Abstract
The expression pattern of the BCL-6 transcription factor has been assessed in normal and neoplastic B-cell populations and in Hodgkin's disease. However, little is known about BCL-6 expression and its biological significance in T-cell neoplasms. In this study, a series of 59 lymphoma samples, including 27 CD30+ anaplastic large-cell lymphomas (ALCLs), 24 other peripheral T-cell neoplasms, and 8 T-cell lymphoblastic lymphomas (T-LBLs), as well as a panel of t(2; 5)-positive lymphoma-derived human cell lines, were evaluated for BCL-6 protein expression by immunohistochemistry on frozen sections and cell smears. To define the relationship between BCL-6 protein and CD30 antigen in CD30+ ALCLs and in non-neoplastic lymph nodes, serial section immunohistochemistry and two-color staining were used in selected CD30+ ALCLs as well as in reactive lymph nodes with non-neoplastic T-cell proliferations. BCL-6 protein was expressed in 12 of 27 (45%) CD30+ ALCL cases, irrespective of their antigenic phenotypes (T-cell or null-cell type), and in the t(2; 5)-positive cell lines. In contrast, the remaining 24 peripheral T-cell neoplasms as well as the 8 T-LBLs were considered negative for BCL-6 expression. Coexpression of CD30 and BCL-6, as detected in CD30+ ALCLs, was also found in a subset of non-neoplastic lymphoid elements, namely the large lymphoid cells scattered in the interfollicular areas of reactive lymph nodes. These findings suggest that CD30+ ALCLs may represent the neoplastic transformation of extrafollicular CD30+ cells and that BCL-6 may provide an additional marker for characterizing CD30+ ALCLs.
THE BCL-6 GENE has been originally identified by virtue of its involvement in chromosomal translocations affecting chromosomal band 3q27 in diffuse large B-cell lymphomas of the immunocompetent host.1-4 The BCL-6 protein, which belongs to the family of transcription factors containing zinc finger motifs,2,3 is a nuclear-located protein.5-9 Within the B-cell compartment, BCL-6 protein is preferentially expressed in follicular germinal center B-cells and related B-cell lymphomas,5-9 whereas it is not expressed in immature B-cell precursors or in differentiated plasma cells.8,9
Expression of BCL-6 in normal lymphoid tissue occurs also in a subset of interfollicular and intrafollicular CD4+ T cells, in a small population of CD30+ cells localized in the interfollicular areas, and in rare CD30+ large cells at the edge of the germinal centers.8 This finding raises questions regarding the biological significance of BCL-6 expression in these subsets of lymphoid cells. In particular, it remains to be defined whether tumor cells showing CD4+ and/or CD30+ phenotypes also express the BCL-6 protein.
Although BCL-6 expression has been extensively studied in normal and neoplastic B-cell populations and in Hodgkin's disease (HD),5-9 little is known about its expression and biological significance in peripheral T-cell neoplasms. Among the latter, CD30+ anaplastic large-cell lymphomas (ALCLs) have been widely characterized (reviewed10-12 ). In particular, based on the finding that these lymphomas are composed solely of tumor cells that have reached the activation state defined by the CD30 antigen,13 CD30+ ALCLs have been proposed to represent the neoplastic proliferation of extrafollicular CD30+ lymphoid cells. In the context of reactive lymphoid tissue, CD30+ cells, which are usually of T-cell origin, are preferentially localized around B-cell follicles.13
This study was aimed at investigating the frequency and the distribution of BCL-6 protein expression throughout the major pathological categories of human peripheral T-cell neoplasms and at comparing it with the expression of CD30 antigen within both reactive and neoplastic lymph nodes.
MATERIALS AND METHODS
Samples.The study was based on tissue samples of 51 human lymphomas including T-cell chronic lymphocytic leukemia/small lymphocytic T-cell lymphoma (1 case), mycosis fungoides (7 cases), peripheral T-cell lymphomas (PTCLs; 16 cases), and CD30+ ALCLs (27 cases; Table 1). A small number of T-cell lymphoblastic lymphomas (CD4+/CD8+, 4 cases; CD4+/CD8−, 3 cases; and CD4−/CD8−, 1 case) were also included in the study for comparative purposes. The mycosis fungoides group displayed the CD4+/CD8− phenotype. Among the remaining peripheral T-cell neoplasms other than CD30+ ALCL, immunologic analysis showed different tumor cell immunophenotypes (CD4+/CD8−, 9 cases; CD4+/CD8+, 5 cases; and CD4−/CD8−, 3 cases). CD4+/CD8+ tumors were TdT negative. Within the CD30+ ALCL group, 19 cases (all CD4+/CD8−) had a T-cell phenotype and lacked B-cell markers; the remaining 8 cases (all CD4−/CD8−) were composed of neoplastic cells that failed to express either B- or T-cell–specific antigens.
BCL-6 Expression in Human T-cell Neoplasms
| Diagnosis* . | No. of Positive/Tested Cases† . |
|---|---|
| Precursor T-cell neoplasm | |
| Precursor T-lymphoblastic lymphoma | 0/8 |
| Peripheral T-cell neoplasms | |
| T-cell chronic lymphocytic leukemia | 0/1 |
| Mycosis fungoides | 0/7 |
| Peripheral T-cell lymphomas | |
| Unspecified | 0/11 |
| AILD-like | 0/4 |
| Angiocentric | 0/1 |
| Anaplastic large-cell CD30+ lymphomas | |
| T cell | 8/19 |
| null cellρ | 4/8 |
| Diagnosis* . | No. of Positive/Tested Cases† . |
|---|---|
| Precursor T-cell neoplasm | |
| Precursor T-lymphoblastic lymphoma | 0/8 |
| Peripheral T-cell neoplasms | |
| T-cell chronic lymphocytic leukemia | 0/1 |
| Mycosis fungoides | 0/7 |
| Peripheral T-cell lymphomas | |
| Unspecified | 0/11 |
| AILD-like | 0/4 |
| Angiocentric | 0/1 |
| Anaplastic large-cell CD30+ lymphomas | |
| T cell | 8/19 |
| null cellρ | 4/8 |
Abbreviation: AILD, angioimmunoblastic T-cell lymphoma.
According to the revised European-American lymphoma classification.14
The percentage of positive neoplastic cells ranged from 10% to 60%.
ρ Null cell group included two cases classified as anaplastic large-cell lymphoma Hodgkin's-like. They were BCL-6 negative.
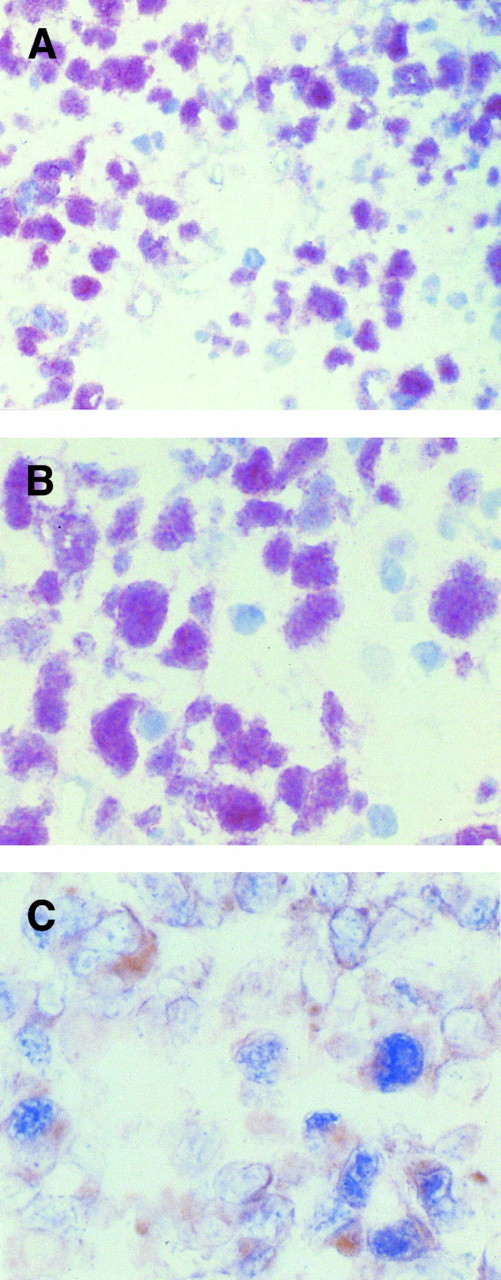
(A and B) Immunohistochemical demonstration of BCL-6 protein expression in a CD30+ anaplastic large-cell lymphoma employing the APAAP method on frozen tissue sections. (A) In this field several tumor cells show a nuclear staining pattern with anti–BCL-6 MoAb PG-B6. (B) Anaplastic large cells show strong nuclear immunoreactivity with the anti–BCL-6 MoAb PG-B6. The positivity is microgranular. (C) Coexpression of BCL-6 protein (nuclear, blue) is observed in a proportion of CD30+ (cytoplasmic and membranous, reddish) anaplastic large tumor cells, as detected by two-color staining (see Materials and Methods). Original magnification (A) ×250, (B) ×400, and (C) ×400.
(A and B) Immunohistochemical demonstration of BCL-6 protein expression in a CD30+ anaplastic large-cell lymphoma employing the APAAP method on frozen tissue sections. (A) In this field several tumor cells show a nuclear staining pattern with anti–BCL-6 MoAb PG-B6. (B) Anaplastic large cells show strong nuclear immunoreactivity with the anti–BCL-6 MoAb PG-B6. The positivity is microgranular. (C) Coexpression of BCL-6 protein (nuclear, blue) is observed in a proportion of CD30+ (cytoplasmic and membranous, reddish) anaplastic large tumor cells, as detected by two-color staining (see Materials and Methods). Original magnification (A) ×250, (B) ×400, and (C) ×400.
The diagnosis of T-cell neoplasm was based on correlative analysis of the morphological, immunophenotypic and, when necessary, genotypic characteristics. The cases were categorized as previously reported by Harris et al.14 The diagnosis of CD30+ ALCLs was based on correlative analysis of the histopathologic and immunophenotypic characteristics.14,15 In particular, expression of the CD30 antigen by all or almost all neoplastic cells was required for the diagnosis of CD30+ ALCLs. CD30+ tumor cells were consistently absent in the peripheral T-cell neoplasms other than CD30+ ALCLs.
Frozen tissue from nine clinical samples with non-neoplastic T-cell proliferations (seven reactive lymph nodes without obvious cause and with a high degree of interfollicular/paracortical expansion and two reactive lymph nodes in infectious mononucleosis) were also included in the study. The expanded interfollicular/paracortical areas of all non-neoplastic (reactive) lymph nodes were predominantly populated by CD4+ T lymphocytes.
Cell lines.The t(2; 5)-positive Karpas 299, SU-DHL1, and DEL human-derived cell lines16 were also included in the study.
Immunohistochemistry.Deparaffinized and cryostat sections were used for immunophenotyping and lineage assignment of lymphoma cases with monoclonal antibodies (MoAbs; βF1, CD3, CD4, CD5, CD8, CD9, CD10, CD15, CD19, CD20, CD21, CD22, CD24, CD30, CD43, CD45, CD45RA, CD45RO, CD68, CD74, CDw75, LN3, MB2, DDBB42, DBA44, OPD4, DRC-1, Leu8, anti-TdT, anti-k and λ immunoglobulin [Ig] light chains, epithelial membrane antigen [EMA], vimentin, and cytokeratin [MNF116]).15,17 Immunohistochemistry was performed with the avidin-biotin-peroxidase complex (ABC-px)18 or alkaline phosphatase antialkaline phosphatase (APAAP)19 methods, as previously described.18,19
Staining with BCL-6.The BCL-6 protein was immunostained using the MoAb PG-B65,7 on frozen sections by the APAAP method.19
Cases were considered positive when ≥10% of neoplastic cells showed nuclear staining for the MoAb PG-B6. A threshold of 10% positive cells was arbitrarily chosen because T-cell neoplasms are almost always either infiltrated by a background population of small, non-neoplastic T cells, or admixed with residual small and large B cells. However, to formally clarify the nature of occasional BCL-6+ cells in samples containing less than 10% of the stained cells, double label studies were performed.
In mycosis fungoides lesions, BCL-6 staining was assessed on the cytologically abnormal lymphoid cells in the epidermis, thus minimizing the potential interference from dermal reactive lymphocytes.20
Cytospin smears of human-derived cell lines were fixed in acetone-chloroform at room temperature for 10 minutes and immunostained with the MoAb PG-B6 by the APAAP method.19
Two-color staining.Multiple immunohistochemical staining was performed to detect BCL-6 protein plus CD30 in selected CD30+ ALCL cases and reactive lymph nodes, as previously reported.21 Double label studies21 (BCL-6 plus CD19; BCL-6 plus CD3) were also performed in CD30+ ALCL cases and other peripheral T-cell neoplasms.
RESULTS
Expression of BCL-6 in T-cell neoplasms.Results of BCL-6 expression in the total series of T-cell neoplasms studied is listed in Table 1. BCL-6 protein expression was restricted to the CD30+ ALCL group, in which 12 of 27 (45%) cases were positive (Fig 1A). In positive cases, 10% to 60% of the tumor cells were labeled with BCL-6. In five cases more than 50% of the tumor cells were stained, whereas in the remaining positive cases 10% to 25% (3 cases) and 25% to 50% (4 cases) of the tumor cells were stained with BCL-6. Anti–BCL-6 MoAb stained the nuclei of large tumor cells. The staining pattern on frozen tissue sections was of moderate to strong intensity. Nuclear positivity was diffuse/microgranular (Fig 1B). Coexpression of BCL-6 protein and CD30 was observed in the tumor cells of all CD30+ ALCL cases tested (7/7; Fig 1C). Both T-cell (8 cases) and null-cell (4 cases) immunophenotypes were found among the BCL-6+ CD30+ ALCL cases (Table 1).
Among the 15 CD30+ ALCL cases that were considered negative (<10%) for BCL-6 protein expression, 11 cases did not show any staining. Conversely, four samples had scattered large cells that stained for BCL-6 protein and accounted for less than 1% to 1% to 2% of the cellularity. Two-color staining with BCL-6 protein plus CD30 and BCL-6 protein plus CD19 showed that a fraction of these scattered BCL-6+ cells were also CD30+ and presumably were tumor cells. The remaining BCL-6 protein expressing cells were host CD19+ B-cells.
The 24 peripheral T-cell neoplasms other than CD30+ ALCLs as well as the eight T-cell lymphoblastic lymphomas were considered negative for BCL-6 expression. Four cases of peripheral T-cell neoplasms, all lacking CD30+ cells, contained a minor component (<1%) of scattered BCL-6 expressing small and large cells. Two-color staining with BCL-6 plus CD19 and BCL-6 plus CD3 confirmed that in two cases the BCL-6 expressing cells were host CD19+ B cells. In the other two cases, the BCL-6 expressing cells were predominantly B cells, although occasional BCL-6+ CD19− cells could be observed. The lineage of these rare cells remains presently undefined.
Expression of BCL-6 in human lymphoma-derived cell lines.On cytospin preparations cultured Karpas 299 ALCLs, SU-DHL1, and DEL cells were characterized by a strong granular nuclear staining with anti–BCL-6 MoAb (not shown).
Expression of BCL-6 and CD30 in non-neoplastic lymph nodes.Serial sections immunohistochemistry was used to analyze the expression of BCL-6 and CD30 in expansions of activated T cells because of chronic and acute inflammatory conditions. BCL-6+ cells as well as CD30+ cells were consistently present in reactive lymph nodes, but varied markedly in number from case to case and area to area.
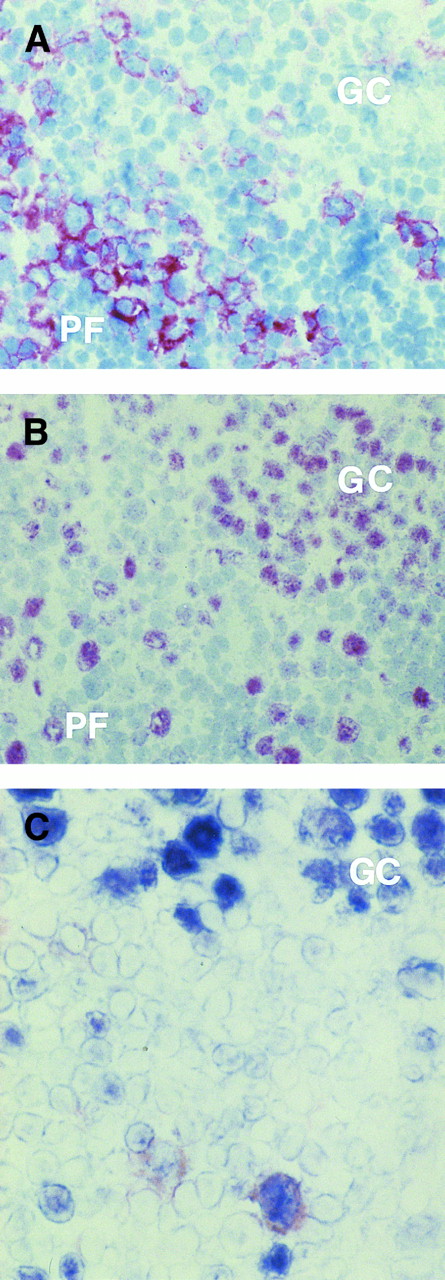
As expected, numerous B cells within the germinal centers consistently stained for BCL-6, whereas cells positive for CD30 were usually absent (Fig 2A and B). On the other hand, BCL-6+ and CD30+ cells were localized around B-cell follicles, although they were also seen at the margins of germinal centers (Fig 2A and B). Coexpression of CD30 and BCL-6 was detected by two-color staining in large cells outside normal germinal centers (Fig 2C) in which, as seen in this and previous studies,13,22 CD30+ cells, larger than most centroblasts, are all of B-cell phenotype.
(A and B) Serial sections from a reactive lymph node with non-neoplastic T-cell proliferation. Several CD30+ (A) and BCL-6+ (B) large lymphocytes are present at the margins of a germinal center (GC), although they are also seen in the perifollicular (PF ) zone. Within the GC numerous B cells stain for BCL-6 (B), whereas cells positive for CD30 are absent (A). (C) A large cell near to a GC coexpresses CD30 antigen (reddish) and BCL-6 protein (blue), as detected by two-color staining (see Materials and Methods). No coexpression by the BCL-6+ GC cells is detectable. (A and B) APAAP immunostaining, hematoxylin counterstain, original magnification ×250; (C) frozen section, original magnification ×400.
(A and B) Serial sections from a reactive lymph node with non-neoplastic T-cell proliferation. Several CD30+ (A) and BCL-6+ (B) large lymphocytes are present at the margins of a germinal center (GC), although they are also seen in the perifollicular (PF ) zone. Within the GC numerous B cells stain for BCL-6 (B), whereas cells positive for CD30 are absent (A). (C) A large cell near to a GC coexpresses CD30 antigen (reddish) and BCL-6 protein (blue), as detected by two-color staining (see Materials and Methods). No coexpression by the BCL-6+ GC cells is detectable. (A and B) APAAP immunostaining, hematoxylin counterstain, original magnification ×250; (C) frozen section, original magnification ×400.
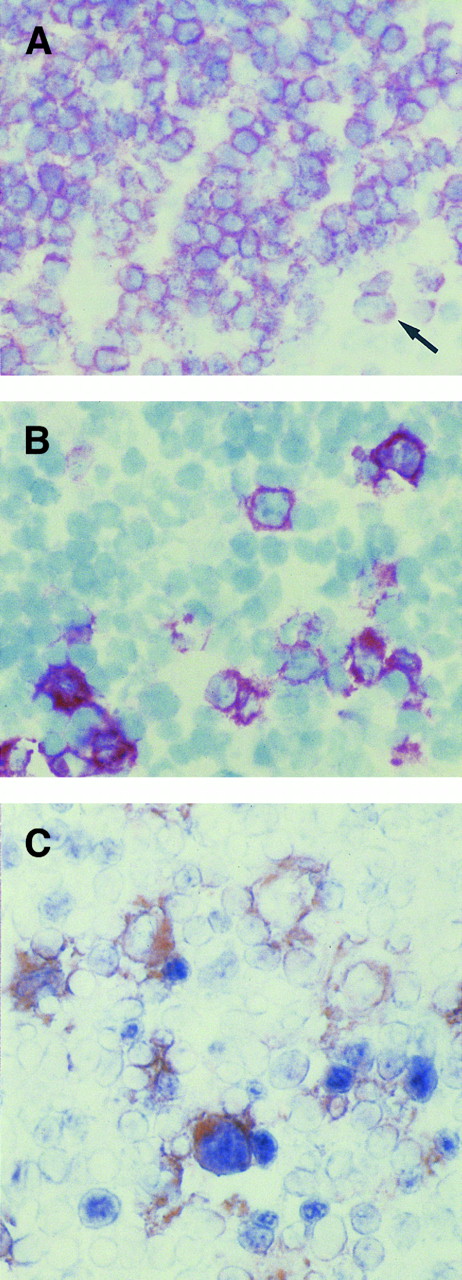
In the interfollicular areas principally populated by CD3+/CD4+ T cells (Fig 3A), CD30+ cells were consistently present, but varied markedly in number from case to case (Fig 3B). Serial sections provided immunoarchitectural evidence of a similar immunostaining with both CD30 and BCL-6 (not shown). Coexpression of BCL-6 protein was observed in a fraction of CD30+ large cells (Fig 3C). These cells were localized in the interfollicular areas, in which B cells were relatively sparse, thus suggesting that most of the cells were, in fact, of T-cell origin.
(A, B, and C) Serial sections from a reactive lymph node with non-neoplastic T-cell proliferation. In the interfollicular area, where almost all cells, including large cells (arrow), are positive for CD3 antigen (A), some CD30+ (B) large lymphocytes are present. (C) Coexpression of BCL-6 protein (nuclear, blue) is observed in a large CD30+ (cytoplasmic and membranous, reddish) cell, as detected by two-color staining (see Materials and Methods). Individual cells expressing either BCL-6 or CD30 are also seen. (A and B) APAAP immunostaining, hematoxylin counterstain, original magnification ×400; (C) frozen section, original magnification ×400.
(A, B, and C) Serial sections from a reactive lymph node with non-neoplastic T-cell proliferation. In the interfollicular area, where almost all cells, including large cells (arrow), are positive for CD3 antigen (A), some CD30+ (B) large lymphocytes are present. (C) Coexpression of BCL-6 protein (nuclear, blue) is observed in a large CD30+ (cytoplasmic and membranous, reddish) cell, as detected by two-color staining (see Materials and Methods). Individual cells expressing either BCL-6 or CD30 are also seen. (A and B) APAAP immunostaining, hematoxylin counterstain, original magnification ×400; (C) frozen section, original magnification ×400.
DISCUSSION
The present study was aimed at defining the expression of BCL-6 protein in distinct pathological categories of T-cell neoplasms. Our data indicate that BCL-6 is expressed by a substantial fraction (45%) of CD30+ ALCLs, whereas it is consistently absent in all other peripheral and precursor T-cell neoplasms tested. Notably, coexpression of CD30 and BCL-6, as detected in CD30+ ALCLs, may also occur in a specific subset of non-neoplastic lymphoid elements, namely the large lymphoid cells scattered in the interfollicular areas of reactive lymph nodes.
The relevance of these data is twofold and concerns the histogenesis of CD30+ ALCLs and the potential diagnostic use of BCL-6 expression in differentiating CD30+ ALCLs from other categories of peripheral T-cell neoplasms.
Regarding CD30+ ALCL histogenesis, the frequent and selective occurrence of BCL-6 expression in CD30+ ALCLs, combined with the evidence that large lymphoid cells localized in the interfollicular areas of non-neoplastic lymph nodes also coexpress CD30 and BCL-6 protein, further corroborate the concept that CD30+ ALCLs, or at least a proportion of these lymphomas, represent the neoplastic transformation of extrafollicular CD30+ cells. As recently shown, BCL-6 expression is detectable also in Reed-Sternberg (RS) CD30+ cells of classical HD,7 another disorder putatively related to extrafollicular CD30+ lymphoid cells.13 However, the frequency of BCL-6+ HD cases (about 30%) and the percentage of BCL-6+ RS cells (about 10%)7 are different from the findings in CD30+ ALCLs (present study).
The biological basis accounting for the expression of BCL-6 protein in human CD30+ ALCLs is currently unknown. In physiological conditions, BCL-6 protein is expressed by a small fraction of normal resting CD4+ T lymphocytes. After activation by 12-O-tetradecanoylphorbol-13-acetate (TPA) and ionomycin, the proportion of BCL-6+ T cells increases significantly, returning to baseline levels by 24 to 48 hours.7 In this respect, our detection of BCL-6 protein in a fraction of interfollicular CD30+ cells in non-neoplastic lymph nodes may reflect the fact that these lymphoid cells are activated by the network of inflammatory cytokines mounted by the underlying disease.23,24 It may be postulated that BCL-6 expression in normal and reactive CD30+ cells localized in the interfollicular areas is a scheduled event occurring as a consequence of time-limited cellular activation, whereas the tumor cells of CD30+, BCL-6+ ALCL would constitutively maintain the activated state associated with expression of CD30 and BCL-6. Alternatively, it may be hypothesized that BCL-6 expression in CD30+ ALCLs occurs as a consequence of a presently unknown genetic lesion causing either directly or indirectly the deregulated expression of the BCL-6 protein. Because BCL-6 expression is detectable in primary CD30+ ALCLs and ALCL cell lines expressing NPM/alk chimeric protein (this study and Falini B et al, unpublished observation, October 1996), it is likely that BCL-6 expression does not represent a second pathogenetic mechanism for CD30+ ALCLs mutually exclusive with t(2; 5) (p23; q35) translocation.14
Independent of histogenetic and pathogenetic implications, the selectivity of the association between BCL-6 expression and CD30+ ALCLs provides a new tool that may prove potentially relevant toward the refinement of the phenotypic criteria separating this category from other types of peripheral T-cell neoplasms.
Supported in part by the Associazione Italiana per la Ricerca sul Cancro, Milan, Italy; by the Ministero della Sanità, Ricerca Finalizzata, Rome, Italy; and by National Institutes of Health Grant No. CA-44029.
Address reprint requests to Antonino Carbone, MD, Division of Pathology, Centro di Riferimento Oncologico, Istituto Diricovero e Cura a Carattere Scientifico, via Pedemontana Occidentale, Aviano I-33081, Italy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal