Abstract
CD44 is a cytotoxic triggering molecule on activated, but not fresh natural killer (NK) cells. In the current study, metabolic pathways used in CD44-directed lysis (CD44DL) were examined using activated human NK cells as effectors. We found that CD44 expressed by activated NK cells was indistinguishable in isoform and molecular weight from CD44 on unactivated cells. However, de novo protein expression was required for the induction of CD44DL, suggesting that activated NK cells contain proteins not present in fresh NK cells that couple CD44 to the lytic machinery. Concanimycin A, a selective inhibitor of perforin-based cytolysis, totally blocked CD44DL, natural cytototoxicity, and antibody-dependent cell-mediated cytolysis (ADCC). Moreover, studies in which kinase inhibitors were added during the effector phase of lysis indicated that protein-tyrosine and ser/thr kinases were required for all three cytolytic activities and that protein kinase C played a nonessential role in lysis. By contrast, wortmannin totally inhibited CD44DL, but failed to block natural cytotoxicity and only partially blocked ADCC, suggesting that phosphatidylinositol 3-kinase (PI 3-kinase) is required at an early, receptor-specific stage of CD44DL. Finally, cytochalasin B enhanced CD44DL, but not ADCC, indicating that CD44DL is modulated by actin polymerization. Taken together, our data suggest that CD44 in NK cells interacts with proteins induced during interleukin-2 activation in a triggering pathway that induces perforin release, requires PI 3-kinase, and is modulated by the cytoskeleton.
NATURAL KILLER (NK) cells mediate a number of cytolytic activities.1 In the absence of antibodies, they lyse virally infected cells and NK-sensitive tumor targets such as K562 cells, but the receptors involved in the triggering of natural cytotoxicity are poorly characterized. Freshly isolated NK cells also mediate antibody-dependent cellular cytotoxicity (ADCC), which involves the binding of antibody-coated target cells to FcγRIIIA (CD16) on the NK cells. FcγRIIIA associates with two signal transducing molecules, CD3ζ and FcεRIγ, that, upon cross-linking, activate several tyrosine kinases and induce an increase in intracellular calcium, ultimately leading to NK cell degranulation and the release of cytolytic substances at the effector:target interface.2-4 Finally, NK cells mediate redirected lysis through adhesion molecules such as CD44 and CD69.5-10 We have shown, for example, that a bispecific antibody (bsAb) that binds both CD44 and 2,4,6 trinitrophenyl (TNP) will attach TNP-coated target cells to CD44 on NK cells and induce their lysis.7 By contrast, a bsAb that binds to MHC rather than CD44 will link the two cells together, but will not induce lysis. CD44-directed lysis (CD44DL) is mediated by interleukin-2 (IL-2)–activated, but not fresh NK cells from most donors,7 some T-cell clones,11,12 and fresh human neutrophils.13 The cross-linking of CD44 on NK or T cells induces little, if any, increase in intracellular calcium, but can enhance the calcium signal resulting from cross-linking of CD2 or FcγRIIIA.10,14 Moreover, CD44DL mediated by T-cell clones is blocked by a tyrosine kinase inhibitor.12
CD44 is a heavily glycosylated, type I transmembrane receptor that is broadly expressed on a large number of cell types, including most leukocytes.15,16 The N-terminal portion of the CD44 extracellular domain bears homology to cartilage link protein and provides a binding site for hyaluronan (HA), the principal ligand of CD44.17-19 Proximal to the transmembrane region, the extracellular domain of CD44 contains an insertion site for one or more variant exons, resulting in the expression of a large number of CD44 isoforms.20-22 Variant exons are commonly found in CD44 molecules on epithelial cells and tumors, where they have been implicated in metastasis.23,24 Most hematopoetic cells express the standard isoform of CD44, which contains no variant exons, but other CD44 isoforms have been observed in activated T and B cells.25 The cytoplasmic portion of CD44 is about 70 residues in length and contains several serine/threonine phosphorylation sites but no tyrosine residues.18,26 CD44 can associate through its cytoplasmic domain with the cytoskeleton, perhaps by interacting with ankyrin or ERM (ezrin, radixin, and moesin) family members.15 27-29
The purpose of the current study is to investigate the molecular mechanisms leading to CD44DL in human NK cells. We have examined the CD44 molecule before and after NK cell stimulation for changes in structure and have used inhibitors to define the pathways used during the generation and effector phases of CD44DL. We found that acquisition of CD44 triggering function requires de novo protein expression but is not accompanied by a change in CD44 isoform or molecular weight. In the effector phase, CD44DL uses a phosphatidylinositol 3-kinase (PI 3-kinase)–dependent pathway that is modulated by protein kinase C (PKC) and the cytoskeleton.
MATERIAL AND METHODS
Antibodies and reagents.Phycoerythrin (PE) anti-Leu 19 (anti-CD56) was purchased from Becton Dickinson (San Jose, CA). NIH44.1 (IgG1 anti-huCD44 monoclonal antibody [MoAb]), MOPC-300 (IgG1 myeloma protein), affinity-purified polyclonal rabbit anti-DNP antibodies, and anti-CD44(Fab) × anti-DNP(Fab) (a chemically cross-linked bsAb) have been described.7 Cytochalasin B, herbimycin A, and GF109203X (bisindolylmaleimide I30) were purchased from Calbiochem (San Diego, CA); wortmannin, staurosporine and actinomycin D were from Sigma (St Louis, MO); and concanamycin A (CMA) was from Wako Pure Chemicals (Richmond, VA).
Effector cells.Peripheral blood lymphocytes (PBL) were isolated from buffy coats or leukopacks from normal National Institutes of Health Blood Bank donors by Ficoll-Hypaque density gradient sedimentation, plastic adherence, and nylon wool adsorption.7 Low-density PBL (LD-PBL), which are typically 30% to 50% CD16+, CD56+ (ie, NK) cells, were isolated on a two-layer discontinuous Percoll density gradient31 and were activated by culturing for 2 to 3 days with 100 U/mL recombinant IL-2 (rIL-2). NK cells were purified from activated LD-PBL by using a Vario MACS system with an NK isolation kit (Miltenyi Biotec, Auburn, CA), which negatively selects NK cells by removing CD3+, CD4+, CD19+, and CD33+ cells, or by staining the cells with PE–anti-CD16 MoAb and electronically sorting using a Becton Dickinson FACStar Plus flow cytometer.
Cytotoxicity assay.The cytotoxicity assay and target cells have been described previously.7 Briefly, effector cells were incubated for 20 to 30 minutes at room temperature with 0.6 μg/mL anti-CD44(Fab) × anti-DNP(Fab) bsAb (for CD44DL), anti-DNP Ab (for ADCC), or no Ab (for natural cytotoxicity) and diluted serially. As required, inhibitors or medium (control) were added to the diluted effector cells and 51Cr-labeled target cells (104 /well) were added immediately thereafter. Target cells were MC1 cells that had been modified for 5 minutes with 3 mmol/L trinitrobenzene sulfonate (TNP-MC1) for CD44DL and ADCC assays7 and with K562 cells for natural cytotoxicity assays. Both target cells were negative for Fas (CD95) expression by fluorescence-activated cell sorting (FACS) analysis (data not shown). Cytotoxicity was followed using a 4-hour 51Cr-release assay.32 The percentage of specific lysis was calculated as the mean ± SEM from triplicate samples using 51Cr released by 2.5% Triton X-100 as the maximum release value and the amount released in culture medium as the spontaneous release. Spontaneous release was 10% or less of the maximum in all experiments reported here.
Reverse transcriptase-polymerase chain reaction (RT-PCR).Total RNA was extracted from 1 × 107 cells using a Qiagen (Chatsworth, CA) RNeasy kit. The CD44 cDNA first strand was produced using Moloney's murine leukemia virus reverse transcriptase (Life Technologies, Gaithersburg, MD) with an antisense primer (GCGAGATCTTTATTATTCTGGAATTTGGGGTGTCCT) annealing to exon 16. cDNA was amplified for 30 cycles using the same antisense primer and a sense-strand primer (TCCACCTGAAGAAGATTGTACATCAGT) annealing to exon 4. Amplified samples were detected by Southern blotting using a 32P-labeled CD44 cDNA probe.
Immunoprecipitation.NK cells (1 mL of 1 to 2 × 107/mL) were surface biotinylated33 by incubating them for 1 hour at 4°C in phosphate-buffered saline, pH 8.0, containing 1 mg/mL of sulfosuccinimidobiotin (Pierce Chemical Co, Rockford, IL). The surface-labeled cells were washed twice, resuspended in 0.8 mL lysing buffer (50 mmol/L Tris, pH 8.5; 5 mmol/L EDTA; 150 mmol/L NaCl; 0.5% NP40; 1 mmol/L phenylmethyl sulfonyl fluoride; 1 μg/mL each of aprotinin, leupeptin, and α-1-antitrypsin; and 1 mmol/L Na3VO4 ) and incubated for 10 minutes at 0°C. Lysates were then centrifuged for 15 minutes at 10,000g and the insoluble fraction was discarded. Supernatants were precleared once for 2 hours with slow tumbling with 50 μL of packed protein-A beads (Pharmacia, Piscataway, NJ) precoated with rabbit IgG. Five micrograms of NIH44.1 or an isotype-matched control MoAb (MOPC 300) was added and supernatants were incubated for 1 hour at 4°C, followed by 2 hours of incubation with slow tumbling with 25 μL of packed protein A-Sepharose beads precoated with rabbit antimouse IgG (Cappel Organon Teknika, Durham, NC). After washing three times in lysis buffer, the beads were boiled in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer, and the released proteins were analyzed by SDS-PAGE on a 12.5% Pharmacia PhastGel. The resulting gel was transferred to a nitrocellulose membrane and blotted with 1:1,500 horseradish peroxidase (HRP)-streptavidin (Amersham, Arlington Heights, IL), using a Pharmacia PhastSystem Western blotting apparatus.
Effector-target conjugates.Effector-target conjugates were detected by flow cytometry using a modification of a previously published procedure.34 Activated LD-PBL and TNP-MC1 cells were fractionated on Ficoll-Hypaque to remove dead cells. LD-PBL were stained with PE — anti–Leu-19 for 30 minutes on ice and washed. The LD-PBL were then incubated with graded concentrations of anti-CD44(Fab) × anti-DNP(Fab) followed by the addition of TNP-MC1 cells at a ratio of 1:1. The cells were spun and incubated at 37°C for 1 hour. The effector-target conjugates were gently resuspended and analyzed by flow cytometry. The CD56+ LD-PBL were easily distinguished from the TNP-MC1 tumor cells on the basis of their smaller forward light scatter. Thus, conjugates were calculated as the percentages of CD56+ particles having the same or higher forward light scatter as the TNP-MC1 cells.
RESULTS
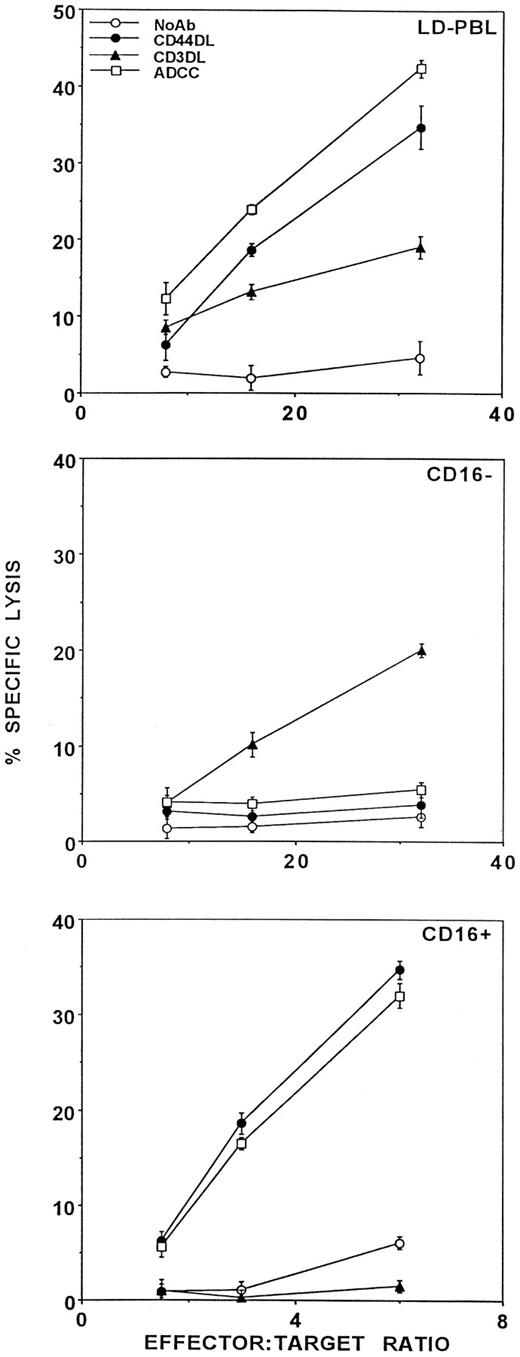
Acquisition of CD44DL activity requires de novo protein expression but is not accompanied by a change in CD44 structure.We previously showed that CD44 gains the ability to trigger lysis when low buoyant density PBL are activated with IL-2 and used as effectors in a standard 4-hour 51Cr release assay.7 Figure 1 shows that, in functionally pure sorted populations of activated LD-PBL, the CD44DL activity is mediated exclusively by the NK cells. Thus, in the upper panel, unseparated cells mediate CD44DL, CD3-directed lysis (CD3DL), and ADCC. When these cells were electronically sorted into CD16+ (lower panel) and CD16− (middle panel) subsets, the CD16+ cells mediated ADCC but not CD3DL, and were therefore functionally pure NK cells, whereas the CD16− cells mediated CD3DL but not ADCC, indicative of functionally pure cytotoxic T-lymphocytes (CTL). As can be seen in Fig 1, only the NK cells mediated CD44DL. Although previous reports have shown that T-cell clones mediate CD44DL,11,12 we have never observed CD44DL activity in CTL derived from resting (high buoyant density) PBL using IL-2 and a TcR cross-linking signal or from tumor-infiltrating lymphocyte lines from several patients (data not shown). However, we have observed that, in rare cases, the T-cell fraction from IL-2–activated LD-PBL (as in Fig 1, middle panel) could mediate a small amount of CD44DL.7
CD44DL is mediated by NK cells. LD-PBL were activated for 3 days with 100 U/mL rIL-2 and tested for the ability to lyse TNP-MC1 target cells in the presence of no antibody (○), anti-CD44(Fab) × anti-DNP(Fab) bsAb (•), anti-CD3(Fab) × anti-DNP(Fab) bsAb (▴), and anti-DNP Ab (□). Effector cells were as follows: upper panel, total LD-PBL; middle panel, electronically sorted CD16− LD-PBL; lower panel, sorted CD16+ LD-PBL.
CD44DL is mediated by NK cells. LD-PBL were activated for 3 days with 100 U/mL rIL-2 and tested for the ability to lyse TNP-MC1 target cells in the presence of no antibody (○), anti-CD44(Fab) × anti-DNP(Fab) bsAb (•), anti-CD3(Fab) × anti-DNP(Fab) bsAb (▴), and anti-DNP Ab (□). Effector cells were as follows: upper panel, total LD-PBL; middle panel, electronically sorted CD16− LD-PBL; lower panel, sorted CD16+ LD-PBL.
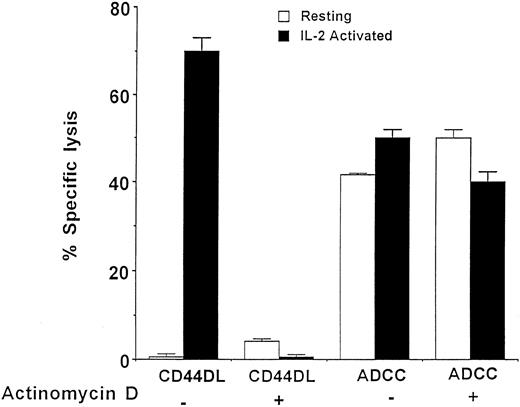
To see whether the acquisition of CD44DL required protein synthesis, LD-PBL were incubated for 3 days with either medium or IL-2 in the presence or absence of the transcription inhibitor, actinomycin D (Fig 2). In the absence of actinomycin D, NK cells acquired the ability to mediate CD44DL only when they were cultured with IL-2, whereas these same cells mediated ADCC regardless of whether IL-2 was present in the medium. The gain in CD44DL activity was totally blocked by low concentrations of actinomycin D. By contrast, actinomycin D had no effect on ADCC, indicating that the NK cells retained their cytolytic competence in the presence of the inhibitor. Therefore, these data suggest that the de novo expression of one or more proteins was required to link CD44 to the killing machinery.
The acquisition of CD44DL requires IL-2 activation and transcription. LD-PBL were incubated for 3 days in the presence or absence of 100 U/mL IL-2 with or without 10 ng/mL actinomycin D, washed, and tested for the ability to lyse TNP-MC1 target cells at an effector:target ratio of 25:1. CD44DL, lysis measured in the presence of anti-CD44(Fab) × anti-DNP (Fab) bsAb; ADCC, lysis measured in the presence of intact rabbit anti-DNP Ab. Similar results were obtained at other effector:target ratios and in two of two other experiments. Data represent the means and standard errors of triplicate samples from a single experiment. (□) Resting; (▪) IL-2 activated.
The acquisition of CD44DL requires IL-2 activation and transcription. LD-PBL were incubated for 3 days in the presence or absence of 100 U/mL IL-2 with or without 10 ng/mL actinomycin D, washed, and tested for the ability to lyse TNP-MC1 target cells at an effector:target ratio of 25:1. CD44DL, lysis measured in the presence of anti-CD44(Fab) × anti-DNP (Fab) bsAb; ADCC, lysis measured in the presence of intact rabbit anti-DNP Ab. Similar results were obtained at other effector:target ratios and in two of two other experiments. Data represent the means and standard errors of triplicate samples from a single experiment. (□) Resting; (▪) IL-2 activated.
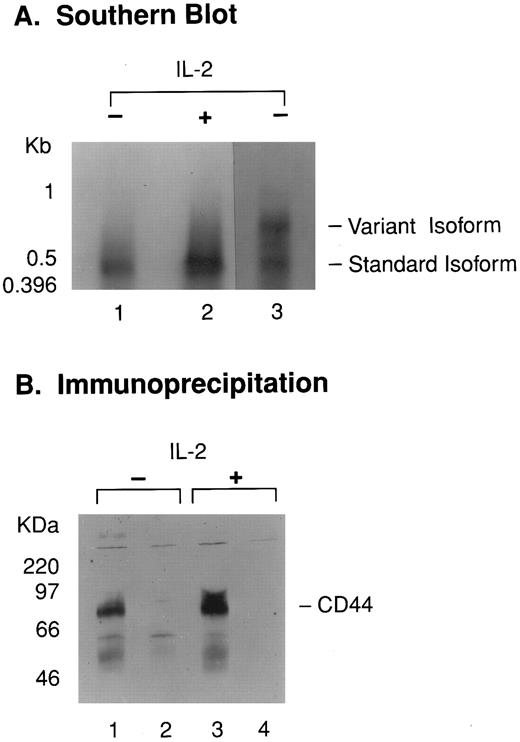
We next asked whether the acquisition of CD44DL activity correlated with a change in the molecular structure of CD44 itself. Primers flanking the variant domain splice site were used in RT-PCR experiments to test for activation-induced changes in isoform expression. As seen in Fig 3A, both activated and resting LD-PBL produced a single 430-bp band by RT-PCR, corresponding to the standard isoform, whereas control (breast cancer) cells gave bands characteristic of larger isoforms. In addition, immunoprecipitation of surface-labeled CD44 from both activated and resting purified NK cells (Fig 3B) gave a single broad band of 85 to 95 kD, the reported molecular weight of the standard form of CD44 protein.15 Thus, no activation-induced changes in isoform expression or in posttranslational modification were detected in the CD44 molecule.
(A) Activated NK cells express message encoding the standard CD44 isoform. RNA from unactivated (lane 1) and IL-2–activated (lane 2) LD-PBL and from MDMB-75 breast cancer cells (lane 3) was analyzed by RT-PCR using primers flanking the CD44 variant domain insertion point. Amplified products were detected by Southern blotting. Positions of standard and variant isoforms are indicated. (B) Unactivated and activated NK cells express similar sized forms of CD44 on their surfaces. Immunoprecipitates from biotin-labeled purified NK cells were analyzed by SDS-PAGE and Western-blotted with streptavidin-HRP. The cells were either unactivated (lanes 1 and 2) or activated (lanes 3 and 4). Anti-CD44 MoAb was used for immunoprecipitation in lanes 1 and 3, and an isotype-matched control antibody (MOPC 300) was used in lanes 2 and 4.
(A) Activated NK cells express message encoding the standard CD44 isoform. RNA from unactivated (lane 1) and IL-2–activated (lane 2) LD-PBL and from MDMB-75 breast cancer cells (lane 3) was analyzed by RT-PCR using primers flanking the CD44 variant domain insertion point. Amplified products were detected by Southern blotting. Positions of standard and variant isoforms are indicated. (B) Unactivated and activated NK cells express similar sized forms of CD44 on their surfaces. Immunoprecipitates from biotin-labeled purified NK cells were analyzed by SDS-PAGE and Western-blotted with streptavidin-HRP. The cells were either unactivated (lanes 1 and 2) or activated (lanes 3 and 4). Anti-CD44 MoAb was used for immunoprecipitation in lanes 1 and 3, and an isotype-matched control antibody (MOPC 300) was used in lanes 2 and 4.
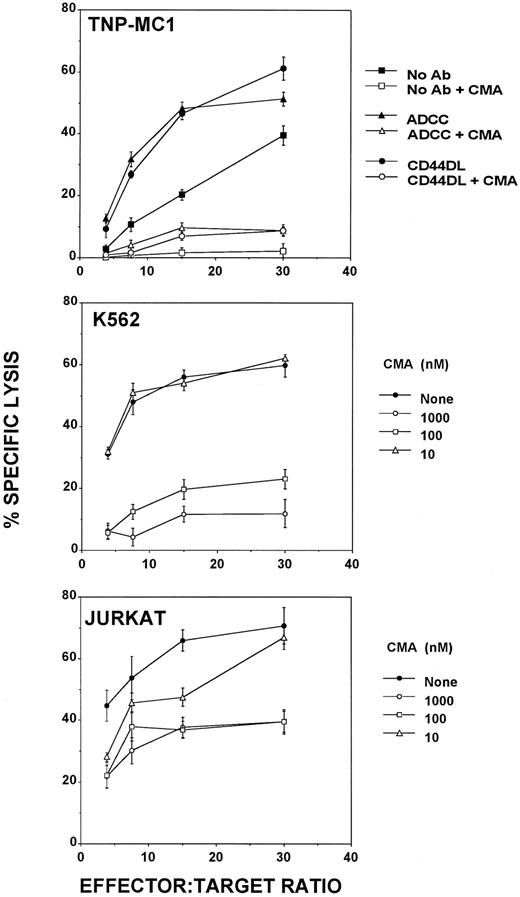
CD44DL, ADCC, and natural cytotoxicity use the perforin-based killing pathway.Kataoka et al35 recently showed that CMA inhibits the perforin-based, but not Fas-dependent pathway of cell-mediated cytolysis. To investigate whether the granule exocytosis (perforin-based) pathway was used in CD44DL, ADCC, and natural cytotoxicity, we tested CMA for the ability to inhibit these activities. Figure 4 shows that CMA at concentrations greater than 100 nmol/L totally blocked the three activities when the Fas-negative targets, K562 and TNP-MC1, were used. By contrast, natural cytotoxicity against Jurkat target cells, which express Fas, was only partially blocked by CMA. This observation is consistent with previous findings35 that Fas-positive targets are lysed by both perforin- and Fas-based mechanisms, but that only the perforin-based component is blocked by CMA. Thus, Fig 4 provides evidence that, under our experimental conditions, natural cytotoxicity, ADCC, and CD44DL all used the perforin-based pathway, exclusively.
CMA blocks CD44DL, ADCC, and natural cytotoxicity mediated by activated NK cells. (Upper panel) Lysis of TNP-MC1 cells by activated NK cells in the presence (open symbols) or absence (solid symbols) of 100 nmol/L CMA. Lysis was measured in the absence of antibody (squares), in the presence of anti-CD44 bsAb (CD44DL; circles), and in the presence of intact anti-DNP Ab (ADCC; triangles). (Middle panel) Blockage of natural cytotoxicity against the Fas-negative, NK-sensitive target, K562 with 10, 100, and 1,000 nmol/L CMA. (Lower panel) Partial inhibition of lysis of Jurkat cells, which are Fas-positive and NK-sensitive, by 10, 100, and 1,000 nmol/L CMA. All experiments were repeated four times, with similar results, except for the inhibition of Jurkat lysis, which was repeated twice. Pretreatment of effector cells with 100 nmol/L CMA for 1 hour at 37°C, followed by washing, gave similar results.
CMA blocks CD44DL, ADCC, and natural cytotoxicity mediated by activated NK cells. (Upper panel) Lysis of TNP-MC1 cells by activated NK cells in the presence (open symbols) or absence (solid symbols) of 100 nmol/L CMA. Lysis was measured in the absence of antibody (squares), in the presence of anti-CD44 bsAb (CD44DL; circles), and in the presence of intact anti-DNP Ab (ADCC; triangles). (Middle panel) Blockage of natural cytotoxicity against the Fas-negative, NK-sensitive target, K562 with 10, 100, and 1,000 nmol/L CMA. (Lower panel) Partial inhibition of lysis of Jurkat cells, which are Fas-positive and NK-sensitive, by 10, 100, and 1,000 nmol/L CMA. All experiments were repeated four times, with similar results, except for the inhibition of Jurkat lysis, which was repeated twice. Pretreatment of effector cells with 100 nmol/L CMA for 1 hour at 37°C, followed by washing, gave similar results.
Serine-threonine, tyrosine, and PI 3-kinases are required for CD44DL.To compare the early events in CD44DL, ADCC, and NK cytotoxicity, we added metabolic inhibitors to the cytotoxicity assays. Inhibition of lysis by staurosporine and herbimycin, broadly reactive serine-threonine and tyrosine kinase inhibitors, respectively, is shown in Table 1. Both inhibitors completely blocked the three types of NK cell-mediated cytotoxicity at concentrations near their reported IC50 ,36 37 suggesting that serine-threonine and tyrosine kinases are required for cytotoxic function in NK cells.
Serine-Threonine and Tyrosine Kinase Inhibitors Block CD44DL
| Inhibitor . | Concentration . | % Inhibition of Lysis . | ||
|---|---|---|---|---|
| . | . | CD44DL . | NK . | ADCC . |
| Experiment no. 1 | ||||
| Staurosporine | 25 nmol/L | 100 | 74 | 100 |
| Staurosporine | 2.5 nmol/L | 39 | 23 | 75 |
| Experiment no. 2 | ||||
| Herbimycin A | 5.2 μmol/L | 99 | 90 | 98 |
| Herbimycin A | 0.52 μmol/L | 64 | 18 | 62 |
| Inhibitor . | Concentration . | % Inhibition of Lysis . | ||
|---|---|---|---|---|
| . | . | CD44DL . | NK . | ADCC . |
| Experiment no. 1 | ||||
| Staurosporine | 25 nmol/L | 100 | 74 | 100 |
| Staurosporine | 2.5 nmol/L | 39 | 23 | 75 |
| Experiment no. 2 | ||||
| Herbimycin A | 5.2 μmol/L | 99 | 90 | 98 |
| Herbimycin A | 0.52 μmol/L | 64 | 18 | 62 |
Activated LD-PBL were tested for the ability to mediate the indicated type of lysis at an E:T of 24:1, except for NK lysis (natural cytotoxicity) in experiment no. 1, which was measured at 5:1. The percentages of lysis in the absence of inhibitors for CD44DL, NK, and ADCC were 52%, 46%, and 30% for experiment no. 1, and 33%, 91%, and 32% for experiment no. 2. Similar percentages of inhibition were obtained at other E:T ratios used in these experiments. Both inhibitors were used in at least four separate experiments with similar results.
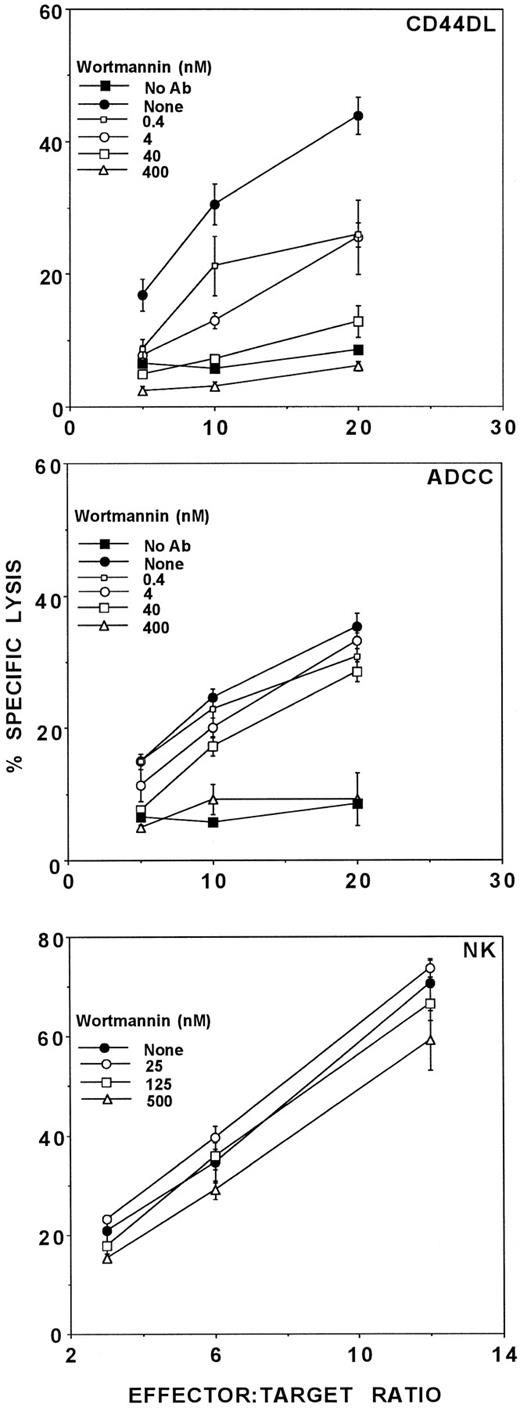
Recently, Bonnema et al,38 using NK clones as effector cells, showed that natural cytotoxicity was sensitive to inhibition by GF109203X, a PKC-specific inhibitor,30 and insensitive to wortmannin, a PI 3-kinase inhibitor, whereas the reverse was true for ADCC. We therefore tested GF109203X and wortmannin for the ability to inhibit CD44DL, ADCC, and natural cytotoxicity. Figure 5 shows that CD44DL was particularly sensitive to inhibition by wortmannin, whereas ADCC was less sensitive and natural cytotoxicity was insensitive. The reported IC50 of wortmannin for PI 3-kinase is 5 nmol/L; other kinases (eg, PI 4-kinase and myosin light chain kinase) require 100-fold greater concentrations for inhibition.39 As seen in Fig 5, 5 nmol/L wortmannin inhibited CD44DL by about 50% and inhibition was nearly complete at higher concentrations. This finding implies that PI 3-kinase is required for CD44DL. Wortmannin only partially Inhibited ADCC at concentrations 10-fold above the IC50 , suggesting that PI 3-kinase is not required for ADCC. In agreement with the earlier findings,38 wortmannin failed to inhibit the antibody-independent lysis of K562 cells, indicating that PI 3-kinase was not involved in natural cytotoxicity.
Inhibition of cytolysis by wortmannin. Activated LD-PBL were tested for lytic activity in the presence of the PI 3-kinase inhibitor, wortmannin. CD44DL (upper panel) and ADCC (middle panel) were tested using TNP-MC1 targets in the presence of 400 (▵), 40 (□), 4 (○), 0.4 nmol/L (□), and 0 nmol/L (•) wortmannin. (▪) Lysis in the absence of antibody or wortmannin. Natural cytotoxicity (lower panel) was measured using K562 target cells in the presence of 500 (▵), 125 (□), 25 (○), and 0 nmol/L (•) wortmannin. This experiment has been repeated five times, using cells from different donors, with similar results.
Inhibition of cytolysis by wortmannin. Activated LD-PBL were tested for lytic activity in the presence of the PI 3-kinase inhibitor, wortmannin. CD44DL (upper panel) and ADCC (middle panel) were tested using TNP-MC1 targets in the presence of 400 (▵), 40 (□), 4 (○), 0.4 nmol/L (□), and 0 nmol/L (•) wortmannin. (▪) Lysis in the absence of antibody or wortmannin. Natural cytotoxicity (lower panel) was measured using K562 target cells in the presence of 500 (▵), 125 (□), 25 (○), and 0 nmol/L (•) wortmannin. This experiment has been repeated five times, using cells from different donors, with similar results.
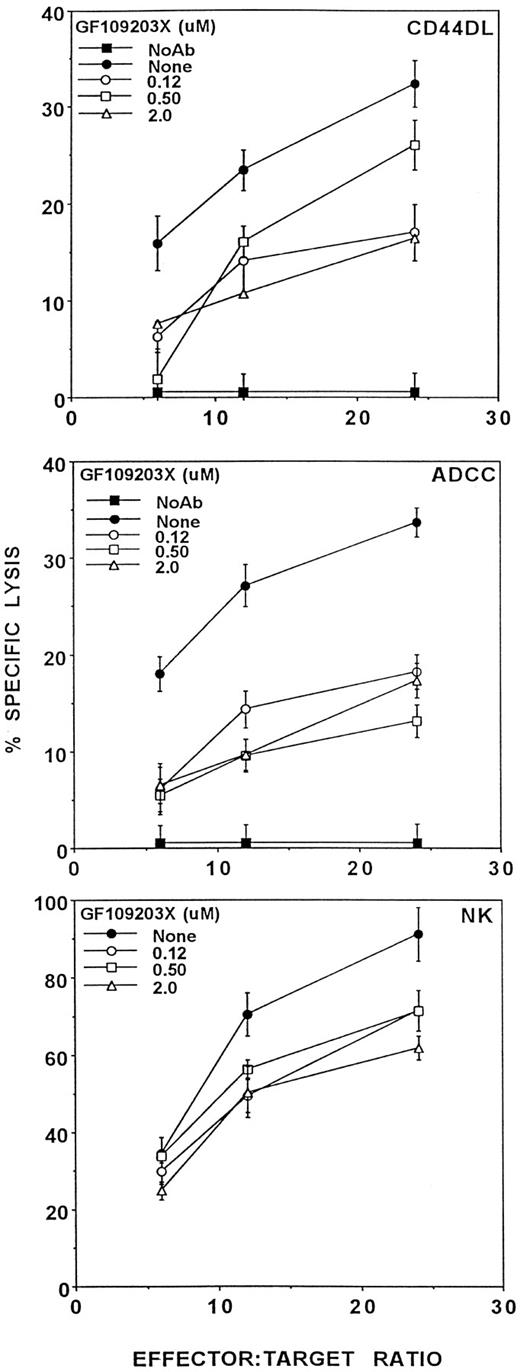
In NK clones, GF109203X inhibits natural cytotoxicity and degranulation induced by phorbol ester plus calcium ionophore (PKC activators) at concentrations of 0.1 μmol/L or greater.38 Figure 6 shows that, in activated peripheral blood NK cells, GF109203X partially inhibited CD44DL and ADCC and natural cytotoxicity. Assuming that GF109203X specifically inhibited PKC, particularly in the range of 0.12 and 0.5 μmol/L, where it is known to act specificially, these data suggest that PKC is used in, but is not required for CD44DL and ADCC.
The effects of GF109203X on CD44DL, ADCC, and natural cytotoxicity. Activated LD-PBL were tested for lytic activity in the presence of the indicated concentrations of the PKC inhibitor, GF109203X. CD44DL (upper panel) and ADCC (middle panel) were tested with TNP-MC1 targets, and natural cytotoxicity (lower panel) was measured using K562 target cells. GF109203X concentrations were 2.0 (▵), 0.5 (□), 0.12 (○), and 0 μmol/L (•). (▪) Lysis in the absence of antibody or GF109203X. Similar results have been obtained in five separate experiments.
The effects of GF109203X on CD44DL, ADCC, and natural cytotoxicity. Activated LD-PBL were tested for lytic activity in the presence of the indicated concentrations of the PKC inhibitor, GF109203X. CD44DL (upper panel) and ADCC (middle panel) were tested with TNP-MC1 targets, and natural cytotoxicity (lower panel) was measured using K562 target cells. GF109203X concentrations were 2.0 (▵), 0.5 (□), 0.12 (○), and 0 μmol/L (•). (▪) Lysis in the absence of antibody or GF109203X. Similar results have been obtained in five separate experiments.
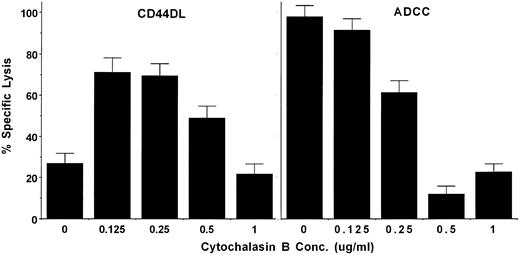
Cytochalasin B enhances CD44DL.Previous studies indicated that both CD44 and PI 3-kinase can associate with the cytoskeleton.27-29,40 41 We therefore used cytochalasin B, an inhibitor of actin polymerization, to test for cytoskeletal involvement in CD44DL. Figure 7 shows that cytochalasin B inhibited ADCC in a dose-dependent manner. By contrast, low concentrations of cytochalasin B enhanced CD44-dependent lysis, but this enhancement decreased to the normal level at higher concentrations. At still higher concentrations, cytochalasin inhibited CD44DL to near background levels (data not shown). The enhancement of CD44DL by cytochalasin B was observed in NK cells from approximately 60% to 70% of donors. To see if cytochalasin affected CD44DL by altering conjugate formation between effector and target cells, we used FACS analysis to quantify the number of CD56+ cells in conjugates at varying bsAb concentrations. The data of Table 2 show that no changes in conjugate formation occurred at the concentration of bsAb used in the cytotoxicity experiments (0.6 μg/mL) as a result of adding either low (experiment no. 2) or high (experiment no. 1) concentrations of cytochalasin B, although some inhibition was observed in experiment no. 1 at higher bsAb concentrations. Thus, cytochalasin B produced an enhancement in CD44DL without altering conjugate formation.
Low concentrations of cytochalasin B enhance CD44DL. Activated LD-PBL were assayed for the ability to mediate CD44DL (left panel) or ADCC (right panel) in the presence of the indicated concentrations of cytochalasin B at an effector:target ratio of 35:1. Qualitatively similar results were obtained at other E:T ratios and with NK cells from at least seven other donors.
Low concentrations of cytochalasin B enhance CD44DL. Activated LD-PBL were assayed for the ability to mediate CD44DL (left panel) or ADCC (right panel) in the presence of the indicated concentrations of cytochalasin B at an effector:target ratio of 35:1. Qualitatively similar results were obtained at other E:T ratios and with NK cells from at least seven other donors.
Effect of Cytochalasin B on Conjugate Formation
| . | bsAb (μg/mL) . | % of CD56+ Cells in Conjugates . | |
|---|---|---|---|
| . | . | Medium . | Cytochalasin B . |
| Experiment no. 1 | 0 | 18 | 18 |
| 0.6 | 28 | 28 | |
| 1.6 | 39 | 31 | |
| 3.2 | 52 | 39 | |
| Experiment no. 2 | 0 | 6 | 5 |
| 0.6 | 21 | 20 | |
| 1.6 | 32 | 34 | |
| 3.2 | 45 | 43 | |
| . | bsAb (μg/mL) . | % of CD56+ Cells in Conjugates . | |
|---|---|---|---|
| . | . | Medium . | Cytochalasin B . |
| Experiment no. 1 | 0 | 18 | 18 |
| 0.6 | 28 | 28 | |
| 1.6 | 39 | 31 | |
| 3.2 | 52 | 39 | |
| Experiment no. 2 | 0 | 6 | 5 |
| 0.6 | 21 | 20 | |
| 1.6 | 32 | 34 | |
| 3.2 | 45 | 43 | |
Activated LD-PBL were surface labeled with PE–anti-CD56 and mixed with TNP-MC1 cells in the presence of the indicated concentrations of anti-CD44(Fab) × anti-DNP(Fab) bsAb for 30 minutes at 37°C with either 0.5 μg/mL (experiment no. 1) or 0.125 μg/mL (experiment no. 2) cytochalasin B. Conjugates were detected as CD56+ cells having forward light scatter greater or equal to that of the TNP-MC1 cells.
DISCUSSION
In this report, metabolic inhibitors were used to identify pathways involved in CD44DL. Two aspects of CD44DL were investigated: (1) the acquisition of triggering function by CD44 during 48 to 72 hours of activation of NK cells with IL-2; and (2) metabolic processes involved in the 4-hour effector phase of lysis. CD44DL was compared with ADCC and natural cytotoxicity to determine which inhibitors were specific for particular triggering pathways. There are two known mechanisms that can lead to significant levels of cell-mediated cytolysis in a 4-hour assay: granule exocytosis and Fas (CD95)-induced programmed cell death.42 CMA blocks acidification of cytolytic granules, resulting in the inactivation of perforin and the selective blockage of the granule exocytosis pathway.35 The observation that CMA totally blocks CD44DL, ADCC, and natural cytotoxicity of Fas-negative targets strongly suggests that all of these processes use the same perforin-based mechanism in the later stages of the lysis. Therefore, the inability of a drug to inhibit one type of lysis while blocking another suggests that it works at an early, receptor-specific phase in the lytic pathway.
Experiments in which actinomycin D was included during the activation of NK cells showed that de novo protein synthesis was an absolute requirement for the development of CD44DL. Before IL-2 treatment, the NK cells did not mediate CD44DL, but were cytolytically active, as shown by their ability to mediate ADCC. The presence of actinomycin D during NK cell activation had no effect on ADCC, indicating that maintenance of the cytolytic machinery did not require gene transcription. Therefore, it is likely that the acquisition of killing function by CD44 involved the IL-2–dependent expression of one or more proteins that coupled CD44 to the cytolytic machinery.
It is known that the CD44 molecule undergoes structural changes that can affect its function. Changes in CD44 isoform expression occur in T and B lymphocytes after antigen stimulation25 and are related to metastasis in tumor cells.23,24,43 However, in the current study, RT-PCR experiments failed to find any change in isoform after activation. Apparently, the standard isoform mediates CD44DL. Posttranslational modifications in the glycosylation and phosphorylation states of CD44 that effect ligand binding have also been observed.44-49 However, immunoprecipitates of CD44 from activated and resting NK cells failed to detect any gross differences in CD44 structure resulting from activation. Thus, if changes in CD44 structure are required for its gain in triggering function, such changes would have to result from relatively small posttranslational modifications.
Metabolic processes involved in the effector phase of lysis were examined by including inhibitors in the 4-hour assays. Staurosporine and herbimycin A blocked all types of lysis, indicating that serine-threonine and tyrosine kinases were required at late and/or early stages in the lytic pathways. Bonnema et al38 recently showed that wortmannin and GF109203X, specific inhibitors of PI 3-kinase and PKC, respectively, differentially inhibited ADCC and natural cytotoxicity in NK clones. Based on that study, we found that CD44DL and ADCC, but not natural cytotoxicity, were blocked by wortmannin and that CD44DL was more susceptible to inhibition than ADCC. The inability of wortmannin to inhibit natural cytotoxicity suggests that it did not inhibit at the degranulation phase of lysis, but rather blocked at early receptor-specific steps in CD44DL and ADCC. Thus, it is likely that PI 3-kinase lies somewhere between receptor signaling and the triggering of degranulation. Because the p85 subunit of PI 3-kinase contains two SH2 domains by which it binds tyrosine-phosphorylated proteins,50,51 it is likely that both CD44 and FcγRIII engagement result in the activation of one or more protein tyrosine kinases, consistent with herbimycin A blocking both types of lysis. FcγRIII (CD16) is known to activate p56lck and other protein tyrosine kinases,3,52 but a protein tyrosine kinase that is activated by CD44 has not yet been identified on NK cells. However, association of p56lck with CD44 on resting T cells has been reported.53
In our studies, GF109203X partially inhibited CD44DL, ADCC, and natural cytotoxicity, suggesting that PKC is involved in all three types of lysis. The activation of PKC per se has been shown to be sufficient to trigger a lytic response,38 and the inability of GF109203X to completely inhibit lysis in our studies may indicate that PKC participates in one of two or more parallel lytic pathways or simply that GF109203X is intrinsically unable to totally inhibit PKC. Our observation that GF109203X partially inhibited all three types of cytotoxicity differs from the results of Bonnema et al38 and may be related to the fact that their studies were performed using NK clones, whereas we used activated peripheral blood NK cells.
It has been reported that both CD44 and PI 3-kinase can associate with the cytoskeleton.27-29,40,41 Therefore, we tested the effect of cytochalasin B, an inhibitor of actin polymerization, on CD44DL. It has long been known that cytochalasin inhibits cell-mediated lysis,54 and at high doses it inhibited ADCC and CD44DL, suggesting that actin polymerization is involved in the degranulation phase of lysis. However, at low concentrations, at which it had no significant effect on conjugate formation, cytochalasin B markedly enhanced CD44DL but inhibited ADCC. This suggests that polymerized actin negatively affects the early stages of CD44DL, but not ADCC. If CD44 is anchored to the cytoskeleton in activated NK cells, then cytochalasin may enhance CD44DL by partially disrupting the cytoskeletal network, perhaps leading to an increase in the motility of CD44 on the cell surface. Cytochalasin also enhances LFA-1 and Mac-1 affinities for ICAM-1.55 An intriguing alternative then, is that β2 integrins are involved in CD44DL and that cytochalasin acts on these molecules rather than on CD44 directly.
The studies presented here provide an initial framework for understanding the early biochemical events in CD44DL. Effector-target conjugate formation leads to the cross-linking of CD44 molecules and perhaps other cosignaling receptors as well. This initiates a sequence of events whose final step is the release of perforin and granzymes into the effector:target interface. The early phase of CD44DL is modulated by the cytoskeleton and requires one or more proteins that are expressed when NK cells are activated with IL-2. The phosphorylation of phosphatidylinositol at the 3 position by PI 3-kinase is an obligatory step in CD44DL, and it is likely that at least one protein tyrosine kinase is used in the early phase of CD44DL to activate PI 3-kinase. How CD44, which is devoid of tyrosine in its cytoplasmic portion, interfaces with this pathway remains an important question for understanding CD44DL.
ACKNOWLEDGMENT
The authors thank Dr Miklos Moses (Metabolic Diseases Branch, NIDDK, Bethesda, MD) for aid with the Southern blots and Dr Charles Zacharchuk (Laboratory of Immune Cell Biology, NCI, Bethesda, MD) for thoughtful comments regarding the manuscript.
Address reprint requests to David M. Segal, PhD, Bldg 10, Room 4B17, NIH, Bethesda, MD 20892-1360.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal