .2328 |
| Ki675-150 | 0 | 1.04 | 1.00-1.09 | .0316 |
Abbreviation: OR, odds ratio.
Rb, p53, and Ki67 expression was analyzed as continuous variables.
Multivariate analysis for overall survival.In the multivariate Cox's regression model for survival, the model had a good fit (χ2 = 33.33, P < .0001) (Table 6). Patients over 60 years had a risk ratio in disease-specific mortality of 3.39 (95% CI:1.32 to 8.67, P = .0104) considering patients under 60 years old. Patients with stage III had a risk-ratio in disease-specific mortality of 7.93 (95% CI:2.54 to 24.71, P = .0005) and patients in stage IV had a risk-ratio in disease-specific mortality of 9.46 (95% CI:2.92 to 30.57, P = .0003), respectively, both considering patients in stage I-II. Increase in Ki67 expression is associated with a risk ratio in disease-specific mortality of 1.05 (95% CI:1.01 to 1.09, P = .0099), when analyzed as a continuous variable. LMP1-EBV expression had a protective influence, with a risk-ratio specific mortality of 0.39 (95% CI:0.17 to 0.92, P = .0307) for positive patients in comparison with negative ones.
Factors Influencing Overall Survival (Multivariate Cox's Regression Model)
| . | Reference . | RR . | 95% CI . | P . |
|---|---|---|---|---|
| Age >60 | <61 | 3.39 | 1.32-8.67 | .0104 |
| Stage III | Stage I + II | 7.93 | 2.54-24.71 | .0005 |
| Stage IV | Stage I + II | 9.46 | 2.92-30.57 | .0003 |
| Growth fraction | 0 | 1.05 | 1.01-1.09 | .0099 |
| LMP1 expression | Unexpression | 0.39 | 0.17-0.92 | .0307 |
| Model χ2 = 33.332, P < .0001 | ||||
| . | Reference . | RR . | 95% CI . | P . |
|---|---|---|---|---|
| Age >60 | <61 | 3.39 | 1.32-8.67 | .0104 |
| Stage III | Stage I + II | 7.93 | 2.54-24.71 | .0005 |
| Stage IV | Stage I + II | 9.46 | 2.92-30.57 | .0003 |
| Growth fraction | 0 | 1.05 | 1.01-1.09 | .0099 |
| LMP1 expression | Unexpression | 0.39 | 0.17-0.92 | .0307 |
| Model χ2 = 33.332, P < .0001 | ||||
Multivariate analysis for treatment response.In the regression model, stage III, stage IV, B symptoms, and Ki67 expression influenced negatively in the probability to achieve a complete remission: stage III had an odds ratio of 5.21 (95% CI:1.49 to 18.26, P = .0095) and stage IV patients 4.67 (95% CI:1.12 to 18.20, P = .0238) considering patients in stage I and II (Table 7). Patients with B symptoms had an OR of 3.99 (95% CI:1.20 to 13.31, P = .0226) for patients without B symptoms. Ki67 expression had a OR of 1.06 (95% CI:1.00 to 1.11, P = .0246) with respect to negative staining. Rb expression had a protective effect with an OR of 0.90 (95% CI:0.84 to 0.97, P = .0001) for absence of expression.
Factors Influencing Failure to Achieve a Complete Remission (Multivariate Logistic Regression)
| . | Reference . | OR . | 95% CI . | P . |
|---|---|---|---|---|
| B symptoms | Absence | 3.99 | 1.20-13.31 | .0226 |
| Stage III | Stage I + II | 5.21 | 1.49-18.26 | .0095 |
| Stage IV | Stage I + II | 4.67 | 1.21-18.20 | .0238 |
| Growth fraction | 0 | 1.06 | 1.00-1.11 | .0246 |
| Rb | 0 | 0.90 | 0.84-0.97 | .0001 |
| Model χ2 = 35.054, P < .0001 | ||||
| . | Reference . | OR . | 95% CI . | P . |
|---|---|---|---|---|
| B symptoms | Absence | 3.99 | 1.20-13.31 | .0226 |
| Stage III | Stage I + II | 5.21 | 1.49-18.26 | .0095 |
| Stage IV | Stage I + II | 4.67 | 1.21-18.20 | .0238 |
| Growth fraction | 0 | 1.06 | 1.00-1.11 | .0246 |
| Rb | 0 | 0.90 | 0.84-0.97 | .0001 |
| Model χ2 = 35.054, P < .0001 | ||||
DISCUSSION
As was the case with previously published studies, we found a statistically significant adverse impact on the overall survival and treatment response for some clinical parameters such as age over 60, B symptoms, and advanced clinical stage. The most outstanding result of our analysis is the identification of different biologic parameters that are related to a favorable outcome in HD patients: LMP1-EBV expression was associated with longer overall survival, and elevated Rb expression was found to be associated with a higher probability to achieve a complete remission. On the other hand, a high proliferation index (Ki67) was found to be unfavorably associated with both achievement of complete remission and overall survival. In our series p53, bcl2, and CD15 expression did not show any significant association with the outcome.
The prognostic relevance of high proliferation index, as measured with the Ki67 antibody, has been extensively shown in diverse types of neoplasms,36,37 including NHLs,21 22 although to our knowledge, data for this in HD have not yet been published. Current data support Ki67 as a relevant prognostic factor among immunohistochemical markers, which predicts both a shorter survival and a lower probability of achieving complete remission.
The Ki67 values scored in this study do not only reflect the growth fraction exclusively of HRSC, but also measures the proliferation index of the other cell subpopulations present in HD-involved lymph nodes. As in other lymphomas, the presence and degree of activation of T and B cells, macrophages, and endothelial cells could be directly involved in the tumor cell growth and apoptotic escape. The whole cell population in HD (HRSC and accompanying cells) is probably involved in a self-regulatory process, which forms the basis for the progression of the disease and the clinical outcome. Although we considered double labelling Ki67-CD30 to be performed, we excluded this possibility, as it does not actually guarantee scoring the proliferation index of the putative neoplastic population in HD. At the same time, the analysis of Ki67 expression of the overall cellularity allows avoiding the introduction of bias derived from the selection of the neoplastic cell subset, facilitating the automatic measure of the histologic sample and the reproductibility of the assay.
The retinoblastoma susceptibility gene (Rb) has been characterized as a tumor suppressor gene involved in cell-cycle control, where it seems to act as a status quo cellular growth fraction control mechanism, regulating gene transcription.38 The status of Rb gene in HD has been recently investigated,11 and an anomalous pattern of Rb expression, a partial loss of Rb expression, has been described in a significant number of HD cases. This data is in keeping with previous cytogenetic studies showing loss of 13q to be one of the most frequent cytogenetic observations in HD.39 40
Low level of Rb expression exerts an unfavorable and statistically independent effect on the achievement of complete remission, while its influence on overall survival was only found for a cutoff point of Rb = 20. This result is in agreement with previously reported data relative to other human neoplasms such as soft tissue sarcomas.18 41-43
There seems to be an opposite situation in these findings concerning the significance of Rb and Ki67 detection. These parameters, identified as significant in relation with prognosis, increase of Ki67, and loss of Rb, could take place in association with, at least some in vitro models. Loss of Rb expression has been found to lead to a higher growth fraction, in consonance with the cell cycle negative regulator role of Rb protein.38 At the same time, both findings seem to validate the technique used for immunostaining and further quantification ot the signal, as cases with stronger staining for one of these proteins have a tendency to show weaker values for the other protein.
The technique used does not guarantee that the Rb protein analyzed comes from HSRC; in fact, these cells express a much higher level of Rb protein than other cell types present in these lymph nodes, and the protein measured derives mainly from the large cells.8
LMP1-EBV expression has also been found, in the multivariate analysis, to be associated with a longer survival. LMP1 is an EBV-induced oncogenic protein, characteristically expressed by HSRC in HD and may be involved in the induction of an antigenic response.44 Indeed, LMP1-EBV mutations, which seem to take place in some HD cases,45,46 are thought to abolish the antigenicity of this protein,47 a more aggressive behavior having been described in tumors harboring LMP1-EBV mutations.46,48,49 The findings of this series could support the interpretation that LMP1-EBV positive cases of HD could induce a more effective immune response.12,46 These results contrasts with those of previous series,26 50 which failed to identify an effect on survival of the expression of EBV-related LMP1, which may be due to the small size of the series or the lack of multivariate analysis.
Hodgkin's lymphoma seems to be a multigenic disease in which progression or the response to treatment is the consequence of the interrelationship of diverse factors, among which LMP1-EBV expression, loss of Rb, and high growth fraction seems to play a more relevant role. Although further and prospective studies are still neccesary to confirm these data, the results of our study support the hypothesis that molecular factors could be crucial in the oncogenesis and in the prognosis of HD. The use of the proposed prognostic markers could allow the identification of the more agressive variants of HD, susceptible to treatment with different chemotherapeutic approaches. The exact nature of Rb alteration in HD has yet to be clarified.
ACKNOWLEDGMENT
The authors would like to thank all of the members of the Spanish Collaborative Group for the Study of Hodgkin's Disease.
APPENDIX
The Spanish Collaborative Group for the Study of Hodgkin's Disease consisted of the following investigators and centers: V. Abraira, Hospital Ramón y Cajal, Universidad de Alcalá, Madrid; A. Acevedo, Hospital de la Princesa, Universidad Autónoma, Madrid; B. Aguilera, Instituto Nacional de Toxicologia, Universidad Autónoma, Madrid; C. Bellas, Hospital Ramón y Cajal, Universidad de Alcalá, Madrid; R. Carrión, Hospital Gregorio Marañón, Universidad Complutense, Madrid; I. Chacón, Hospital Virgen de la Salud, Toledo; E. Cristobal, Hospital Gregorio Marañón, Universidad Complutense, Madrid; E. Flores, Hospital Gregorio Marañón, Universidad Complutense, Madrid; J. Forteza, Hospital Xeral de Galicia, Universidad de Santiago de Compostela; M. Fraga, Hospital Xeral de Galicia, Universidad de Santiago de Compostela; R. Garcia-del-Moral, Hospital Clı́nico San Cecı́lio, Universidad de Granada; F. Gomez-Marcos, Hospital Ramón y Cajal, Universidad de Alcalá, Madrid; A.I. Manzanal, Hospital Ramón y Cajal, Universidad de Alcalá, Madrid; B. Martinez, Hospital Virgen de la Salud, Toledo; J. Menarguez, Hospital Gregorio Marañón, Universidad Complutense, Madrid; C. Montalbán, Hospital Ramón y Cajal, Universidad de Alcalá, Madrid; M. Morente, Hospital General Universitario, Universidad de Alcalá, Guadalajara; H. Oliva, Fundación Jimenez Diaz, Universidad Autónoma de Madrid; F. O'Valle, Hospital Clı́nico San Cecı́lio, Universidad de Granada; M.A. Piris, Hospital Virgen de la Salud, Toledo; C. Ramirez, Hospital Clı́nico San Cecı́lio, Universidad de Granada; F. Revelles, Hospital Clı́nico San Cecı́lio, Universidad de Granada; V. Romagosa, Hospital Principes de España, Universidad Central de Barcelona; C. Ruiz-Marcellán, Hospital Valle de Hebrón, Universidad Autónoma de Barcelona; M. Sanchez-Beato, Hospital Virgen de la Salud, Toledo; A. Santón, Hospital Ramón y Cajal, Universidad de Alcalá, Madrid; M. Vaquero, Hospital G.Trias i Pujol, Universidad Autónoma de Barcelona, Badalona.
Supported by Grant No. 93/0016 from the Fondo de Investigacion Sanitaria (FIS), Spain.
Address reprint requests to Manuel M. Morente, MD, Servicio de Anatomia Patológica, Hospital General Universitario, Calle del Donante de Sangre, s/n, 19002 Guadalajara, Spain.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal