Abstract
Many experimental and clinical protocols are being developed that involve ex vivo culture of human hematopoietic cells on stroma or in the presence of cytokines. However, the effect of these manipulations on primitive hematopoietic cells is not known. Our severe combined immune-deficient mouse (SCID)-repopulating cell (SRC) assay detects primitive human hematopoietic cells based on their ability to repopulate the bone marrow (BM) of immune-deficient non-obese diabetic/SCID (NOD/SCID) mice. We have examined here the maintenance of SRC, colony-forming cells (CFC), and long-term culture-initiating cells (LTC-IC) during coculture of adult human BM or umbilical cord blood (CB) cells with allogeneic human stroma. Transplantation of cultured cells in equivalent doses as fresh cells resulted in lower levels of human cell engraftment after 1 and 2 weeks of culture for BM and CB, respectively. Similar results were obtained using CD34+-enriched CB cells. By limiting dilution analysis, the frequency of SRC in BM declined sixfold after 1 week of culture. In contrast to the loss of SRC as measured by reduced repopulating capacity, the transplanted inocula of cultured cells frequently contained equal or higher numbers of CFC and LTC-IC compared with the inocula of fresh cells. The differential maintenance of CFC/LTC-IC and SRC suggests that SRC are biologically distinct from the majority of these in vitro progenitors. This report demonstrates the importance of the SRC assay in the development of ex vivo conditions that will allow maintenance of primitive human hematopoietic cells with repopulating capacity.
INCUBATION OF human bone marrow (BM) under appropriate conditions results in the establishment of a stromal layer that provides a microenvironment that supports primitive human hematopoietic cells. Until recently, the long-term BM culture (LTBMC) system has provided one of the only means to detect and investigate the proliferation and differentiation properties of primitive human hematopoietic cells. There has been much interest in the development of ex vivo culture conditions that permit the support or expansion of primitive cells for clinical applications. For example, because primitive normal and leukemic cells are differentially maintained on BM stroma, procedures have been developed to purge the BM of some chronic myeloid leukemia (CML) or acute myeloid leukemia (AML) patients before autologous transplantation.1-4 Moreover, retroviral infection during culture of human hematopoietic cells over an LTBMC stromal layer has been shown to increase the efficiency of gene transfer into primitive cells compared with simply using viral supernatants.5,6 This gene transfer method has been used clinically for the transduction of the human adenosine deaminase (ADA) gene into the BM cells of an ADA-deficient patient followed by transplantation of the infected cells.7 There has also been interest in expanding the number of primitive cells present in small samples such as umbilical cord blood (CB) collections before transplantation.8
In the mouse, quantitative studies suggest that the long-term repopulating ability (ie, stem cell function) of cultured hematopoietic cells decreases during incubation in primary LTBMC or after recharging the stroma cultures with BM cells.9,10 For human LTBMC, only the kinetics of primitive in vitro hematopoietic progenitors capable of initiating long-term cultures (long-term culture-initiating cells [LTC-IC]) have been studied. There appears to be a steady decline in the recovery of LTC-IC from the stromal layer over 5 weeks in culture.11 Although there is a close correlation in the mouse between LTC-IC and in vivo long-term repopulating cells,10 in humans the relationship between LTC-IC and pluripotent repopulating stem cells is not established. The standard 5-week LTC-IC assay only assesses the myeloid differentiation potential.
Recently, our laboratory has developed a repopulation assay for primitive human hematopoietic cells by transplantation into sublethally irradiated severe combined immune-deficient (SCID) or non-obese diabetic/SCID (NOD/SCID) mice.12-15 The transplanted human cells home to and engraft the murine BM, where they proliferate and differentiate producing large numbers of LTC-IC,16 colony-forming cells (CFC), immature CD34+Thy1+ and CD34+CD38− cells and mature myeloid, erythroid, and lymphoid cells.12-15 We have defined the engrafting human cell as an SCID-repopulating cell (SRC).14,17 Our studies strongly suggest that the SRC is a very primitive hematopoietic cell, although the exact place of SRC in the stem cell hierarchy and its relation to early in vitro hematopoietic progenitors such as LTC-IC is not fully understood. Gene-marking experiments have shown that, whereas both LTC-IC and CFC are efficiently transduced with retroviruses, these progenitors make only minor contributions to the engraftment of immune-deficient mice.15 By contrast, the SRC that were able to repopulate the mice were poorly infected with retrovirus vectors. Furthermore, cell purification experiments showed that SRC belong exclusively to the CD34+CD38− cell population.15,18 In contrast, whereas the LTC-IC are relatively enriched in the CD34+CD38− fraction, LTC-IC and large numbers of CFC are also present in the CD34+CD38+ fraction.19 20 Together, these results provide strong evidence that SRC possess extensive proliferative and differentiative capacity and are biologically distinct from most CFC and LTC-IC.
To investigate the ability of SRC to be supported in cultures with allogeneic BM stroma, we established conventional human LTBMC recharged with mononuclear BM, CB, and enriched CD34+ CB cells. The maintenance of SRC, LTC-IC, and CFC was investigated for each sample, before and after culturing on BM stroma for a period of 1 to 3 weeks.
MATERIALS AND METHODS
Mice.The NOD/LtSz-scid/scid (NOD/SCID)21 mice were maintained under sterile conditions at the Ontario Cancer Institute (Toronto, Ontario, Canada). All mice were housed in microisolator cages and provided with autoclaved food and water ad libitum.
Cells.CB samples were obtained from umbilical tissues according to approved procedures. Normal adult BM cells were obtained as leftover cells from allogeneic transplants according to procedures approved by the Human Experimentation Committee at the Princess Margaret Hospital (Toronto, Ontario, Canada). Low-density BM or CB cells were collected after separation on Ficoll-Hypaque (Pharmacia, Piscataway, NJ). Human CB cells were enriched for CD34+ cells by negative selection using a cocktail of lineage antibodies22 and the StemSep device according to the manufacturers' protocol (StemCell Technologies Inc, Vancouver, British Columbia, Canada).
BM stromal layers.BM stromal layers were established as previously described.23 Briefly, Ficoll-separated BM cells were suspended in myeloid long-term culture (LTC) medium (StemCell Technologies Inc) supplemented with 10−6 mol/L hydrocortisone sodium succinate (HSS; Sigma-Aldrich Canada, Ltd, Missisauga, Ontario, Canada). Cells were placed into 25-cm2 tissue culture flasks (Nunc; GIBCO BRL, Gaithersburg, MD) and incubated for 3 days at 37°C in a humidified atmosphere with 5% CO2 and afterwards at 33°C with weekly culture refeeding. Once confluent (3 to 6 weeks after initiation of the cultures), stromal layers were trypsinized and irradiated (1,500 cGy) using a 137Cs source. Stromal cells were subcultured in LTC medium in 25-cm2 flasks (1 to 1.7 × 106 cells/flask) or in 96-well tissue culture plates (Nunc) at a concentration of 2.5 to 3 × 104 cells/well. Subcultured stromal layers were used 2 to 6 days later for recharging with fresh hematopoietic cells.
Coculture of human CB or BM cells with stromal layers.Mononuclear CB or BM cells (1 × 106 cells/mL) or Lin−CD34+ CB (0.6 to 1.3 × 105 cells/mL) were placed above the irradiated allogeneic stromal layers in 25-cm2 flasks in LTC medium supplemented with 10−6 mol/L HSS. In two experiments, Ficoll-separated BM cells were placed in parallel into stroma-contained and empty culture flasks (no stroma [NS] LTBMC). The reconstituted flasks were maintained at 33°C in 5% CO2 , with weekly half-medium change. After 1, 2, or 3 weeks of incubation, nonadherent (NA) cells were removed, followed by trypsinization of adherent cell layers (ACL). To avoid the presence of allogeneic stromal cells in the resulting cell suspensions, a 30-minute adhesion procedure was performed at 37°C. Hematopoietic cells from this suspension were mixed with the NA cells. In some experiments, hematopoietic cells from ACLs were removed using cell-dissociation buffer (CDB; enzyme-free/EDTA-based buffer; GIBCO BRL), according to the manufacturer's instructions. Dissociated hematopoietic cells were mixed with NA cells, whereas stromal layers were treated additionally with two cycles of pipetting and stroma sedimentation. Supernatants of hematopoietic cells were removed and mixed with previously obtained fractions. Resulting suspensions were washed, counted, and processed for CFC and LTC-IC assays, flow cytometric analysis, and injection into NOD/SCID mice. Occasionally, the suspension fraction cells were also analyzed.
Human-specific hematopoietic progenitor cell assay.Fresh BM and CB cells, Lin−CD34+ CB cells, cell suspensions obtained from the long-term cocultures, and BM cells from the transplanted mice (see below) were assayed in culture conditions that are selective for the growth of human cells as previously described.12 Cell numbers plated from each cell population were as follows: human BM or CB cells, 2 × 104 cells/mL; Lin−CD34+ CB cells, 1 × 103 cells/mL; and BM cells from transplanted mice, 1 × 105/mL. After 10 to 12 days of incubation, the cultures were scored for the number of CFC. Duplicate or triplicate plates were established.
Limiting dilution analysis for quantitation of LTC-IC.This assay for fresh and cultured hematopoietic cells was performed as described,24 25 with some modifications. Irradiated human allogeneic stromal layers in 96-well plates were overlaid with cell suspensions in 150 μL of LTC medium with HSS at the following concentrations: 103, 2.5 × 103, 5 × 103, and 10 × 103 BM or CB cells/well. Lin−CD34+ CB cells were seeded at 100, 200, 500, and 1,000 cells/well. Plates were incubated at 37°C for 5 weeks with weekly culture refeeding. Fifteen replicate wells per dilution were established.
LTC-IC were quantified in two ways. The first method determined the percentage of wells containing cobblestone areas (CA), which were defined as regions of closely attached dark and bright cells beneath and above stromal layers, respectively. The second method determined the presence (and the content) of progenitors in each LTC well. The entire contents of each well were harvested (by trypsinization of ACLs) and plated into 24-well plates containing 0.5 mL/well of complete methylcellulose medium (see above) for 12 days. A positive well was defined as a well that contained at least one clonogenic progenitor. The absolute number of LTC-IC present in the cell populations was calculated using Poisson statistics (see below). The comparison of the LTC-IC frequency in 11 samples obtained by the two assays showed a good correlation: 92% of LTC-IC were estimated in the replating assay versus the direct CA-counting assay. The range of CFC production by individual LTC-IC was analyzed only in cultures containing less than 0.31 LTC-IC per well (as calculated by data from the entire experiment) to ensure that any positive well contained no more than 1 LTC-IC.26 The mean number of CFC per 1 LTC-IC was 6.0 (range, 1 to 40). No obvious differences were observed in the size of CFC produced by LTC-IC from fresh versus cultured hematopoietic cells.
Flow cytometry.Two-color flow cytometry was performed on a FACScan (Becton Dickinson, Mountain View, CA) as previously described.13 Briefly, Lin− CB cells were incubated on ice for 30 minutes in presence of saturating amounts of monoclonal anti-CD34–fluorescein isothiocyanate (FITC; HPCA-1; Becton Dickinson) and anti-CD38–phycoerythrin (PE; Leu-17; Becton Dickinson) antibodies. IgG1 isotype controls conjugated to FITC and PE were also included.
Transplantation of fresh and cultured BM and CB cells into immune-deficient mice.In accordance with our previously described transplantation protocols for adult BM12 or CB13 cells, 8- to 10-week-old NOD/SCID mice were sublethally irradiated with 400 cGy from a 137Cs source following by intravenous injection of fresh or cultured BM cells, CB cells, or Lin−CD34+ CB cells. Cytokines (granulocyte-macrophage colony-stimulating factor at 6 μg/mouse, interleukin-3 at 6 μg/mouse, and human stem cell factor at 10 μg/mouse; all from Amgen, Thousand Oaks, CA) were administered by intraperitoneal injection every other day. Mice were killed 4 to 5 weeks posttransplantation and BM cells were obtained from the pelvis, femurs, and tibiae. Suspensions of BM cells were processed for DNA analysis. To assure the multilineage character of human SRC engraftment into recipient BM, the presence of human macrophage (M), granulocyte (G), granulocyte-macrophage (GM), erythroid, and mixed hematopoietic colonies was estimated in each transplanted animal by means of methylcellulose assay (see above).
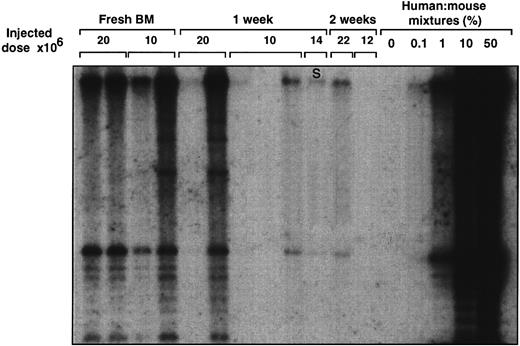
Transplantation of NOD/SCID mice with freshly isolated human BM or BM cultured on pre-established allogeneic stroma for 1 or 2 weeks. Mice were transplanted with the indicated number of cells. After 4 to 5 weeks, human cell engraftment of the murine BM was estimated by Southern blot. The extent of human cell engraftment was determined by comparing hybridization in the sample lines with the control human/mouse mixtures using densitometric methods. S, cells from suspension culture fraction only were injected.
Transplantation of NOD/SCID mice with freshly isolated human BM or BM cultured on pre-established allogeneic stroma for 1 or 2 weeks. Mice were transplanted with the indicated number of cells. After 4 to 5 weeks, human cell engraftment of the murine BM was estimated by Southern blot. The extent of human cell engraftment was determined by comparing hybridization in the sample lines with the control human/mouse mixtures using densitometric methods. S, cells from suspension culture fraction only were injected.
DNA extraction and analysis.DNA extracted from BM cells was digested with EcoRI and Southern blot analysis was performed using a human chromosome 17-specific α-satellite probe (p17H8) as described.12 To quantify the presence of human cells, the intensity of the characteristic 2.7-kb band in samples was compared with that of human/mouse control mixtures (0%, 0.1%, 1.0%, 10%, and 50% human DNA) using densitometric analyses and calculated by means of Imagequant software (Molecular Dynamics, Sunnyvale, CA).
Statistical analysis.Comparisons were made using standard statistical tests. Results are expressed as the mean ± standard error. For purposes of limiting dilutions assays (LTC-IC and SRC), Poisson statistics for the single-hit model were applied. The frequency of LTC-IC and SRC in cell suspensions was calculated using maximum likelihood estimator.27
RESULTS
Comparison of SRC, LTC-IC, and CFC maintenance during BM coculture with allogeneic stroma.To assess the ability of allogeneic human BM stroma to support the growth of early human hematopoietic progenitors including SRC, LTC-IC, and CFC, their content was estimated in each cell sample before and after 1 to 3 weeks of culturing on preformed irradiated stromal layers. The SRC assay involved intravenous injection of cells into sublethally irradiated NOD/SCID mice using standard conditions. Four to 5 weeks after transplantation, the murine BM was analyzed by Southern blot analysis to determine the level of human cell engraftment and by methylcellulose plating to assess the presence of multilineage human CFC. In the representative experiment shown, the BM of mice receiving a transplant of both 10 and 20 × 106 fresh BM cells contained human cells (Fig 1). However, mice receiving a transplant with 10 × 106 cells from the pooled suspension and adherent culture fractions or 14 × 106 cells from the suspension fraction after culture were engrafted to much lower levels (Fig 1). BM cells cultured on stroma for 2 weeks showed a further decline in their ability to engraft immune-deficient mice even after the injection of 22 × 106 cells.
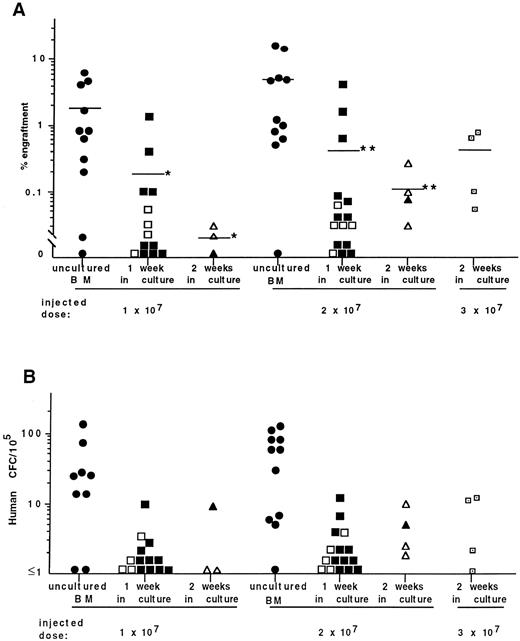
Human cell engraftment and CFC content of the BM of mice receiving transplants of cells from a total of six BM coculture experiments are summarized in Fig 2. In agreement with our previous observations,12,13,15 human CFC were found in the BM of engrafted mice showing the multilineage engraftment of NOD/SCID mice (Fig 2B). Overall, strong positive correlation (r = .6) exists between the engraftment level and the frequency of human CFC in the BM of mice undergoing transplantation. Although the level of engraftment in mice injected with 10 or 20 × 106 fresh BM cells/mouse was somewhat low, there was an association between the engraftment level and number of injected cells (Fig 2A). The mean percentage of engraftment in mice receiving transplants of 10 × 106 fresh BM cells was 1.8% ± 0.7% (n = 11), whereas those injected with 20 × 106 cells contained 4.9% ± 2% (n = 11) human cells. Cells cultured for 1 week on stroma and injected into mice at identical cell doses as fresh BM showed a significant decrease in their ability to engraft mice. The method of dissociating hematopoietic cells from the adherent cell layer did not influence the engraftment level. No significant differences were observed in experiments in which trypsin versus CDB was used to harvest hematopoietic cells from cultures. Overall, only 0.2% ± 0.1% (n = 13) of human cells were detected in mice receiving transplants of 10 × 106 cultured cells and 0.4% ± 0.3% in animals injected with 20 × 106 cells (n = 16). Neither CFC nor human cells were detected in the BM from 6 of 13 recipients receiving 10 × 106 cultured cells. In the group of mice injected with 20 × 106 of cultured cells, 5 of 16 animals were not engrafted. These limiting dilution data can be used to calculate the frequency of SRC using Poisson statistics. The frequency of SRC in the cultured cell population was 1 in 18.7 × 106 (χ2 = .37), representing an approximately sixfold reduction compared with fresh BM.28
Summary of DNA analysis (A) and frequency of human CFC (B) in the BM of NOD/SCID mice receiving transplants of freshly isolated BM cells and those isolated after 1 and 2 weeks of culture on stroma. Each symbol represents the level of engraftment (A) or CFC concentration (B) in a single mouse. Solid symbols represent mice receiving transplants of cultured cells treated with trypsin. Open symbols represent mice receiving transplants of cells treated with CDB. The horizontal lines indicate the mean level of engraftment. *Statistically significant differences (P < .05) in the mean levels of engraftment in mice injected with 10 × 106 cells cultured for 1 and 2 weeks compared with fresh BM cells. **Statistically significant differences in the mean levels of engraftment in mice injected with 20 × 106 cells after 1 and 2 weeks of coculture (P < .01 and P < .05, respectively) compared with 20 × 106 uncultured BM cells.
Summary of DNA analysis (A) and frequency of human CFC (B) in the BM of NOD/SCID mice receiving transplants of freshly isolated BM cells and those isolated after 1 and 2 weeks of culture on stroma. Each symbol represents the level of engraftment (A) or CFC concentration (B) in a single mouse. Solid symbols represent mice receiving transplants of cultured cells treated with trypsin. Open symbols represent mice receiving transplants of cells treated with CDB. The horizontal lines indicate the mean level of engraftment. *Statistically significant differences (P < .05) in the mean levels of engraftment in mice injected with 10 × 106 cells cultured for 1 and 2 weeks compared with fresh BM cells. **Statistically significant differences in the mean levels of engraftment in mice injected with 20 × 106 cells after 1 and 2 weeks of coculture (P < .01 and P < .05, respectively) compared with 20 × 106 uncultured BM cells.
The incubation of mononuclear BM cells in the absence of pre-established stromal layers (NS-LTBMC) also led to a decline in the repopulating ability of the cultured cells. Whereas injection of 20 × 106 fresh BM cells resulted in 2% to 5% human cell engraftment (n = 3), 1-week NS-LTBMC cells gave only 0% to 0.2% human cell engraftment (n = 3). Furthermore, transplantation of 12 × 106 cells after 3 weeks of culturing in NS-LTBMC led to the complete loss of engrafting ability, whereas an equivalent number of fresh BM cells resulted in 0.1% to 5% engraftment. Stromal support also did not provide long-term (3 weeks) maintenance of SRC: 3 mice injected with 10 to 13 × 106 cultured on stroma cells were not engrafted (data not shown).
For each of the experiments described above, total cell production, CFC, and LTC-IC content (estimated using limiting dilution assays) were assessed. These measurements provide assurance of the quality of the cultures and enable comparison with other reports in the literature. After 1 week of incubation of BM cells on pre-established stroma, cell production decreased to a level of 72% ± 11% of the input number of cells (data of 6 independent experiments). The content of CFC (6 BM samples) and LTC-IC (3 samples) had decreased 49% ± 7% and 49% ± 11% of the input numbers, respectively. Interestingly, the number of CFC injected from fresh or cultured individual BM samples did not correlate with the level of engraftment achieved in mice (Table 1). Thus, in experiments 1 and 2, the frequency of CFC in cell suspensions after 1 week of culturing increased compared with fresh BM (data not shown). However, despite the injection of increased numbers of CFC, the level of engraftment in mice receiving transplants of cultured cells decreased.
Determination of the Cell Number, CFC, LTC-IC, and the Ability to Engraft the BM of NOD/SCID Recipients by Human BM Cells Cultured on Allogeneic Stroma
| No. of Experiment . | Source of Cells . | In Culture . | % of Engraftment† . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| . | . | Cells ×106 . | CFC . | LTC-IC . | Injected Into Mice . | . | |||
| . | . | per Flask (%)* . | per Flask (%)* . | per 106 . | per Flask (%)* . | Cells ×106 per Mouse . | CFC per Mouse . | LTC-IC . | . |
| . | . | . | . | . | . | . | . | per Mouse . | . |
| 1 | Fresh BM | 10.0 | 44,600 | 83.9 | 839 | 20.0 | 89,200 | 1,678 | 1; 1 |
| 1 wk in culture | 3.7 (37%) | 25,245 (57%) | 87.5 | 327 (39%) | 20.0 | 135,000 | 1,750 | 0; 0.1 | |
| 2 | Fresh BM | 10.0 | 74,000 | 209.0 | 2,090 | 10.2 | 75,480 | 2,132 | 0; 1 |
| 1 wk in culture | 5.8 (58%) | 29,870 (40%) | 134.9 | 782 (37%) | 20.0 | 103,000 | 2,698 | 0; 0.03 | |
| 10.0 | 51,500 | 1,349 | 0; 0; 0.1 | ||||||
| 3 | Fresh BM | 10.0 | 48,000 | 118.1 | 1,181 | 19.5 | 93,600 | 2,303 | 17; 18 |
| 10.0 | 48,000 | 1,151 | 0; 5 | ||||||
| 1 wk in culture | 8.7 (87%) | 22,837 (48%) | 97.7 | 850 (72%) | 20.0 | 52,500 | 1,954 | 0; 0; 0.1; 2; 4 | |
| 10.0 | 26,250 | 977 | 0; 0.4; 2 | ||||||
| 3 wk in culture | 0.98 (9.8%) | 3,714 (8%) | 51.2 | 50 (5%) | 13.5 | 51,165 | 691 | 0 | |
| No. of Experiment . | Source of Cells . | In Culture . | % of Engraftment† . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| . | . | Cells ×106 . | CFC . | LTC-IC . | Injected Into Mice . | . | |||
| . | . | per Flask (%)* . | per Flask (%)* . | per 106 . | per Flask (%)* . | Cells ×106 per Mouse . | CFC per Mouse . | LTC-IC . | . |
| . | . | . | . | . | . | . | . | per Mouse . | . |
| 1 | Fresh BM | 10.0 | 44,600 | 83.9 | 839 | 20.0 | 89,200 | 1,678 | 1; 1 |
| 1 wk in culture | 3.7 (37%) | 25,245 (57%) | 87.5 | 327 (39%) | 20.0 | 135,000 | 1,750 | 0; 0.1 | |
| 2 | Fresh BM | 10.0 | 74,000 | 209.0 | 2,090 | 10.2 | 75,480 | 2,132 | 0; 1 |
| 1 wk in culture | 5.8 (58%) | 29,870 (40%) | 134.9 | 782 (37%) | 20.0 | 103,000 | 2,698 | 0; 0.03 | |
| 10.0 | 51,500 | 1,349 | 0; 0; 0.1 | ||||||
| 3 | Fresh BM | 10.0 | 48,000 | 118.1 | 1,181 | 19.5 | 93,600 | 2,303 | 17; 18 |
| 10.0 | 48,000 | 1,151 | 0; 5 | ||||||
| 1 wk in culture | 8.7 (87%) | 22,837 (48%) | 97.7 | 850 (72%) | 20.0 | 52,500 | 1,954 | 0; 0; 0.1; 2; 4 | |
| 10.0 | 26,250 | 977 | 0; 0.4; 2 | ||||||
| 3 wk in culture | 0.98 (9.8%) | 3,714 (8%) | 51.2 | 50 (5%) | 13.5 | 51,165 | 691 | 0 | |
The value in parentheses represents the percentage of input value in the fresh BM sample.
Each value represents the human cell engraftment in the BM of each individual mouse.
LTC-IC frequency after 1 week of coculture was similar to that in fresh BM, being 84% ± 11% of input level (Table 1). Consequently, the number of injected LTC-IC in inocula of fresh and cultured BM samples was similar. However, the quantity of injected LTC-IC from fresh and cultured BM did not correlate with the ability of these two cell populations to repopulate BM of recipient mice. Thus, injection of mice with 1,678 LTC-IC derived from fresh BM (experiment 1) resulted in 1% engraftment, whereas the BM of mice injected with 1,750 LTC-IC derived from 1-week cocultures contained only 0% and 0.1% human cells. Experiments 2 and 3 showed the same tendency; there was poor correlation between the quantity of LTC-IC in freshly isolated and cultured BM cells and the ability of these samples to engraft murine BM. Overall, there was moderate positive correlation between the level of engraftment and the number of LTC-IC injected from fresh BM cells (r = .54), whereas no correlation was observed for cultured cells (r = −.03).
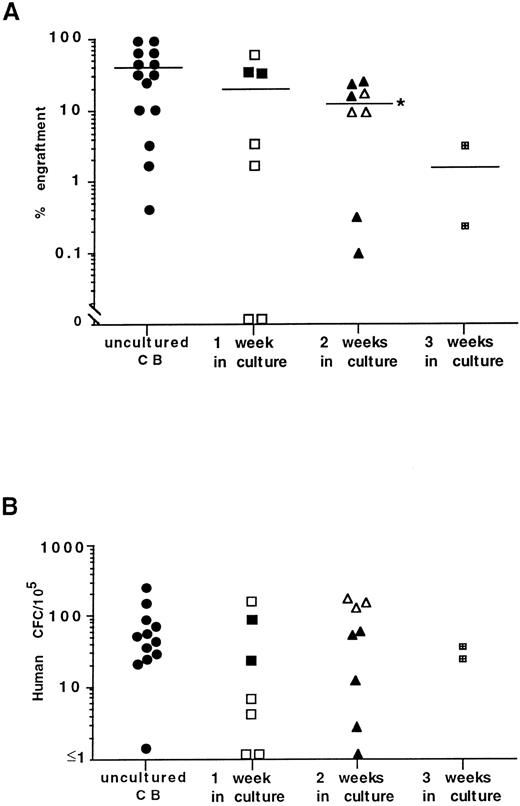
Culture of human CB with allogeneic stroma.Mononuclear CB cells, freshly isolated or cultured for 1 to 3 weeks on pre-established allogeneic BM stroma, were transplanted into NOD/SCID mice at various cell concentrations to assess the SRC activity of these samples. A summary from five independent experiments is shown in Fig 3. Significant numbers of CFC were detected in the BM of engrafted mice (Fig 3B). The mean level of human cell engraftment in mice receiving transplants of 8 to 16 × 106 fresh CB cells was 40% ± 10% and did not differ significantly from the engraftment level in mice injected with cells cultured for 1 week (20% ± 10%; Fig 3A). However, the ability of CB cells cultured for 2 weeks to engraft NOD/SCID mice was significantly reduced (12% ± 3%) when compared with fresh CB cells. After 3 weeks of incubation, a further decrease in engrafting capacity of cultured CB cells was observed. Similar to BM cocultures, no significant differences were detected comparing trypsin versus CDB treatment for harvesting hematopoietic cells from adherent cell layers.
Summary of DNA analysis (A) and frequency of human CFC (B) in the BM of NOD/SCID mice receiving transplants of freshly isolated CB cells and those isolated after 1 to 2 weeks of culture on stroma. Each symbol represents the level of engraftment (A) or CFC concentration (B) in a single mouse. Solid symbols represent mice receiving transplants of cultured cells treated with trypsin. Open symbols represent mice receiving transplants of cultured cells treated with CDB. The horizontal lines indicate the mean level of engraftment. *Statistically significant differences in the mean level of engraftment in mice receiving transplants of CB cells cultured for 2 weeks (P < .05) compared with uncultured CB cells.
Summary of DNA analysis (A) and frequency of human CFC (B) in the BM of NOD/SCID mice receiving transplants of freshly isolated CB cells and those isolated after 1 to 2 weeks of culture on stroma. Each symbol represents the level of engraftment (A) or CFC concentration (B) in a single mouse. Solid symbols represent mice receiving transplants of cultured cells treated with trypsin. Open symbols represent mice receiving transplants of cultured cells treated with CDB. The horizontal lines indicate the mean level of engraftment. *Statistically significant differences in the mean level of engraftment in mice receiving transplants of CB cells cultured for 2 weeks (P < .05) compared with uncultured CB cells.
The data on CFC and LTC-IC production in CB cultures and the ability of cultured cells to engraft mice are presented in Table 2. Although there was a significant decline in cellularity in 1-week cultures, the average production of CFC was increased to 137% compared with input level. When compared with BM cocultures, the support of CFC from CB was significantly higher (P = .015). The maintenance of LTC-IC in CB cultures was also higher than that in BM cultures (83% and 49%, respectively). Interestingly, in two of three experiments, the frequency of LTC-IC dramatically increased during the first week of culturing and, consequently, the number of LTC-IC was considerably higher in the transplanted inocula from cultured cells than from fresh CB. Similar to BM cultures, the increased number of CFC and LTC-IC in the inoculum of 1-week cultured cells did not necessarily result in the increased human cell engraftment in recipient mice (experiments 1 and 2). In another experiment (no. 3), a significant decrease in the frequency and content of LTC-IC was observed after 2 weeks in coculture, but this was not accompanied by a decrease in the percentage of human cell engraftment in mice injected with a high (16 × 106) cell dose. Although there was a high positive correlation between the engraftment level and the number of LTC-IC in the inoculum of fresh CB cells (r = .88), only low correlation was observed for cells derived from 1-week cultures (r = .17).
Determination of the Cell Number, CFC, LTC-IC, and the Ability to Engraft the BM of NOD/SCID Recipients by Human CB Cells Cultured on Allogeneic Stroma
| No. of Experiment . | Source of Cells . | In Culture . | % of Engraftment† . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| . | . | Cells ×106 per Flask (%)* . | CFC per Flask (%)* . | LTC-IC . | Injected Into Mice . | . | |||
| . | . | . | . | per 106 . | per Flask (%)* . | Cells ×106 per Mouse . | CFC per Mouse . | LTC-IC per Mouse . | . |
| 1 | Fresh CB | 5.9 | 19,470 | 237.6 | 1,402 | 16.0 | 52,800 | 3,802 | 36 |
| 1 wk in culture | 1.83 (31%) | 34,404 (176%) | 883.7 | 1,617 (115%) | 16.0 | 300,800 | 14,139 | 62 | |
| 8.0 | 150,400 | 7,070 | 4 | ||||||
| 2 | Fresh CB | 8.0 | 15,200 | 135.7 | 1,086 | 16.8 | 31,920 | 2,280 | 3; 11 |
| 8.4 | 15,960 | 1,140 | 2; 10 | ||||||
| 1 wk in culture | 2.15 (27%) | 26,768 (176%) | 598.9 | 1,288 (119%) | 16.8 | 208,538 | 10,062 | 0; 2 | |
| 8.4 | 104,269 | 5,031 | 0 | ||||||
| 3 | Fresh CB | 10.0 | 53,500 | 313.8 | 3,138 | 16.8 | 89,880 | 5,272 | 41; 46 |
| 8.4 | 44,940 | 2,636 | 24; 34 | ||||||
| 1 wk in culture | 2.4 (24%) | 31,529 (59%) | 206.6 | 496 (16%) | 14.6 | 189,435 | 3,016 | 33; 37 | |
| 2 wk in culture | 4.4 (44%) | 16,133 (30%) | 10.7 | 47 (7%) | 16.0 | 58,400 | 170 | 13; 20; 25 | |
| 7.7 | 28,105 | 82 | 0.1 | ||||||
| 3.7 | 13,505 | 39 | 0.3 | ||||||
| No. of Experiment . | Source of Cells . | In Culture . | % of Engraftment† . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| . | . | Cells ×106 per Flask (%)* . | CFC per Flask (%)* . | LTC-IC . | Injected Into Mice . | . | |||
| . | . | . | . | per 106 . | per Flask (%)* . | Cells ×106 per Mouse . | CFC per Mouse . | LTC-IC per Mouse . | . |
| 1 | Fresh CB | 5.9 | 19,470 | 237.6 | 1,402 | 16.0 | 52,800 | 3,802 | 36 |
| 1 wk in culture | 1.83 (31%) | 34,404 (176%) | 883.7 | 1,617 (115%) | 16.0 | 300,800 | 14,139 | 62 | |
| 8.0 | 150,400 | 7,070 | 4 | ||||||
| 2 | Fresh CB | 8.0 | 15,200 | 135.7 | 1,086 | 16.8 | 31,920 | 2,280 | 3; 11 |
| 8.4 | 15,960 | 1,140 | 2; 10 | ||||||
| 1 wk in culture | 2.15 (27%) | 26,768 (176%) | 598.9 | 1,288 (119%) | 16.8 | 208,538 | 10,062 | 0; 2 | |
| 8.4 | 104,269 | 5,031 | 0 | ||||||
| 3 | Fresh CB | 10.0 | 53,500 | 313.8 | 3,138 | 16.8 | 89,880 | 5,272 | 41; 46 |
| 8.4 | 44,940 | 2,636 | 24; 34 | ||||||
| 1 wk in culture | 2.4 (24%) | 31,529 (59%) | 206.6 | 496 (16%) | 14.6 | 189,435 | 3,016 | 33; 37 | |
| 2 wk in culture | 4.4 (44%) | 16,133 (30%) | 10.7 | 47 (7%) | 16.0 | 58,400 | 170 | 13; 20; 25 | |
| 7.7 | 28,105 | 82 | 0.1 | ||||||
| 3.7 | 13,505 | 39 | 0.3 | ||||||
The value in parentheses represents the percentage of input value in the fresh BM sample.
Each value represents the human cell engraftment in the BM of each individual mouse.
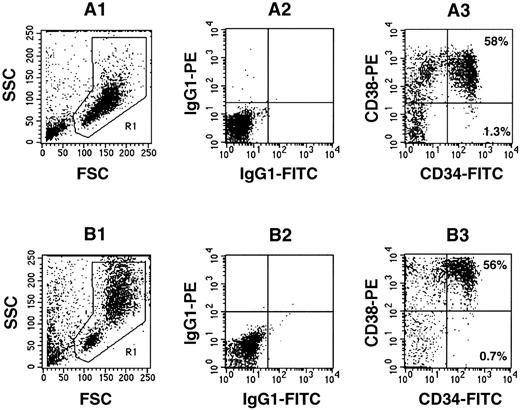
Coculture of Lin−CD34+ CB cells with allogeneic BM stroma.Enrichment for CD34+ cells is increasingly being used in many experimental and clinical procedures, including gene therapy.6,15 29 The absence of accessory cells in purified CD34+ cells could impact on the support of early hematopoietic progenitors. In the present study, human CB cells were enriched for CD34+ cells to approximately 60% purity. The phenotype of purified cells was assayed by flow cytometry; a representative sample is shown in Fig 4A. Injection of 3.5 × 105 freshly isolated Lin−CD34+ cells led to high levels of human cell engraftment. As few as 5 × 104 CD34+-enriched cells resulted in highly engrafted mice (Table 3).
Flow cytometric analysis of surface marker expression on Lin−CD34+-enriched CB cells before (A) and after (B) 1 week of incubation on pre-established allogeneic stroma. (A1 through A3) Freshly isolated Lin−CD34+-enriched CB cells. (A1) Forward and side scatter plot showing the gate (R1) used for the analysis. (A2) Isotype control for nonspecific IgG1 staining. (A3) CD34 and CD38 expression on the cells gated in R1. (B1 through B3) Lin−CD34+-enriched CB cells were cultured for 1 week. (B1) Forward and side scatter plot showing the gate (R1) used for the analysis. (B2) Isotype control for nonspecific IgG1 staining. (B3) CD34 and CD38 expression on the cells gated in R1.
Flow cytometric analysis of surface marker expression on Lin−CD34+-enriched CB cells before (A) and after (B) 1 week of incubation on pre-established allogeneic stroma. (A1 through A3) Freshly isolated Lin−CD34+-enriched CB cells. (A1) Forward and side scatter plot showing the gate (R1) used for the analysis. (A2) Isotype control for nonspecific IgG1 staining. (A3) CD34 and CD38 expression on the cells gated in R1. (B1 through B3) Lin−CD34+-enriched CB cells were cultured for 1 week. (B1) Forward and side scatter plot showing the gate (R1) used for the analysis. (B2) Isotype control for nonspecific IgG1 staining. (B3) CD34 and CD38 expression on the cells gated in R1.
Determination of the Cell Number, CFC, LTC-IC, and the Ability to Engraft the BM of NOD/SCID Recipients by Human Lin−CD34+ CB Cells Cultured on Allogeneic BM Stroma
| No. of Experiment . | Source of Cells . | In Culture . | % of Engraftment3-151 . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| . | . | Cells ×105 . | CFC . | LTC-IC . | Injected Into Mice . | . | |||
| . | . | per Flask (%)3-150 . | per Flask (%)3-150 . | per 105 . | per Flask (%)3-150 . | Cells ×105 per Mouse . | CFC . | LTC-IC . | . |
| . | . | . | . | . | . | . | per Mouse . | per Mouse . | . |
| 1 | Lin−CD34+ CB | 6.3 | 149,310 | 231.9 | 1,461 | 0.5 | 11,850 | 116 | 3; 26 |
| 1 wk in culture | 7.4 (117%) | 95,090 (64%) | 128.1 | 948 (65%) | 2.0 | 25,700 | 256 | 1 | |
| 1.0 | 12,850 | 128 | 0; 0 | ||||||
| 0.5 | 6,425 | 64 | 0; 0; 0 | ||||||
| 2 | Lin−CD34+ CB | 13.0 | 327,600 | 1,900.1 | 24,701 | 3.5 | 88,200 | 6,650 | 29; 71 |
| 1 wk in culture | 15.6 (120%) | 113,880 (35%) | 1,367.6 | 21,344 (86%) | 7.8 | 56,940 | 10,667 | 30; 47 | |
| 3.9 | 28,470 | 5,334 | 11; 23 | ||||||
| No. of Experiment . | Source of Cells . | In Culture . | % of Engraftment3-151 . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| . | . | Cells ×105 . | CFC . | LTC-IC . | Injected Into Mice . | . | |||
| . | . | per Flask (%)3-150 . | per Flask (%)3-150 . | per 105 . | per Flask (%)3-150 . | Cells ×105 per Mouse . | CFC . | LTC-IC . | . |
| . | . | . | . | . | . | . | per Mouse . | per Mouse . | . |
| 1 | Lin−CD34+ CB | 6.3 | 149,310 | 231.9 | 1,461 | 0.5 | 11,850 | 116 | 3; 26 |
| 1 wk in culture | 7.4 (117%) | 95,090 (64%) | 128.1 | 948 (65%) | 2.0 | 25,700 | 256 | 1 | |
| 1.0 | 12,850 | 128 | 0; 0 | ||||||
| 0.5 | 6,425 | 64 | 0; 0; 0 | ||||||
| 2 | Lin−CD34+ CB | 13.0 | 327,600 | 1,900.1 | 24,701 | 3.5 | 88,200 | 6,650 | 29; 71 |
| 1 wk in culture | 15.6 (120%) | 113,880 (35%) | 1,367.6 | 21,344 (86%) | 7.8 | 56,940 | 10,667 | 30; 47 | |
| 3.9 | 28,470 | 5,334 | 11; 23 | ||||||
The value in parentheses represents the percentage of input value in the fresh BM sample.
Each value represents the human cell engraftment in the BM of each individual mouse.
Incubation of Lin−CD34+ cells for 1 week on preformed stroma did not drastically alter their cell surface phenotype, although there was an approximately twofold decrease in the percentage of CD34+CD38− cells (Fig 4B). After 1 week of coculture, increased production of mature cells and decrease in the number of CFC was observed (Table 3). The absolute number and frequency of LTC-IC slightly decreased during this period. Interestingly, although a small number of freshly isolated Lin−CD34+ cells, equivalent to 116 LTC-IC, were able to generate high levels of human cell engraftment (Table 3, experiment 1), equal numbers of 1-week cultured cells did not engraft mice. The injection of fourfold more cultured cells, corresponding to a doubling in the number of LTC-IC injected per mouse, resulted in lower levels of engraftment when compared with fresh Lin−CD34+ cells. The transplantation of comparable numbers of LTC-IC per inoculum of fresh and cultured Lin−CD34+ cells (Table 3, experiment 2) resulted in decreased levels of human cell engraftment in mice transplanted with cultured cells.
DISCUSSION
The development of the SRC assay for primitive human hematopoietic cells capable of repopulating the BM of NOD/SCID immune-deficient mice with myeloid and lymphoid lineages provides a new approach to investigate the organization of the human hematopoietic system and to characterize primitive cells. We report here that there was a differential maintenance of SRC compared with CFC and LTC-IC after 1 to 3 weeks of culture of human BM or CB on stromal layers. This result supports our earlier conclusions using gene marking and cell purification that SRC represents a different population of hematopoietic cells compared with the clonogenic progenitors and most 5-week LTC-IC.
One to 3 weeks of culturing of mononuclear BM cells on allogeneic BM stroma led to a decrease in the repopulation capacity of the cultured hematopoietic cells. Our culture conditions were similar to those previously reported and yielded similar numbers of CFC and LTC-IC, attesting to the quality of our cultures.30,31 Nevertheless, the injection of cultured BM cells at the same doses (10 to 20 × 106 cells/mouse) as freshly isolated cells resulted in a significant decrease in the level of human cell engraftment. Moreover, in contrast with the mice receiving transplants of fresh BM cells, a proportion of the mice injected with cultured cells were not engrafted. This observation enabled application of Poisson statistics to estimate that there was an approximately sixfold reduction in the frequency of SRC in 1-week cultured BM cell suspensions (1 SRC in 18.7 × 106 cells) compared with freshly isolated mononuclear BM cells (1 SRC in 3 × 106).28 Our data on the decline of repopulating ability of human cultured BM cells correspond to the quantitative studies of mouse long-term BM cultures.9,10,32,33 The murine studies have shown recovery of only 5% to 15% long-term repopulating cells (or competitive repopulating units) after 3 or 4 weeks in culture.10,32,33 Our data suggest approximately 15% recovery of human BM-derived SRC after incubation on human stroma after only 1 week. Cells cultured from selected Ph+ CML patients for 10 days on stroma resulted in successful transplantation of normal hematopoietic cells, although there was obviously no data on the exact number of repopulating cells present in this culture system.4 Our data suggest that the usage of protocols involving stroma-dependent long-term human cultures must be applied cautiously. At best, a significant excess of cells should be used to take into account the loss of repopulating cells during the culture.
The mechanism that underlies the loss of SRC during culture on stroma is not known. It could be due to differentiation of the primitive cells during culture or to changes in the expression and/or function of adhesion receptors that could affect the engraftment potential of the primitive cells. Interestingly, it has been shown that engineering stromal cells to express cytokines34 or the use of completely stromal-free cultures35 can improve the maintenance of LTC-IC. The SRC assay will be useful in assessing these conditions.
Comparative analysis of freshly isolated BM and CB cells showed that the latter possesses significantly higher repopulating ability. This finding is in agreement with quantitative data using limiting dilution analysis showing that the frequency of SRC is about threefold higher in CB compared with BM.28 The maintenance of SRC in cultures with human mononuclear CB cells was superior to that with BM. There was no significant decrease in the mean level of human cell engraftment after transplantation of 1-week cultured CB cells compared with freshly isolated samples, although a decline was noticeable after 2 weeks of incubation. Similar doses of cultured CB cells transplanted into NOD/SCID mice provided much higher engraftment compared with BM cells, suggesting that CB may be a better source of stem cells for ex vivo manipulations. This could be due to the maintenance of higher numbers of SRC or to the fact that CB cells seem to have an intrinsically higher proliferative capacity.20 36
CB cells purified on the basis of CD34 antigen expression are used extensively in gene therapy studies.15,29 The injection of as few as 5 × 104 of freshly isolated Lin−CD34+ CB cells into NOD/SCID mice resulted in 3% to 26% human cell engraftment indicating purification of SRC in this fraction. One week of culture of Lin−CD34+ CB cells led to a decreased engraftment ability of cultured cells that was especially noticeable when limited numbers of cells (0.5 to 2 × 105 cells/mouse) were injected. Most of the cells remained CD34+ during culture, although there was a decline in the CD34+CD38− population. The CD34+CD38− phenotype defines a rare quiescent subset of CB cells20 and SRC belongs exclusively to this cell population.15 18 Therefore, the decrease of CD34+CD38− population in stroma-dependent cultures likely reflects the reduction we observed in the repopulating ability of the cultured cells.
To assess the relationship between CFC, LTC-IC, and SRC, the behavior of these subsets of hematopoietic cells was investigated in stromal-dependent cultures of BM, CB, and Lin−CD34+CB cells. There was no direct correlation between the number of CFU-C present in the inoculum of cultured cells and repopulation of NOD/SCID recipients. CFC define a relatively mature population of committed hematopoietic progenitors, nonoverlapping with LTC-IC.35 The LTC-IC have been generally considered to be the most primitive population detected by in vitro assays and representative of the stem cell subset.24,34,37 The frequency of LTC-IC in BM and CB samples estimated in the present study was similar to published reports,24,26,37 with slightly increased numbers of LTC-IC in CB compared with BM (230 ± 51 per 106 cells [n = 3] and 150 ± 29 per 106 cells [n = 4], respectively). The maintenance of LTC-IC was approximately twofold higher in 1-week CB cocultures compared with BM cocultures (83% and 49%, respectively). The average support of LTC-IC in Lin−CD34+ cultures (76%) was consistent with previous findings.11 However, for all sources of hematopoietic cells, there was a differential maintenance of LTC-IC and SRC. The transplantation of similar or even increased numbers of LTC-IC in the inoculum of cultured cells (compared with freshly isolated cells) frequently resulted in a decreased level of human cell engraftment compared with mice receiving transplants of fresh cells. Moderate or high positive correlation between the numbers of LTC-IC in inocula of fresh BM or CB cells and their ability to engraft immune-deficient mice was observed; however, no correlation was observed with cells cultured for 1 week.
Examination of the behavior of different subsets of primitive murine hematopoietic cells in LTBMC has resulted in contradictory observations on the similar10 or differential33 maintenance of LTC-IC and repopulating cells. Our data correspond to both studies in view of the gradual loss of cells with repopulating potential during culture and provide evidence for the differential maintenance of primitive human repopulating cells and LTC-IC. Interestingly, no correlation was observed between the speed of hematopoietic recovery after transplantation of cells cultured for 10 days from CML patients and the number of infused nucleated cells, clonogenic progenitors, or LTC-IC.4 These data, together with experiments showing that gene-marked LTC-IC do not contribute to the human graft in NOD/SCID mice,15 provide strong evidence that LTC-IC and SRC represent distinct cell populations. Recent studies have suggested that the LTC-IC assay detects a heterogeneous population of early cells and that those that are able to sustain hematopoiesis for up to 100 days represent a more primitive subset.20 Interestingly, these extended LTC-IC (ELTC-IC) are found in the CD34+CD38− fraction and are poorly infected with retrovirus vectors.20 38 Thus, there is likely to be overlap between the cells detected in the SRC and ELTC-IC assays.
The explosion of interest in manipulating human stem cells before autologous transplantation highlights the need for an assay that conclusively detects cells with repopulating potential. The data we report here indicate that the SRC may provide a more reliable assay for such a primitive cell than the standard 5-week LTC-IC. The rapid decline of SRC during the culture of hematopoietic cells on stroma indicates that some caution must be applied to the clinical application of ex vivo expansion protocols.
ACKNOWLEDGMENT
The authors thank I. McNiece (Amgen) for cytokines, L. McWhirter and M. Oskamp for providing CB specimens, N. Jamal and H. Messner for BM samples, J.C.Y. Wang for assistance with statistical analysis, and members of the laboratory for critically reviewing the manuscript.
Supported by grants from the Medical Research Council of Canada (MRC), the National Cancer Institute of Canada (NCIC) with funds from the Canadian Cancer Society, the Canadian Genetic Diseases Network of the National Centers of Excellence, the Blood Gene Therapy Program of the Hospital for Sick Children, a Research Scientist award from the NCIC (to J.E.D.), an MRC Scientist award (to J.E.D.), and a studentship from the Fonds pour la Formation des Chercheurs et l'Aide à la Recherche (to A.L.).
Address reprint requests to John E. Dick, PhD, Department of Genetics, Research Institute, Hospital for Sick Children, 555 University Ave, Toronto, Ontario, Canada M5G 1X8.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal