Abstract
We analyzed herein whether antibodies to HLA class I α1 domain, which trigger apoptosis of activated T cells, may also control the growth/survival of human B lymphocytes. Addition of monoclonal antibody (MoAb) 90 (mouse IgG1) or YTH862 (rat IgG2b) was found to strongly inhibit the proliferation of CD40-activated total tonsil B cells as well as that of purified naive, germinal center, and memory B-cell subsets. This inhibitory effect was not prevented by addition of B-cell tropic factors, such as interleukin-2 (IL-2), IL-4, and IL-10, and was a result of induced B-cell apoptosis as shown by using a TUNEL assay and DNA electrophoresis. In contrast, engagement of another epitope of the α1 domain, as well as that of the α2 and α3 domains by specific anti-HLA class I MoAbs, failed to inhibit DNA synthesis and to induce apoptosis of CD40-activated B cells. As recently reported for acquisition of sensitivity to Fas (APO-1/CD95) -dependent apoptosis, susceptibility to MoAb90-and YTH862-induced death was restricted to CD40-activated B cells, because resting and anti–IgM-activated B cells did not undergo apoptosis after HLA class I engagement. Moreover, ligation of the B-cell receptor protected CD40-activated B cells from both HLA class I- and Fas-mediated growth inhibition and apoptosis. Taken together, these results show that engagement of the α1 domain of HLA class I induces apoptotic cell death of CD40-activated, but not of antigen-activated B cells, and would, therefore, suggest a possible role for HLA class I molecules in the control of B-cell homeostasis.
THE HOMEOSTASIS OF B and T lymphocytes is regulated by different checkpoints that avoid autoimmunity, that control the size of B- and T-cell clones generated during an ongoing immune response, and that ensure their antigen specificity.1,2 Among the different mechanisms involved in these processes, triggering of the antigen receptor on both T (TCR) and B (BCR) lymphocytes can transduce agonistic or antagonistic signals leading either to activation/survival or anergy/death depending on the stage of lymphocyte maturation and on the nature, valency, concentration, and affinity of the antigen. In addition, several lines of evidence indicate that the Fas (APO-1/CD95)-Fas ligand system3,4 plays a role in regulating bystander B-cell activation and the emergence of autoreactive B-cell clones.5,6 Indeed, B cells are induced to express high levels of Fas and acquire sensitivity to Fas-mediated apoptosis after engagement of their CD40 antigen,7-9 but not when costimulated through their CD40 and their BCR.6 9 Therefore, signals generated by the interaction of the appropriate antigen with BCR control B-cell sensitivity to different death triggers, such as the Fas-Fas ligand system.
Because different studies have shown that engaging polymorphic or monomorphic determinants of HLA class I inhibits T-cell proliferation induced by anti-CD3 antibodies or lectins,10-17 we wondered whether engagement of HLA class I determinants may also affect the growth/survival of activated human B cells. To this end, we used a panel of antiHLA class I antibodies, including monoclonal antibody (MoAb)90, an MoAb generated after imunizing mice with tonsillar B cells, which recognizes the α1 domain of HLA class I molecules and induces apoptosis of activated CD45RA+RO+ human T cells (Genestier et al, submitted). The results presented herein show that engagement of a restricted epitope of the HLA class I α1 domain induced apoptosis of CD40-activated, but not resting B cells. In contrast, ligation of the BCR by anti-IgM antibodies prevented HLA class I-induced apoptosis of CD40-activated human B lymphocytes.
MATERIALS AND METHODS
Antibodies and Cytokines
MoAb90 was produced after immunizing mice with three consecutive intraperitoneal injections of human tonsil B cells (106 cells). The isotype of MoAb90 (IgG1) was determined by immunoassay with the Isotype Ab-Stat kit (Sangstat Medical, Menlo Park, CA). MoAb90 was purified from ascitic fluids by diethyl aminoethyl chromatography. The YTH862 MoAb (anti–HLA-A, -B, and -C, rat IgG2b), kindly provided by H. Waldmann (University of Oxford, Oxford, England), was produced by immunizing rats (DA strain) with consecutive intraperitoneal injections of phytohemagglutinin (PHA)-activated human peripheral blood lymphocytes (PBLs; 20 × 106). TP25.99 MoAb (anti–HLA-A, -B, and -C, IgG1)18 was provided by Dr R. Buelow (Sangstat Medical, Menlo Park, CA), W6/32 MoAb (anti–HLA-A, -B, and -C, IgG2a) was obtained from American Type Culture Collection (ATCC; Rockville, MD), and B9.12.1 MoAb (anti–HLA-A, -B, and -C, IgG2a) was purchased from Immunotech (Marseille, France). Epitope specificity of the different anti-HLA class I antibodies were analyzed by using transfected C1R cells that express HLA-A2.1 or hybrid mouse/human exon-shuffled HLA-A2.1/H-2Dd genes19 and has been previously reported (Genestier et al, submitted). Briefly, MoAb90 and YTH862 bind to the same epitope of the α1 domain, whereas B9.12.1 recognizes a different epitope of the α1 domain. W6/32 and TP25.99 bind to a nonpolymorphic epitope of the α2 and α3 domain, respectively. Purified anti-Fas MoAb (IgM, clone CH11) and fluorescein isothiocyanate (FITC)-conjugated anti-Fas MoAb (IgG1, clone UB2) were purchased from Immunotech. IgG1 control MoAb (for bioassays) and FITC-conjugated IgG control MoAbs (for flow cytometric analyses) were from Dako (Glostrup, Denmark). FITC-conjugated anti-CD19, anti-CD20, anti-CD2, anti-CD3, anti-CD14, and anti-CD56 were from Becton Dickinson (Mountain View, CA). Purified human recombinant Interleukin (IL)-4 (107 U/mg) and IL-10 (2 × 107 U/mg) were from Schering-Plough Research Institute (Kenilworth, NJ) and were used at 50 U/mL and 100 ng/mL, respectively. Purified human recombinant IL-2 (3 × 106 U/mg, Amgen Biologicals, Thousand Oaks, CA) was used at 20 U/mL.
MoAb90 inhibits CD40-dependent B-cell proliferation. Tonsil B cells (2 × 104/well) were cultured on irradiated CD32-transfected L cells (5 × 103/well) in the presence of 1 μg/mL anti-CD40 MoAb and (A) with increasing concentrations of MoAb90 or W6/32 added at the onset of the culture; (B) with 5 μg/mL of control IgG1, W6/32 or MoAb90 added on day 0 or 4. Proliferation was measured by [3H]TdR incorporation after a pulse with [3H]TdR during the last 16 hours of the culture on day 6 (A) or at the time indicated (B), and results are expressed as mean ± standard deviation (SD) of triplicate determinations. In each experiment, background of [3H]TdR uptake of CD32-L cells was less than 500 counts per minute (cpm). Arrow in B indicates time of MoAb90 addition. Data presented are representative of three independent experiments. (⋄) indicates IgG1 control, (○) indicates W6/32, and (•) indicates MoAb90. (C) Tonsil B cells vere cultured for 5 days on CD32-L cells in the presence of 1 μg/mL anti-CD40 MoAb with 1 μg/mL of W6/32 or MoAb90. Photos of culture wells (original magnitude ×100) show the formation of B-cell clumps induced by proliferation.
MoAb90 inhibits CD40-dependent B-cell proliferation. Tonsil B cells (2 × 104/well) were cultured on irradiated CD32-transfected L cells (5 × 103/well) in the presence of 1 μg/mL anti-CD40 MoAb and (A) with increasing concentrations of MoAb90 or W6/32 added at the onset of the culture; (B) with 5 μg/mL of control IgG1, W6/32 or MoAb90 added on day 0 or 4. Proliferation was measured by [3H]TdR incorporation after a pulse with [3H]TdR during the last 16 hours of the culture on day 6 (A) or at the time indicated (B), and results are expressed as mean ± standard deviation (SD) of triplicate determinations. In each experiment, background of [3H]TdR uptake of CD32-L cells was less than 500 counts per minute (cpm). Arrow in B indicates time of MoAb90 addition. Data presented are representative of three independent experiments. (⋄) indicates IgG1 control, (○) indicates W6/32, and (•) indicates MoAb90. (C) Tonsil B cells vere cultured for 5 days on CD32-L cells in the presence of 1 μg/mL anti-CD40 MoAb with 1 μg/mL of W6/32 or MoAb90. Photos of culture wells (original magnitude ×100) show the formation of B-cell clumps induced by proliferation.
Cell Preparations
Total B lymphocytes were purified from tonsils as described earlier.20 Briefly, mononuclear cells separated by standard FicollHypaque gradient were first rosetted with sheep red blood cells. Nonrosetting cells were further incubated with anti-CD2, anti-CD3, anti-CD4, anti-CD14, anti-CD56, and anti-CD57 MoAbs and then submitted to negative selection performed with magnetic beads coated with antimouse IgG (Dynabeads; Dynal, Oslo, Norway). Purified B-cell preparations contained greater than 98% B cells; less than 2% T cells; monocytes and natural killer (NK) cells as determined by staining with FITC-conjugated anti-CD19, anti-CD20, anti-CD2, anti-CD3, anti-CD14, and anti-CD56 MoAbs; and fluorescence analysis performed with a FACScan (Becton Dickinson). Separation of resting versus germinal center (GC) B cells was done by further incubating cells with, respectively (1) anti-CD38 (OKT10) or (2) anti-CD44 (A2) + anti-IgD (Nordic Immunological Laboratories, Tilburg, The Netherlands) MoAbs before depletion with magnetic beads. The resting (CD44+, CD38−) B-cell population could be further subdivided into naive or memory cells by depletion after staining with (1) anti-IgG + anti-IgA or (2) anti-IgD antibodies, respectively, as previously described.20-22
Engagement of HLA class I α1 domain inhibits DNA synthesis of CD40-activated total, naı̈ve, GC, and memory B cells in response to B-cell growth factors. Tonsil B cells (2 × 104/well) from each subset were cultured on CD32-L cells (5 × 103/well) in the presence of 1 μg/mL anti-CD40 MoAb with either IL-10 (20 ng/mL), IL-4 (50 U/mL), IL-2 (20 U/mL) plus IL-10 or IL-4 plus IL-10, and with control IgG1 or MoAb90 at 1 μg/mL added at the onset of the culture. Proliferation was measured by [3H]TdR incorporation after a pulse with [3H]TdR during the last 16 hours of the culture at the time indicated. Results are expressed as means ± SD of culture triplicates. [3H]TdR uptake of CD32-L cells was less than 500 cpm in the different experiments. This figure is representative of three independent experiments.
Engagement of HLA class I α1 domain inhibits DNA synthesis of CD40-activated total, naı̈ve, GC, and memory B cells in response to B-cell growth factors. Tonsil B cells (2 × 104/well) from each subset were cultured on CD32-L cells (5 × 103/well) in the presence of 1 μg/mL anti-CD40 MoAb with either IL-10 (20 ng/mL), IL-4 (50 U/mL), IL-2 (20 U/mL) plus IL-10 or IL-4 plus IL-10, and with control IgG1 or MoAb90 at 1 μg/mL added at the onset of the culture. Proliferation was measured by [3H]TdR incorporation after a pulse with [3H]TdR during the last 16 hours of the culture at the time indicated. Results are expressed as means ± SD of culture triplicates. [3H]TdR uptake of CD32-L cells was less than 500 cpm in the different experiments. This figure is representative of three independent experiments.
Cell Cultures and Proliferation Assays
All cultures were performed in DMEM-F12 medium (Life Technologies, Gaithersburg, MD) supplemented with 2-mmol/L L-glutamine (Eurobio, Le Ullis, France), 10% horse serum (Life Technologies), 80 μg/mL gentamicin (Gentalline, Schering-Plough, Levallois-Perret, France), and 1% additive culture medium (CRTS, Lyon, France) in the presence of 5 × 103 irradiated (75 Gy) CD32-L cells as previously described.23 Anti-CD40 MoAb8924 or anti-IgM (anti-μ beads) (Bio-Rad, Richmond, CA) were used at 1 μg/mL and 5 μg/mL, respectively. In some experiments, B cells were activated by CD40 ligand (CD40-L)-transfected L cells25 or by CD40-L/CD32-double transfected L cells.26 Cytokines and antibodies were added at the initiation of the culture or at the time indicated within the text to reach a final volume of 200 μL/well. For proliferation assays, resting B cells were seeded in triplicates in 96-well flat bottom plates (Falcon, Oxnard, CA) at 2 × 104 cells/well in the presence of 5 × 103 irradiated (75 Gy) CD32-L cells, and proliferation was determined by [ 3 H]TdR uptake after pulsing cells with [3 H]TdR (1 μCi/well; specific activity: 25 Ci/mmol, Amersham, Buckinghamshire, England) during the last 16 hours of the culture. After harvesting cells on glass-fiber filters, [3H]TdR incorporation was measured by standard liquid scintillation counting techniques.
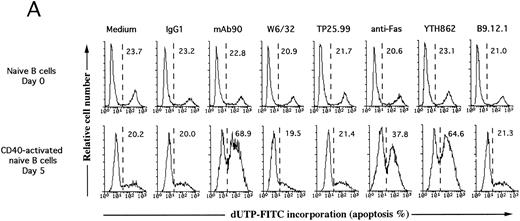
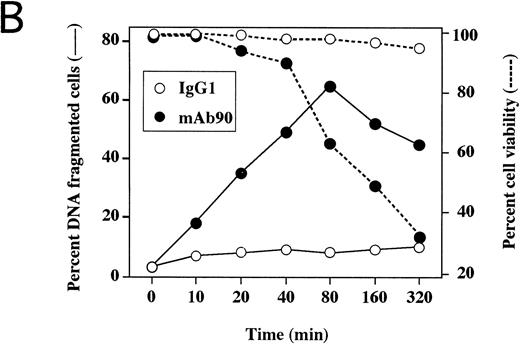
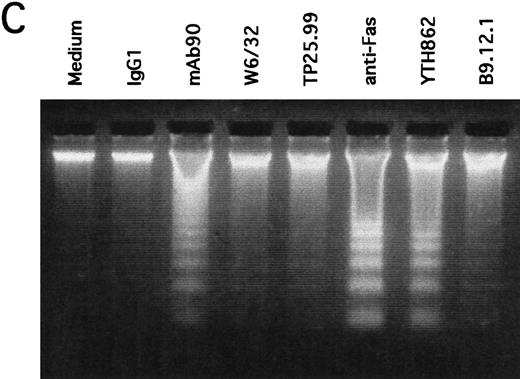
MoAb90 induces apoptosis of CD40-activated B cells. Resting or 5-day CD40-activated B cells were cultured in the presence of 5 μg/mL control IgG1, MoAb90, W6/32, TP25-99, and 1 μg/mL anti-Fas MoAb CH11. (A) Cells were collected after 2 hours of treatment with the different MoAbs, and incorporation of dUTP-FITC was measured by TUNEL assay as described in Materials and Methods. Each histogram represents 104cells and percentage of apoptotic cells are indicated above to the histogram profiles. (B) Kinetics of MoAb90-induced B-cell apoptosis are as follows: 5 μg/mL of control IgG1, W6/32, TP25.99, and MoAb90 were added to the cultures, and the percentage of DNA fragmented cells was measured by TUNEL assay after the indicated time. In parallel, the percentage of cell viability was measured by trypan blue dye exclusion after treatment with MoAb90 at 5 μg/mL for indicated time. Results represent average of duplicate determinations. This figure is representative of three independent experiments. (C) Cells were collected after 12 hours of treatment with the different MoAbs and DNA from 2 × 106 cells was run on 2% agarose gel and stained with ethidium bromide.
MoAb90 induces apoptosis of CD40-activated B cells. Resting or 5-day CD40-activated B cells were cultured in the presence of 5 μg/mL control IgG1, MoAb90, W6/32, TP25-99, and 1 μg/mL anti-Fas MoAb CH11. (A) Cells were collected after 2 hours of treatment with the different MoAbs, and incorporation of dUTP-FITC was measured by TUNEL assay as described in Materials and Methods. Each histogram represents 104cells and percentage of apoptotic cells are indicated above to the histogram profiles. (B) Kinetics of MoAb90-induced B-cell apoptosis are as follows: 5 μg/mL of control IgG1, W6/32, TP25.99, and MoAb90 were added to the cultures, and the percentage of DNA fragmented cells was measured by TUNEL assay after the indicated time. In parallel, the percentage of cell viability was measured by trypan blue dye exclusion after treatment with MoAb90 at 5 μg/mL for indicated time. Results represent average of duplicate determinations. This figure is representative of three independent experiments. (C) Cells were collected after 12 hours of treatment with the different MoAbs and DNA from 2 × 106 cells was run on 2% agarose gel and stained with ethidium bromide.
Preparation of F(ab)′2and Fab′ Fragments
MoAb90 was digested by Ficin to yield F(ab)′2 and Fab′ fragments following manufacturer's instructions (Pierce, Rockford, IL). Briefly, purified MoAb90, at the concentration of 1.5 mg/mL, was digested with immobilized Ficin in the presence of 1 mmol/L cysteine for F(ab)′2 fragments for 20 hours and 10 mmol/L cysteine for Fab′ fragments for 5 hours. F(ab)′2 and Fab′ fragments were purified by chromatography using protein A column and the purity (98% for (ab′ )2 and 96% for Fab′ preparation) was controlled by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under nonreducing conditions.
Measurement of Apoptosis
DNA fragmentation assays.Cells were incubated in DMEM-F12 medium for 12 hours with the MoAbs indicated. DNA preparations were obtained following a procedure previously described.27 Briefly, 107 cells were lysed in lysis buffer containing 10 mmol/L EDTA, 100 mmol/L NaCl, 0.5% (wt/vol) SDS, 100 mmol/L Tris-HCl pH 7.4, and 0.1 mg/mL proteinase K (Boehringer Mannheim, Mannheim, Germany). The DNA was extracted twice with phenol and twice with chloroform:isoamyl alcohol. The aqueous phase was precipitated with two volumes of ethanol. Unfragmented DNA was discarded, and one tenth of the volume of 3 mol/L sodium acetate was added to the supernatant, which was left at −20°C overnight. The precipitate containing fragmented DNA was centrifuged and the pellet was dried under vacuum and resuspended in 100 μL RNAse buffer containing 10 mmol/L Tris and 1 mmol/L EDTA pH 7.5. The samples were diluted in loading buffer and loaded on the gel. Electrophoresis was carried out in 2% agarose gel containing 0.1 μg/mL ethidium bromide (Sigma, St Louis, MO). After electrophoresis, gels were examined under ultraviolet light.
Engagement of HLA Class I by MoAb90 and YTH862 Induces Apoptosis of CD40 Ligand-Activated B Cells
| . | CD40 Ligand* . | CD40 Ligand-CD32* . | ||
|---|---|---|---|---|
| . | Apoptotic Cells . | Viable Cells . | Apoptotic Cells . | Viable Cells . |
| . | (%) . | (%) . | (%) . | (%) . |
| Medium | 25.2 | 63.2 | 36.0 | 57.2 |
| Control IgG1 | 20.5 | 62.0 | 35.3 | 60.5 |
| MoAb90 | 49.1 | 12.4 | 57.7 | 21.2 |
| YTH862 | 45.3 | 37.0 | 51.0 | 38.0 |
| W6/32 | 23.3 | 63.6 | 30.7 | 59.4 |
| B9.12.1 | 28.2 | 63.7 | 39.4 | 59.8 |
| TP25.99 | 28.5 | 59.7 | 36.6 | 59.9 |
| . | CD40 Ligand* . | CD40 Ligand-CD32* . | ||
|---|---|---|---|---|
| . | Apoptotic Cells . | Viable Cells . | Apoptotic Cells . | Viable Cells . |
| . | (%) . | (%) . | (%) . | (%) . |
| Medium | 25.2 | 63.2 | 36.0 | 57.2 |
| Control IgG1 | 20.5 | 62.0 | 35.3 | 60.5 |
| MoAb90 | 49.1 | 12.4 | 57.7 | 21.2 |
| YTH862 | 45.3 | 37.0 | 51.0 | 38.0 |
| W6/32 | 23.3 | 63.6 | 30.7 | 59.4 |
| B9.12.1 | 28.2 | 63.7 | 39.4 | 59.8 |
| TP25.99 | 28.5 | 59.7 | 36.6 | 59.9 |
Total B cells were activated for 5 days with CD40-L–transfected L cells or with CD40-L/CD32-double transfected L cells as described in Materials and Methods. Percentages of apoptotic cells and viable cells were determined by TUNEL and propidium iodide incorporation, respectively, after 4 hours of treatment with the different antibodies.
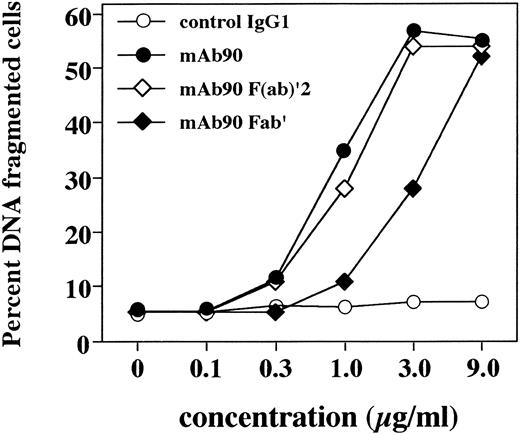
Dose response of MoAb90-induced B-cell apoptosis. Resting B cells were cultured for 5 days over irradiated CD32-L cells in the presence of 1 μg/mL anti-CD40 MoAb. Increasing concentrations of control IgG1, W6/32, or intact Ig, F(ab′)2 , and F(ab)′ fragments from MoAb90 were then added to the cultures. Cells were collected after 2 hours of treatment with the different MoAbs and the percentage of DNA fragmented cells was measured by TUNEL assay.
Dose response of MoAb90-induced B-cell apoptosis. Resting B cells were cultured for 5 days over irradiated CD32-L cells in the presence of 1 μg/mL anti-CD40 MoAb. Increasing concentrations of control IgG1, W6/32, or intact Ig, F(ab′)2 , and F(ab)′ fragments from MoAb90 were then added to the cultures. Cells were collected after 2 hours of treatment with the different MoAbs and the percentage of DNA fragmented cells was measured by TUNEL assay.
TdT-Mediated dUTP-FITC Nick End Labeling (TUNEL)
DNA fragmentation was detected in the nucleus of apoptotic cells as described earlier.28 At the time indicated, approximately 106 cells were collected, washed in cold phosphate-buffered saline (PBS), and transferred to V-bottom microtiter plates (Falcon). After centrifugation, pellets were resuspended in 200 μL of PBS 1% paraformaldehyde. After fixation (15 minutes at 4°C), cells were washed twice in cold PBS and permeabilized with 200 μL of 0.1% Triton X-100 and 0.1% sodium citrate. Cells were washed twice in PBS and resuspended in 25 μL of FITC–12-dUTP staining mixture, which contained 3 nmol FITC–12-dUTP, 30 nmol dATP, 80 μL CaCl2, 200 μL 5× TdT buffer, and distilled water to 1 mL. All reagents were from Boehringer Mannheim. The TdT reaction was performed by addition of 25 U TdT (Promega, Madison, WI) followed by 1 hour incubation at 37°C. The reaction was then stopped by adding 2 μL 0.5 mol/L EDTA and cells were washed twice in cold PBS 1% bovine serum albumin. Samples were analyzed by flow cytrometry on a FACScan. Cell debris and fibroblastic L cells were excluded according to their forward and right angle scatter parameters.
Analysis of Class I and Fas Expression
Cell-surface expression of class I molecules were determined on freshly isolated or cultured B cells by standard immunofluorescence staining with MoAb90 or W6/32 for 10 minutes at 4°C followed by staining with FITC-conjugated antimouse antibodies. Fas expression was determined by direct immunofluorescence staining with FITC-conjugated anti-Fas MoAb UB2. Nonspecific staining was determined using a FITC-conjugated IgG1 control MoAb. Flow cytometry analysis was done with a FACScan. Dead cells and fibroblastic L cells, used in the different culture systems, were excluded during cytometric analysis by addition of 2 μg/mL propidium iodide and according to their forward and right angle scatter parameters.
RESULTS
MoAb90 Inhibits CD40-Induced B-Cell DNA Synthesis
To determine whether HLA class I may also signal in activated human B cells, purified tonsil B cells were cultured with anti-CD40 MoAb and irradiated CD32-L cells23 in the presence of MoAb90. As shown in Fig 1A, addition of MoAb90 at the onset of the culture inhibited CD40-dependent B-cell proliferation in a dose-dependent manner, with an optimum obtained for approximately 50 ng/mL. The time kinetics of [3H]TdR uptake indicated that MoAb90 completely blocked B-cell DNA synthesis from day 3 to 7 (Fig 1B). Futhermore, a delayed addition of MoAb90 after 4 days of CD40 activation prevented the subsequent increase of [3H]TdR incorporation and resulted in a strong and rapid blockade of the CD40-initiated DNA synthesis (Fig 1B). In contrast, addition of the anti-class I MoAb W6/32, which recognizes the α2 domain,18 did not inhibit CD40-dependent B-cell proliferation at any time point tested, even at high concentration (Fig 1). Furthermore, daily microscopic examination of cell cultures from day 1 to day 7 showed the antiproliferative effect of MoAb90 to be associated with a complete inhibition of CD40-induced B-cell clump formation, an observation not reproduced with MoAb W6/32 (Fig 1C).
Because tonsillar B cells are composed of naive, germinal center, and memory B cells,29 we further analyzed the effects of MoAb90 on CD40-induced proliferation of those three B-cell subsets. As shown in Fig 2, the DNA synthesis of the three B-cell populations was almost completely inhibited by addition of 1 μg/mL MoAb90. Addition of optimal concentrations of IL-4, IL-10, IL-2, and their combinations failed to protect the total tonsil B cells as well as the purified populations from the anti-proliferative effect of MoAb90.
Ligation of the BCR does not prime B cells to HLA class I-mediated apoptosis and protects CD40-activated B cells. Naive B cells were cultured on irradiated CD32-L cells in the presence of 1 μg/mL anti-CD40 MoAb alone, 5 μg/mL anti-BCR MoAbs alone, or the combination of both and with 5 μg/mL control IgG1, W6/32, TP25.99, MoAb90, or 1 μg/mL anti-Fas MoAb CH11 added at the onset of the culture. Proliferation was measured by [3H]TdR incorporation after a pulse with [3H]TdR during the last 16 hours of the culture at the time indicated. Results are expressed as means ± SD of culture triplicates. [3H]TdR uptake of CD32-L cells was less than 500 cpm in the different experiments. (⋄) indicates IgG1, (○) indicates W6.32, (□) indicates TP25.99, (▿) indicates B9.12.1, (•) indicates MoAb90, (▪) indicates YTH 862, and (▴) indicates anti-Fas.
Ligation of the BCR does not prime B cells to HLA class I-mediated apoptosis and protects CD40-activated B cells. Naive B cells were cultured on irradiated CD32-L cells in the presence of 1 μg/mL anti-CD40 MoAb alone, 5 μg/mL anti-BCR MoAbs alone, or the combination of both and with 5 μg/mL control IgG1, W6/32, TP25.99, MoAb90, or 1 μg/mL anti-Fas MoAb CH11 added at the onset of the culture. Proliferation was measured by [3H]TdR incorporation after a pulse with [3H]TdR during the last 16 hours of the culture at the time indicated. Results are expressed as means ± SD of culture triplicates. [3H]TdR uptake of CD32-L cells was less than 500 cpm in the different experiments. (⋄) indicates IgG1, (○) indicates W6.32, (□) indicates TP25.99, (▿) indicates B9.12.1, (•) indicates MoAb90, (▪) indicates YTH 862, and (▴) indicates anti-Fas.
Ligation of HLA class I by MoAb90 and MoAb YTH862 Induces Apoptosis of CD40-Activated But Not Resting B Cells
To determine whether the B cell growth inhibitory effect of MoAb90 was a consequence of apoptotic cell death, freshly isolated resting (day 0) and CD40-activated (day 5) naive tonsil B cells were incubated with 5 μg/mL MoAb90 or control MoAb for 2 hours, and the percentage of cells with fragmented DNA was analyzed by a TUNEL assay and fluorescence-activated cell sorting (FACS) analysis. Cells were also treated with 5 μg/mL of anti-HLA class I MoAbs W6/32, TP25.99, YTH862, and B9.12.1 or, as positive control, with 1 μg/mL anti-Fas MoAb CH11 that induces apoptosis of CD40-activated B cells.7 As shown in Fig 3A, CD40-activated but not resting B cells treated with MoAb90 or anti-Fas MoAb showed incorporation of FITC-dUTP, a typical feature of apoptotic cells. Moreover, MoAb YTH862, which binds to the same epitope on the α1 domain as MoAb90, also specifically induced apoptosis of CD40-activated B cells (Fig 3A). In contrast, MoAbs B9.12.1 (which recognizes a different epitope of the α1 domain than MoAbs 90 and YTH862), W6/32 (which binds to the α2 domain) and TP25.99 (which binds to the α3 domain) failed to induce apoptosis in both resting and CD40-activated B cells (Fig 3A). Time kinetic studies showed that the percentage of cells with fragmented DNA increased from 10 to 80 minutes of exposure to MoAb90, but not thereafter (Fig 3B). This was associated with a rapid decrease of CD40-activated B-cell viability, which was observed as soon as 20 minutes after addition of MoAb90 and reached its maximum after 320 minutes (Fig 3B). Moreover, typical ladder patterns of internucleosomal DNA cleavage were observed on DNA electrophoresis of CD40-activated B cells further cultured for 12 hours with either MoAb90, YTH862, or anti-Fas but not with the other anti-HLA class I MoAbs (Fig 3C). Taken together, these results indicate that engagement of HLA class I α1 domain induces prompt apoptosis of CD40-activated B cells.
We next extended theses observations by using CD40-L transfected or CD40-L/CD32 double transfected L cells. In these two experimental conditions MoAb90 and YTH862 induce apoptosis and decrease the percentage of viable cells (Table 1). Therefore, apoptosis can be equally induced in these two systems using cross-linking or soluble MoAbs.
HLA Class I-Induced Apoptosis Does Not Require Cross-Linking
To determine whether MoAb90-induced B-cell apoptosis required HLA class I cross-linking, resting B cells were CD40-activated for 5 days and then treated with control MoAb, intact MoAb90, or Fab′, F(ab′)2 fragments thereof. Results presented in Fig 4 show that MoAb90-induced apoptosis was a dose-dependent process and that F(ab′)2 fragments were as efficient as intact IgG in inducing apoptosis of CD40-activated B cells, whereas Fab′ fragments were slightly less potent (about threefold). The AC50 (concentration required to induced 50% of maximal apoptosis) of intact IgG and F(ab′)2 fragments was about 1 μg/mL, and that of Fab′ fragments 3 μg/mL (Fig 4). Therefore, monovalent engagement of the HLA class I α1 domain is sufficient to induce apoptosis in CD40-activated B cells, because the contamination of Fab′ fragments by F(ab′)2 was lower than 4% as determined by SDS-PAGE (data not shown).
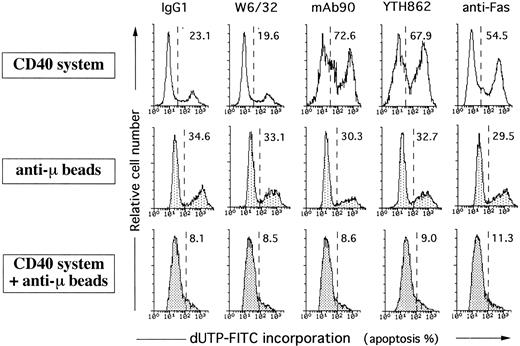
BCR-dependent B-cell activation antagonizes HLA class I-mediated apoptosis. Naive B cells were cultured on irradiated CD32-L cells in the presence of 1 μg/mL anti-CD40 MoAb alone, 5 μg/mL anti-BCR MoAb alone, or the combination of both. After 5 days of culture, cells were collected, treated by 5 μg/mL control IgG1, W6/32, MoAb90, YTH862, or 1 μg/mL anti-Fas MoAb CH11 for 2 hours and then the percentage of DNA fragmented cells was measured by TUNEL assay. Each histogram represents 104 cells and percentage of apoptotic cells are indicated above to the histogram profiles.
BCR-dependent B-cell activation antagonizes HLA class I-mediated apoptosis. Naive B cells were cultured on irradiated CD32-L cells in the presence of 1 μg/mL anti-CD40 MoAb alone, 5 μg/mL anti-BCR MoAb alone, or the combination of both. After 5 days of culture, cells were collected, treated by 5 μg/mL control IgG1, W6/32, MoAb90, YTH862, or 1 μg/mL anti-Fas MoAb CH11 for 2 hours and then the percentage of DNA fragmented cells was measured by TUNEL assay. Each histogram represents 104 cells and percentage of apoptotic cells are indicated above to the histogram profiles.
Engagement of the BCR Protects CD40-activated B Cells from HLA Class I-Mediated Apoptosis
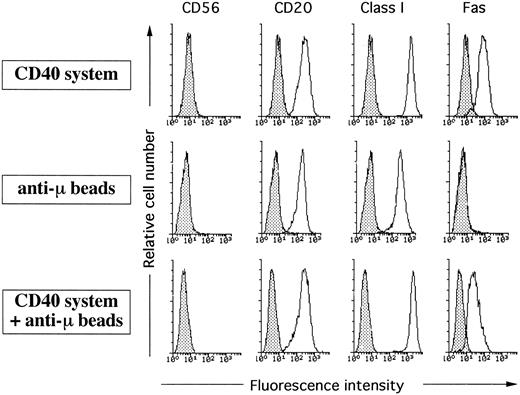
Engagement of HLA class I α1 domain by MoAb YTH862 inhibited CD40-induced proliferation of naive tonsil B cells as previously observed with MoAb90, whereas engagement of HLA class I by MoAbs W6/32, TP25.99, and B9.12.1 did not (Fig 5). However, and as obtained with anti-Fas MoAb, none of the anti-HLA class I MoAbs, including MoAb90 and YTH862, were able to inhibit [3H]TdR uptake of naive B cells activated with anti-IgM antibody coupled to beads (anti-μ beads) (Fig 5). Moreover, addition of anti-μ beads almost completely prevented the inhibitory effect of MoAb90, YTH862, and anti-Fas on the proliferation of CD40-activated naive B cells (Fig 5). Analysis of apoptotic cell death further showed that naive B cells activated for 3 days through their CD40, but not their BCR, had acquired sensitivity to HLA class I- and anti-Fas–mediated apoptosis (Fig 6). Importantly, ligation of the BCR (by anti-μ beads) fully prevented MoAb90- and YTH862-induced apoptosis of CD40-activated naive B cells (Fig 6). As previously reported,6 9 the dual ligation of the CD40 and the BCR also inhibited the Fas-dependent apoptosis in our culture system (Fig 6). Although this protection from Fas-induced apoptosis could be, at least in part, explained by a lower expression of Fas on BCR and CD40 coactivated B cells, naive B cells costimulated with anti-CD40 and anti-μ beads expressed similar high levels of HLA class I as determined by staining with either W6/32 or MoAb90 (Fig 7). Taken together, these results show that engagement of the BCR fails to prime B cells for HLA class I-mediated apoptosis and protects CD40-activated B cells from both HLA class I- and Fas-induced apoptosis.
BCR-dependent activation did not inhibit HLA class I expression. Naive B cells were cultured on irradiated CD32-L cells in the presence of 1 μg/mL anti-CD40 MoAb alone, 5 μg/mL anti-BCR MoAb alone, or the combination of both. After 5 days cells were collected and stained with FITC-conjugated anti-CD56, anti-CD20, anti-Fas UB2, or MoAb90 followed by staining with FITC-conjugated antimouse antibodies. Filled histograms represent staining with a negative control MoAb. This figure is representative of three independent experiments.
BCR-dependent activation did not inhibit HLA class I expression. Naive B cells were cultured on irradiated CD32-L cells in the presence of 1 μg/mL anti-CD40 MoAb alone, 5 μg/mL anti-BCR MoAb alone, or the combination of both. After 5 days cells were collected and stained with FITC-conjugated anti-CD56, anti-CD20, anti-Fas UB2, or MoAb90 followed by staining with FITC-conjugated antimouse antibodies. Filled histograms represent staining with a negative control MoAb. This figure is representative of three independent experiments.
DISCUSSION
The present report shows that engagement of human HLA class I molecules triggers apoptosis of CD40-activated but not resting B lymphocytes. Apoptotic cell death was selectively induced by two antibodies (MoAb90 and YTH862) that bind to a restricted epitope of the α1 domain but not by other anti-HLA class I antibodies that bind to either the α1, α2, or α3 domains (Genestier et al, submitted). However, in contrast to activated T cells whose mitogen-induced proliferation is inhibited by all anti-HLA class I antibodies, only MoAb90 and YTH862 inhibited DNA synthesis of CD40-activated B cells. B-cell growth factors (IL-2, IL-4, and IL-10) were unable to prevent the B-cell growth inhibition and apoptosis (data not shown) induced by the engagement of the α1 domain. However, although all B lymphocytes constitutively express high levels of HLA class I, MoAb90 failed to induce apoptosis in total and naive B cells, as well as in germinal center and memory B cells (data not shown), unless cells were activated through their CD40. The notion that activation is necessary for HLA class I-mediated B-cell death has been recently described with a MoAb reactive against mouse major histocompatability complex class I, RE2, which induces cell death of transformed B-cell lines, and splenic or lymph node B cells only after activation.30 Although, the characteristics of RE2 MoAb-induced cell death were reported to be different from both apoptosis and necrosis, our present results show that activated human B lymphocytes enter into apoptosis after HLA class I engagement as shown by the induction of DNA fragmentation and internucleosomal DNA cleavage.
In contrast to CD40-activated B cells, B cells activated through their BCR did not acquire sensitivity to HLA class I-mediated apoptosis. Similar results were obtained when B cells were activated with Staphylococcus aureus Cowan I particules (data not shown). Moreover, ligation of the BCR confers resistance of CD40-activated B cells to class I-induced cell death and allows CD40-activated B cells to proliferate in the presence of anti-class I MoAbs. These results lead to the conclusion that B cells can actively participate in regulating their own susceptibility to HLA class I α1 domain-mediated apoptosis by engagement of their antigen receptor.
Therefore, the present results show a similar regulation of B-lymphocyte sensitivity to HLA class I- and Fas-mediated apoptosis. Indeed, CD40 engagement, but not BCR ligation, induces susceptibility to the Fas apoptotic pathway in both human and mouse B lymphocytes, whereas engagement of the BCR protects CD40-activated B cells from Fas-induced apoptosis.6-9 However, there are several lines of evidence indicating that the HLA class I-induced apoptosis is Fas independent. Indeed, (1) MoAb90 does not bind to the HLA class I-deficient B-cell line Daudi that express the Fas antigen; (2) MoAb90 does not induce apoptosis of Fas-sensitive B- and T-cell lines, such as Ramos and Jurkat (data not shown); (3) addition of an antagonistic anti-Fas, MoAb ZB431 does not inhibit MoAb90-induced apoptosis of activated T cells (Genestier et al, submitted) and activated B cells (data not shown); and (4) in contrast to the Fas-dependent apoptosis that requires oligomerization of the Fas antigen by multivalent antibodies or antibodies capable of self-aggregation,32 HLA class I-dependent apoptosis does not require cross-linking by the IgG1 MoAb 90 and can be induced by monovalent Fab′ fragments of MoAb90. Further studies are planned to determine whether HLA class I- and anti-Fas–mediated apoptosis use common intracellular pathways.
The physiological significance of the striking proapoptotic effects of anti-HLA class I α1 domain on CD40-activated B cells remains to be established. Although binding of HLA class I to receptors of NK cells represents a mechanism to inhibit the NK cell lytic activity,33 the absence of NK cells from tonsil B-cell populations (Fig 7), indicates that the inhibitory effects of antibodies to HLA class I α1 domain on activated lymphocytes are independent of NK-cell activation. Whether this system represents a mechanism to eliminate B cells that have been activated in a noncognate fashion during T-B–cell interactions or to eliminate autoreactive/anergic B cells that possess desensitized BCR, as suggested for the Fas-Fas ligand system,5,6 34 remains to be determined. The identification of a specific HLA class I α1 domain ligand would greatly contribute to address this puzzling finding.
ACKNOWLEDGMENT
We thank S. Saeland and N. Bonnefoy-Berard for careful reading of the manuscript and S. Bonnet-Arnaud for editorial assistance.
L.G. and M.G. contributed equally to this project.
G.M. was recipient of a grant from the Fondation Mérieux (Lyon, France). This work was supported by a contract from the Région Rhône-Alpes (H0987 30000) and by a grant from the European Biotech Programm “In Vitro Immunotoxicology” (Bio 2. CT 92-0316) to J.P.R.
Address reprint requests to Jean-Pierre Revillard, MD, INSERM U80, Hôpital E. Herriot, Pav P, 5 place d'Arsonval, 69437 Lyon Cedex 03, France.

![Fig. 1. MoAb90 inhibits CD40-dependent B-cell proliferation. Tonsil B cells (2 × 104/well) were cultured on irradiated CD32-transfected L cells (5 × 103/well) in the presence of 1 μg/mL anti-CD40 MoAb and (A) with increasing concentrations of MoAb90 or W6/32 added at the onset of the culture; (B) with 5 μg/mL of control IgG1, W6/32 or MoAb90 added on day 0 or 4. Proliferation was measured by [3H]TdR incorporation after a pulse with [3H]TdR during the last 16 hours of the culture on day 6 (A) or at the time indicated (B), and results are expressed as mean ± standard deviation (SD) of triplicate determinations. In each experiment, background of [3H]TdR uptake of CD32-L cells was less than 500 counts per minute (cpm). Arrow in B indicates time of MoAb90 addition. Data presented are representative of three independent experiments. (⋄) indicates IgG1 control, (○) indicates W6/32, and (•) indicates MoAb90. (C) Tonsil B cells vere cultured for 5 days on CD32-L cells in the presence of 1 μg/mL anti-CD40 MoAb with 1 μg/mL of W6/32 or MoAb90. Photos of culture wells (original magnitude ×100) show the formation of B-cell clumps induced by proliferation.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/2/10.1182_blood.v90.2.726/4/m_bl_0009f1a.jpeg?Expires=1769104395&Signature=2SXpSVi6D-SMhN9XO2CalZrdejjduTCsNRTZOEVBQv1giRUjRbAhz-2GuiISTBihREeljbuFDiIbOPDtcS0obRYLr75VtmKUVm7mgGTUy4zF1sxzQSrorPmBy8uHvHuWjQzHp4x-AaNWi9OPS24S~xxl5K1xwg52J4EkdJ5K9ALiVfI0YbKfpj1YS5sSkpzSR6fa9Hjo7V3-yBodOVuiqDSE851jiPcVzOjHEtJDbbWgHLeTsY79wBRl7~ZoJpEOkG4DOm400EmGM4kYfDVa5VM9YYF5y1P6XZ21xs2-fBoCo0vf7cBrSa3zM9pghdEER-es8XNvukq2VsQ06lJLxw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 1. MoAb90 inhibits CD40-dependent B-cell proliferation. Tonsil B cells (2 × 104/well) were cultured on irradiated CD32-transfected L cells (5 × 103/well) in the presence of 1 μg/mL anti-CD40 MoAb and (A) with increasing concentrations of MoAb90 or W6/32 added at the onset of the culture; (B) with 5 μg/mL of control IgG1, W6/32 or MoAb90 added on day 0 or 4. Proliferation was measured by [3H]TdR incorporation after a pulse with [3H]TdR during the last 16 hours of the culture on day 6 (A) or at the time indicated (B), and results are expressed as mean ± standard deviation (SD) of triplicate determinations. In each experiment, background of [3H]TdR uptake of CD32-L cells was less than 500 counts per minute (cpm). Arrow in B indicates time of MoAb90 addition. Data presented are representative of three independent experiments. (⋄) indicates IgG1 control, (○) indicates W6/32, and (•) indicates MoAb90. (C) Tonsil B cells vere cultured for 5 days on CD32-L cells in the presence of 1 μg/mL anti-CD40 MoAb with 1 μg/mL of W6/32 or MoAb90. Photos of culture wells (original magnitude ×100) show the formation of B-cell clumps induced by proliferation.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/2/10.1182_blood.v90.2.726/4/m_bl_0009f1c.jpeg?Expires=1769104395&Signature=1wxUpb8CuKuQnbmB5grl3s1F93~YgVRFinzg8vpdXhY80~q8FfPXls29D7ZOswNongnUynwlhD3dMVbHB0hJowcUUgxQomw65CmbK97klkDJGkRPZrCr8ECSSDqs7Lrkp48h7RnzqmSzJ3X74rIEs4jdhH5j5uWqFS3J3ii6OIXzSAOf~bxk5VuocCVOjw1oztC--bJ8D7w9CAc72XlQZ6otlpjSvwS43zHHkZXdVkWKc-jXbRWL6ai-~cZJ5ilG-HsJzxsAl1zIzHnrjr769B8n8yi5f0R-x7CQ8bVOWHE03HPpuOP7rjScZGtb2BV9BNlgmaAM18~5BtPkM1fyYA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. Engagement of HLA class I α1 domain inhibits DNA synthesis of CD40-activated total, naı̈ve, GC, and memory B cells in response to B-cell growth factors. Tonsil B cells (2 × 104/well) from each subset were cultured on CD32-L cells (5 × 103/well) in the presence of 1 μg/mL anti-CD40 MoAb with either IL-10 (20 ng/mL), IL-4 (50 U/mL), IL-2 (20 U/mL) plus IL-10 or IL-4 plus IL-10, and with control IgG1 or MoAb90 at 1 μg/mL added at the onset of the culture. Proliferation was measured by [3H]TdR incorporation after a pulse with [3H]TdR during the last 16 hours of the culture at the time indicated. Results are expressed as means ± SD of culture triplicates. [3H]TdR uptake of CD32-L cells was less than 500 cpm in the different experiments. This figure is representative of three independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/2/10.1182_blood.v90.2.726/4/m_bl_0009f2.jpeg?Expires=1769104395&Signature=Y2Vk4~tV3jQAAq3ukDFJAjrGnnJ0VlSsENm3zNjhtru3k4axKR~fJkyQ2R~OQo7z44zUMxMKeHfmsAudwBu4ckReeln2ht53rJw34D43qQJvTZ9f2SjuS3ku~IwPMnK5kjXQkq~qD7oAxuiK9mJz4cPvftAOr3dQYZvreBP88ZM53iF6fFrBSYAtalCqXU4-wWo2YVNfd7WL8HU0KrX6EFVC7LXQEUMi7Q7W3Wf69eerwWPUkqV5tWeMaQ0JMyHp3kpvIOGiomcLf7sl~v3Wyoe46hL8Gu8M~5DLKCKilzwHP4ZAyLPm~eQug6zcYSalyCf9U7nIpjj4KOZQ0wnnPA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Ligation of the BCR does not prime B cells to HLA class I-mediated apoptosis and protects CD40-activated B cells. Naive B cells were cultured on irradiated CD32-L cells in the presence of 1 μg/mL anti-CD40 MoAb alone, 5 μg/mL anti-BCR MoAbs alone, or the combination of both and with 5 μg/mL control IgG1, W6/32, TP25.99, MoAb90, or 1 μg/mL anti-Fas MoAb CH11 added at the onset of the culture. Proliferation was measured by [3H]TdR incorporation after a pulse with [3H]TdR during the last 16 hours of the culture at the time indicated. Results are expressed as means ± SD of culture triplicates. [3H]TdR uptake of CD32-L cells was less than 500 cpm in the different experiments. (⋄) indicates IgG1, (○) indicates W6.32, (□) indicates TP25.99, (▿) indicates B9.12.1, (•) indicates MoAb90, (▪) indicates YTH 862, and (▴) indicates anti-Fas.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/2/10.1182_blood.v90.2.726/4/m_bl_0009f5.jpeg?Expires=1769104395&Signature=U-1~32IM8~s0KfUZz-R7iSU902jWy0c6KBVHI7bFaSixBZWvHGihzuV9FHghsdB5mX33ct0NVLQuzqcVFRmKt5gfJbpT4R7QAJZN5Q7nsuX7sssFQFHHDGCYVfCHAVhytRRE33-zUYLxuGaZ2aLHqXyCxtIyuW4t0mt5pleCXI0i~dejTtL13qM9AvgwkKF6bdZaIWjsdrZ1LA3hQP3~135qwGw8ssNZGJuWpX1lsK3uaqbZNB6Y6PHkVkOrbOkzwfECibGs3q1jZTf3mPIqci5CrZ9QA7Hc6VZywOfRfqr4CD60FXCcw5TeKqnWb-vpahHUZRM8utoqy3ngwfRpxQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal