Abstract
Polycythemia vera (PV) is a clonal hematologic disease characterized by hyperplasia of the three major bone marrow lineages. PV erythroid progenitor cells display hypersensitivity to several growth factors, which might be caused by an abnormality of tyrosine phosphorylation. In the present study, we have investigated protein tyrosine phosphatase (PTP) activity in highly purified erythroid progenitor cells and found that the total PTP activity in the PV cells was twofold to threefold higher than that in normal cells. Protein separation on anion-exchange and gel-filtration columns showed that the increased activity was due to a major PTP eluted at approximately 170 kD. This enzyme was sensitive to PTP inhibitors and it did not cross-react with antibodies to SHP-1, SHP-2, or CD45. Subcellular fractionation showed that the PTP localized with the membrane fraction, where its activity was increased by threefold in PV erythroid progenitors when compared with normal cells. As the erythroid progenitors progressively matured, activity of the PTP declined rapidly in the normal cells but at a much slower rate in the PV cells. These studies suggest that a potentially novel membrane or membrane-associated PTP, representing a major PTP activity, may have an important role in proliferation and/or survival of human erythroid progenitors and that its hyperactivation in PV erythroid progenitors might be responsible for the increased erythropoiesis in PV patients.
POLYCYTHEMIA VERA (PV) is a clonal myeloproliferative disorder that leads to trilineage (erythroid, myeloid, and megakaryocytic) hyperplasia in the bone marrow with a principal clinical manifestation of erythrocytosis and plethora. Although some new insights into the pathogenesis of the disease have been achieved over recent years, little is known about the mechanism by which increased production of peripheral blood cells develops in PV.1,2 Previous studies by us and others have shown that PV erythroid progenitor cells are hypersensitive to a number of growth factors, including interleukin-3 (IL-3), erythropoietin (EPO), stem cell factor (SCF ), granulocyte-macrophage colony-stimulating factor (GM-CSF ), and insulin-like growth factor I (IGF-I).3-7 However, binding experiments and autoradiographic analyses showed that the binding affinities of EPO and SCF, and the numbers of these receptors in PV erythroid progenitor cells were not any greater than those in normal erythroid progenitors, suggesting that the hypersensitivity of PV erythroid progenitor cells to this wide variety of growth factors may reside in an as yet unknown intracellular molecular abnormality within a shared signaling pathway.
In the human hematopoietic system, the extent of the proliferation and differentiation of hematopoietic stem cells and progenitor cells, as well as the production of mature blood cells, are believed to be regulated by various hematopoietic growth factors signaling through their receptors.8,9 Signals of SCF, EPO, IL-3, IGF-I, and IL-6/soluble IL-6 receptor (via gp130) have been shown to play an important role in proliferation and terminal maturation of erythroid progenitor cells.10-12 A common feature of these hematopoietic growth factors is that, upon binding to their receptors, they initiate tyrosine phosphorylation of their receptors and transduction of their signals occurs through shared downstream pathways such as RAS/MAPK and JAK/STAT to targets in the nucleus. The regulation of tyrosine phosphorylation in the signal pathways is accomplished by a delicate interplay between protein tyrosine kinases (PTKs) and protein tyrosine phosphatases (PTPs).13-15 Impaired balance due to abnormalities of either PTKs or PTPs may lead to disorders of cell growth. Although the knowledge about PTPs is still incomplete, recent data indicate that PTPs play a crucial role in hematopoietic growth factor signaling. For instance, SHP-1 has been shown to negatively regulate signaling from a number of receptors, including EPO, IL-3, SCF, and interferon receptors, whereas SHP-2, by binding to the EPO receptor and c-Kit, presumably plays a positive role in their signaling.16-24 However, little is known about the role of PTPs in the proliferation and differentiation of primary human hematopoietic stem cells and progenitor cells. Our recent observation that orthovanadate, a PTP inhibitor, has a diminished effect on PV erythroid progenitors, compared with normal progenitors, directs our attention to this area even more cogently.25 In the present study, we analyzed the PTP activity in highly purified human erythroid colony-forming cells (ECFC)11 and found that total PTP activity in the PV samples was significantly higher than that in normal cells. Further studies showed that the increased activity was due to a major, and potentially novel, membrane or membrane-associated PTP that may have an important role in the proliferation and/or survival of human erythroid cells.
MATERIALS AND METHODS
Blood samples and reagents.Peripheral blood was obtained from healthy volunteers and PV patients after informed consent was obtained, which was approved by the Vanderbilt Committee for the Protection of Human Subjects and the Department of Veteran Affairs Research and Development Committee. Patients met the established criteria for PV26 and had no other disease histories. Three patients were treated with phlebotomy only; one (67 years old) received hydroxyurea at 500 mg every other day in addition to phlebotomy; one (70 years old) had received 32P 5 years earlier, but since then was treated with phlebotomy only. The blood samples (400 mL) were collected in sodium heparin at a final concentration of 20 U/mL and were used within 24 hours. Recombinant human (rh) SCF and rhEPO were kindly provided by Amgen, Inc (Thousand Oaks, CA) and Ortho Biotech, Inc (Raritan, NJ), respectively. Rabbit anti–SHP-1 and anti–SHP-2 polyclonal antibodies (2303 and 1263) were prepared as previously reported.27 28 Monoclonal anti-CD45 was a gift from Dr C.-L. Law (University of Washington, Seattle, WA). Monoclonal antiphosphotyrosine (PY) was purchased from Upstate Biotechnology Inc (Lake Placid, NY). [γ-32P]-ATP and horseradish peroxidase-conjugated secondary antibodies were from Amersham Corp (Arlington Heights, IL).
Preparation of ECFC.Purification of normal and PV ECFC was performed as previously reported.11,29 Briefly, the heparinized blood was layered onto Ficoll-Hypaque (1.077 g/mL), and light-density mononuclear cells were separated by centrifugation. The collected mononuclear cells were then centrifuged through 10% bovine serum albumin to deplete platelets. Depletion of lymphocytes and adherent cells was performed by sheep erythrocyte rosetting with overnight incubation in polystyrene tissue culture flasks at 37°C. Further enrichment of the ECFC was achieved by negative panning with monoclonal antibodies CD11b, CD2, CD45R, and CD16. The purified cells were cultured in a suspension mixture containing 30% fetal calf serum, 1% deionized bovine serum albumin, 2 U/mL of rhEPO, 10 ng/mL of rhSCF, 50 U/mL of rhIL-3, 10 μg/mL of insulin, 10−4 mol/L of 2-mercaptoethanol, 500 U/mL of penicillin, and 40 μg/mL of streptomycin in Iscove's modified Dubecco's medium at 37°C in a high humidity 5% CO2 , 95% air incubator. The cultured cells were usually collected after 6 days of suspension culture, and these cells are referred to as day-6 ECFC. For some experiments, the cultures were maintained up to 3 weeks to allow further differentiation and maturation of the erythroid cells. In such cases, the cultures were depopulated every other day from day 6 by removing half of the cell suspension and replacing this with newly prepared medium containing the same combination of growth factors described above. A small portion of the collected cells was analyzed for colony formation in plasma clot cultures supplemented with 2 U/mL of EPO.29 The remaining cells were washed with phosphate-buffered saline and were then either used immediately or preserved at −70°C for PTP analysis. The plasma clot cultures were incubated at 37°C in a high-humidity, 5% CO2 , 95% air incubator for 7 days and were then fixed and stained with 3,3′ dimethoxybenzidine and hematoxylin for enumeration of erythroid colonies. The purity of the ECFC was determined by erythroid colony-forming efficiency in the plasma clot assay.
Fractionation of cell extracts.The collected ECFC were suspended in a buffer containing 25 mmol/L Tris-HCl, 5 mmol/L EDTA, 2 mmol/L EGTA, 1.5 mmol/L MgCl2 , 10 mmol/L 2-mercaptoethanol, 1.0 mmol/L benzamidine, 0.1 mmol/L phenylmethylsulfonyl fluoride, 20 μg/mL leupeptin, 1 mmol/L pepstatin A, and 1 μg/mL aprotinin (buffer A) with or without 1% Triton X-100. The extracts made in buffer A with 1% Triton X-100 were cleared by centrifugation at 10,000g for 10 minutes and are referred to as whole cell extracts. The cells suspended in buffer A without Triton X-100 were lysed by using a Dounce homogenizer and centrifuged at 800g for 20 minutes to remove the nuclear pellets. The postnuclear extracts were further centrifuged at 100,000g for 60 minutes to give rise to a clear cytosolic supernatant and a pelleted membrane fraction. The pellets, washed once with buffer A without Triton X-100 and then dissolved in buffer A with 1% Triton X-100, were referred to as membrane extracts. The various cell extracts were either subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) for Western blot analyses or fractionated on an anion-exchange Mono-Q-Sepharose column (Pharmacia, Uppsala, Sweden). The column was equilibrated with a buffer containing 25 mmol/L Tris-HCl (pH 7.5), 0.1% Triton X-100, 1 mmol/L EDTA, and 2 mmol/L 2-mercaptoethanol, and bound materials were eluted with a linear gradient of 0 to 1.0 mol/L NaCl. Fractions containing PTP activity were combined, concentrated with an Amicon YM10 filter, and further fractionated on a gel-filtration Superose-12-Column (Pharmacia) equilibrated with a buffer containing 25 mmol/L Tris-HCI (pH 7.5), 0.15 mol/L NaCl, 1 mmol/L EDTA, 2 mmol/L 2-mercaptoethanol, and 0.1% Triton X-100. Column fractions were subjected to PTP activity assays and Western blotting analyses.
PTP activity assay.A nanopeptide ENDYINASL derived from a highly conserved region of the T-cell phosphatase (TC-PTP) was used as a substrate for the determination of PTP activity. Phosphorylation and purification of the 32P-labeled substrate was performed as previously reported.30 Briefly, the substrate at a concentration of 0.1 mmol/L was incubated with soluble EGF receptor kinase in the presence of 1 mmol/L [γ-32P]-ATP (600 cpm/pmol) and 10 mmol/L MnCl2 . The reaction was allowed to proceed for 4 hours at room temperature. The reaction mixture was then precipitated by adding trichloroacetic acid to a final concentration of 20% (wt/vol). After centrifugation in a microcentrifuge, the supernatant was loaded onto a C18 cartridge (Waters, Milford, MA) and was prewashed with acetonitrile/0.1% trifluoroacetic acid (TFA) followed by water/0.1% TFA. Unbound radioactivity was washed off with water/0.1% TFA. The 32P-labeled peptide was then eluted with 20% acetonitrile/0.1% TFA and vacuum-dried. The phosphatase activity assays were performed with 1 μmol/L 32P-labeled ENDYINASL in a buffer containing 20 mmol/L MES-NaOH, 1 mmol/L EDTA, 1 mmol/L dithiothreitol, and 0.1% Triton X-100. The reactions in a total volume of 60 μL were allowed to proceed for 15 minutes at room temperature before the addition of 300 μL of a stop solution containing 10% activated, acid-washed Norit A charcoal suspended in 0.9 mol/L HCl. The mixtures were cleared by centrifugation for 5 minutes, and the radioactivity of 200 μL of the supernatants was determined with a Beckman LS 7000 liquid scintillation counter (Beckman Instruments, Fullerton, CA). For immunodepletion of SHP-1, samples were incubated with anti–SHP-1 antibody (2303) in the presence of protein A-Sepharose beads for 3 hours. The supernatants were separated from the beads by low-speed centrifugation and then subjected to PTP activity analyses. One unit of activity was defined as the release of 1 nmol inorganic phosphate per minute. Protein concentration was determined by using the Bradford method.31
SDS-gel and Western blotting.Protein extracts were separated by 10% SDS-PAGE and transferred to a polyvinylidene difluoride membrane (Amersham). The membrane was probed with various primary antibodies and these were detected using the enhanced chemiluminescence system with horseradish peroxidase-conjugated secondary antibodies (Amersham) according to the manufacturer's protocol. To quantify the expression levels of SHP-1 and SHP-2, given amounts (5 to 50 ng) of purified recombinant SHP-1 and SHP-2 were run as standards in parallel.
RESULTS
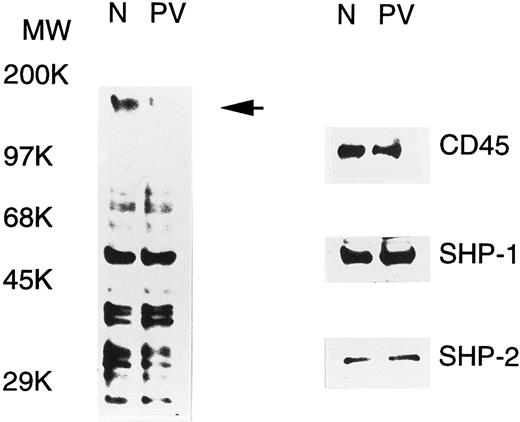
PV ECFC have increased PTP activity.PV erythroid progenitors display hypersensitivity to growth factors and cytokines, and this could be due to abnormal protein tyrosine phosphorylation, a major event in the signal transduction of growth factors and cytokines. To examine this possibility, tyrosine phosphorylation of cellular proteins in day-6 normal and PV ECFC was determined by Western blot analyses with antiphosphotyrosine. As shown in Fig 1, a number of tyrosine-phosphorylated proteins were detected, and most of them displayed an essentially equivalent degree of phosphorylation when normal and PV samples were compared. An intense band at approximately 160 kD was seen in the normal cells, but was hardly detected in PV samples (indicated by an arrow in Fig 1), suggesting that tyrosine phosphorylation or the expression level of the corresponding protein was significantly decreased in PV ECFC. The alteration in tyrosine phosphorylation of cellular proteins could be caused by an increased tyrosine phosphatase activity or a decreased tyrosine kinase activity. To determine the possible role of known PTPs, we examined the expression of three well-known enzymes, namely SHP-1, SHP-2, and CD45, by Western blotting analyses. All three enzymes were detected in both the PV and normal cells, and the levels of expression were comparable (Fig 1). By calibrating the Western blot analyses with purified SHP-1 and SHP-2, it is estimated that SHP-1 and SHP-2 represented approximately 0.11% and 0.02% of total proteins in the cell extracts, respectively. Similar results were obtained from peripheral blood mononuclear cells and day-8 ECFC (data not shown). To address whether the activity of PTPs in PV erythroid progenitor cells was altered, we determined the total PTP activity in the whole extracts of day-6 ECFC with the 32P-ENDYINASL peptide, a commonly used substrate specific for PTPs.30 In all 10 samples (5 normal and 5 PV, each from different individuals) examined, the total PTP activities in PV ECFC were twofold to threefold higher than those observed in the normal cells (P < .001, Table 1). Although the mean age of these groups was slightly different, no relationship between ages and PTP activities was apparent in both patient and control groups. Similar results were obtained with day-8 ECFC, but no difference in PTP activity was obtained with peripheral blood mononuclear cells (data not shown). These data indicate that PV ECFC have a consistently higher PTP activity compared with normal ECFC.
Tyrosine phosphorylation of cellular proteins and expression of SHP-1, SHP-2, and CD45 in normal (N) and PV ECFC. Day-6 ECFC were collected from suspension cultures, washed with phosphate-buffered saline, and lysed in buffer A with 1% Triton X-100. Equal amounts of extracts (25 μg) were subjected to SDS-PAGE and analyzed with antiphosphotyrosine (left panel) or the indicated anti-PTP antibodies (right panel). Purities of the ECFC were 75% and 70% for normal and PV samples, respectively. A representative figure from three experiments with similar results is shown. Each experiment was performed with blood samples from different normal volunteers and PV patients.
Tyrosine phosphorylation of cellular proteins and expression of SHP-1, SHP-2, and CD45 in normal (N) and PV ECFC. Day-6 ECFC were collected from suspension cultures, washed with phosphate-buffered saline, and lysed in buffer A with 1% Triton X-100. Equal amounts of extracts (25 μg) were subjected to SDS-PAGE and analyzed with antiphosphotyrosine (left panel) or the indicated anti-PTP antibodies (right panel). Purities of the ECFC were 75% and 70% for normal and PV samples, respectively. A representative figure from three experiments with similar results is shown. Each experiment was performed with blood samples from different normal volunteers and PV patients.
Total PTP Activity in Normal and PV Erythroid Progenitor Cells
| Sample No. . | Normal . | PV . | ||||||
|---|---|---|---|---|---|---|---|---|
| . | Age (yr) . | Sex . | Erythroid Colony Efficiency (%) . | Activity (U/mg) . | Age (yr) . | Sex . | Erythroid Colony Efficiency (%) . | Activity (U/mg) . |
| 1 | 48 | M | 57 | 0.22 | 67 | M | 59 | 0.34 |
| 2 | 28 | M | 57 | 0.12 | 69 | M | 67 | 0.25 |
| 3 | 52 | M | 63 | 0.09 | 38 | M | 70 | 0.32 |
| 4 | 35 | M | 75 | 0.07 | 49 | M | 57 | 0.27 |
| 5 | 28 | M | 65 | 0.13 | 70 | M | 54 | 0.28 |
| Mean ± SD | 38 ± 11 | 63 ± 7.9 | 0.13 ± 0.06 | 58 ± 14 | 61 ± 6.8 | 0.28 ± 0.05* | ||
| Sample No. . | Normal . | PV . | ||||||
|---|---|---|---|---|---|---|---|---|
| . | Age (yr) . | Sex . | Erythroid Colony Efficiency (%) . | Activity (U/mg) . | Age (yr) . | Sex . | Erythroid Colony Efficiency (%) . | Activity (U/mg) . |
| 1 | 48 | M | 57 | 0.22 | 67 | M | 59 | 0.34 |
| 2 | 28 | M | 57 | 0.12 | 69 | M | 67 | 0.25 |
| 3 | 52 | M | 63 | 0.09 | 38 | M | 70 | 0.32 |
| 4 | 35 | M | 75 | 0.07 | 49 | M | 57 | 0.27 |
| 5 | 28 | M | 65 | 0.13 | 70 | M | 54 | 0.28 |
| Mean ± SD | 38 ± 11 | 63 ± 7.9 | 0.13 ± 0.06 | 58 ± 14 | 61 ± 6.8 | 0.28 ± 0.05* | ||
Normal and PV day-6 ECFC were collected and lysed in buffer A with 1% Triton X-100. PTP activity was assayed with 32P-labeled ENDYINASL as a substrate and presented as units per milligram of protein. Erythroid colony efficiency represents the percentage of erythroid colonies formed per cell number plated in the plasma clot cultures.
Significantly different from that of normal control group (P < .001, Student's t-test).
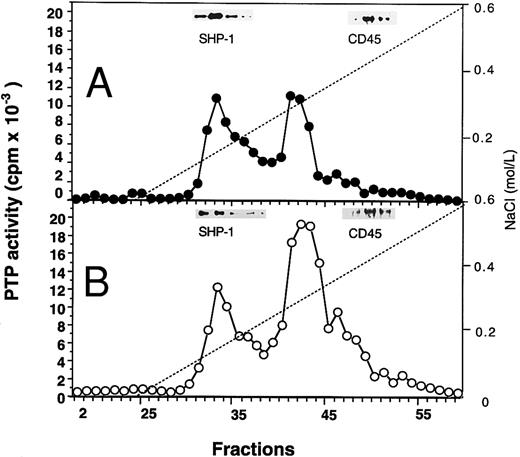
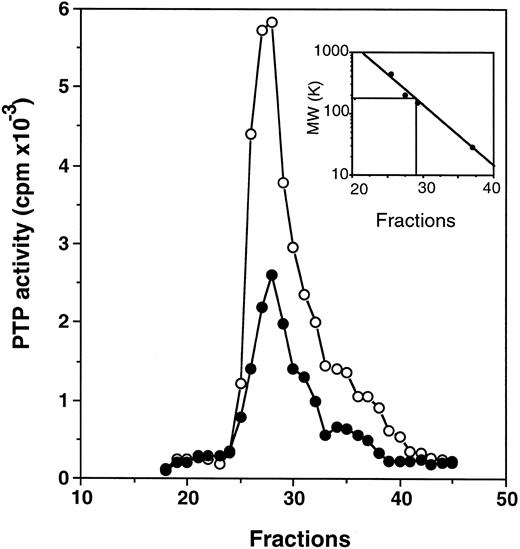
Profiles of PTP activities on anion-exchange mono-Q and Superose-12 gel-filtration columns.ECFC may contain many PTPs among which only one or a few might be affected in the PV cells. To determine which PTPs might be involved, we separated the whole cell extracts on a mono-Q column. As shown in Fig 2, for both normal and PV samples, two major activity peaks were observed at comparable column positions. The first peak eluting at 0.19 mol/L NaCl contained nearly equal amounts of PTP activity when normal and PV samples were compared, and it represented about 50% of the total PTP activity in normal cells but only 20% of the total activity in PV cells. The second peak, which eluted at 0.31 mol/L NaCl and represented more than 70% of the total phosphatase activity in PV cells, was nearly threefold greater than the corresponding peak observed in the normal cells. This major peak activity is responsible for the elevation of the total phosphatase activity in PV ECFC. Western blot analyses of the column fractions showed that the first peak corresponded to SHP-1 (Fig 2). Incubation of the collected peak fractions (nos. 41 through 43) with anti–SHP-1 antibody and protein A-Sepharose beads resulted in 70% to 90% precipitation of SHP-1 accompanied by at least 70% depletion of PTP activity, thus providing further evidence that SHP-1 represented the major PTP activity in the first peak. SHP-2 eluted slightly ahead of SHP-1 (not shown), and it did not give rise to a recognizable activity peak. This is probably due to its lower expression level as described earlier and its lower specific activity as previously reported.27 CD45 did not correspond to a major activity peak and was eluted at 0.42 mol/L NaCl (Fig 2). Together, the data indicate that SHP-1 appears to be normal in expression and activity in PV cells compared with normal cells, and increased PTP activity in PV ECFC is attributed to a major activity that does not cross-react with SHP-1, SHP-2, or CD45. To show further the identity of the second peak, mono-Q column fractions (fractions no. 41 through 43) were combined and then fractionated on a Superose-12 gel-filtration column. Only a single activity peak was observed, and 10-fold enrichment of specific PTP activity was achieved (Fig 3). Based on the positions of standard molecular weight markers, linear regression analyses showed that the increased PTP activity ran at approximately 170 kD (inset of Fig 3). Because this enzyme represents a major PTP activity in erythroid progenitor cells, we defined it as erythroid PTP (E-PTP).
Mono-Q-Sepharose separation profiles of whole extracts from normal (A) and PV (B) day-6 ECFC. Approximately 2 mg of whole cell extracts were loaded onto the column. PTP activity was measured with 32P-labeled ENDYINASL as a substrate and data are expressed as cpm obtained from 2 μL of each 0.5-mL fraction eluted from the column. Solid lines represent PTP activity; dashed lines denote NaCI gradients. (Insets) Western blot analyses of corresponding fractions with anti–SHP-1 and anti-CD45 antibodies. A representative figure from four experiments with similar results is shown. Each experiment was performed with blood samples from different normal volunteers and PV patients.
Mono-Q-Sepharose separation profiles of whole extracts from normal (A) and PV (B) day-6 ECFC. Approximately 2 mg of whole cell extracts were loaded onto the column. PTP activity was measured with 32P-labeled ENDYINASL as a substrate and data are expressed as cpm obtained from 2 μL of each 0.5-mL fraction eluted from the column. Solid lines represent PTP activity; dashed lines denote NaCI gradients. (Insets) Western blot analyses of corresponding fractions with anti–SHP-1 and anti-CD45 antibodies. A representative figure from four experiments with similar results is shown. Each experiment was performed with blood samples from different normal volunteers and PV patients.
Purification on a Superose 12 column. Fractions no. 41 through 43 from the mono-Q column (see Fig 2) were combined, concentrated, and loaded onto a Superose 12 column. PTP activity of fractions from normal (•) and PV (○) samples represents the data obtained from 2 μL of each 0.5-mL fraction eluted from the column and assayed with 32P-labeled ENDYINASL as a substrate. The inset represents the calibration of the Superose 12 column with standard molecular weight markers (apoferritin, 443,000; b-amylase, 200,000; alcohol dehydrogenase, 150,000; and carbonic anhydrase, 29,000). The PTP activity peaked at fraction 27.25, which corresponded to 170 kD based on linear regression analyses. A representative figure from three experiments with similar results is shown. Each experiment was performed with blood samples from different normal volunteers and PV patients.
Purification on a Superose 12 column. Fractions no. 41 through 43 from the mono-Q column (see Fig 2) were combined, concentrated, and loaded onto a Superose 12 column. PTP activity of fractions from normal (•) and PV (○) samples represents the data obtained from 2 μL of each 0.5-mL fraction eluted from the column and assayed with 32P-labeled ENDYINASL as a substrate. The inset represents the calibration of the Superose 12 column with standard molecular weight markers (apoferritin, 443,000; b-amylase, 200,000; alcohol dehydrogenase, 150,000; and carbonic anhydrase, 29,000). The PTP activity peaked at fraction 27.25, which corresponded to 170 kD based on linear regression analyses. A representative figure from three experiments with similar results is shown. Each experiment was performed with blood samples from different normal volunteers and PV patients.
Enzymatic properties of E-PTP.To confirm that the increased activity is indeed from a PTP, we analyzed the response of the partially purified E-PTP samples towards typical inhibitors of PTPs. As shown in Table 2, the PTP activity of samples from both normal and PV ECFC was almost totally inhibited by vanadate, pervanadate, ATP-γ-S, heparin, and Zn2+, all of which have been shown to be the inhibitors of protein tyrosine phosphatases.27,30,32 On the contrary, the activity was not influenced by NaF and microcystin, which are serine/threonine protein phosphatase inhibitors. These results indicate that the partially purified E-PTP has all the typical properties of PTPs. Additional results showed that, like other PTPs,27,33 34 E-PTP exhibited a pH optimum of 4.5 to 5.0 when low molecular weight para-nitrophenylphosphate was used as a substrate (data not shown).
Effects of Various Effectors on the PTP Activity
| Effectors . | Normal ECFC . | PV ECFC . |
|---|---|---|
| Control | 1 | 1 |
| Vanadate (0.1 mmol/L) | 0.03 | 0.02 |
| Pervanadate (0.1 mmol/L) | 0.06 | 0.03 |
| ATP-γ-S (0.1 mmol/L) | 0.05 | 0.02 |
| Heparin (0.2 mg/ml) | 0.06 | 0.05 |
| ZnCl2 (1 mmol/L) | 0.22 | 0.19 |
| Idoacetic acid (1 mmol/L) | 0.05 | 0.04 |
| NaF (50 mmol/L) | 0.96 | 0.86 |
| Microcystin (1 mmol/L) | 1.12 | 1.21 |
| Effectors . | Normal ECFC . | PV ECFC . |
|---|---|---|
| Control | 1 | 1 |
| Vanadate (0.1 mmol/L) | 0.03 | 0.02 |
| Pervanadate (0.1 mmol/L) | 0.06 | 0.03 |
| ATP-γ-S (0.1 mmol/L) | 0.05 | 0.02 |
| Heparin (0.2 mg/ml) | 0.06 | 0.05 |
| ZnCl2 (1 mmol/L) | 0.22 | 0.19 |
| Idoacetic acid (1 mmol/L) | 0.05 | 0.04 |
| NaF (50 mmol/L) | 0.96 | 0.86 |
| Microcystin (1 mmol/L) | 1.12 | 1.21 |
Partially purified normal and PV E-PTP elutes obtained from a Mono-Q column were incubated with various effectors at the indicated concentrations. PTP activity was measured with 32P-labeled ENDYINASL as a substrate. Data represent activity relative to activity in the absence of effectors defined as 1.0.
Subcellular fractionation of E-PTP.To show the cellular localization of E-PTP, cell extracts were fractionated into cytosolic and membrane fractions by ultracentrifugation. The cytosolic fractions from normal and PV ECFC appeared to have equal amounts of PTP activity, representing about 65% of the total activity of normal ECFC and 20% of PV ECFC. However, when the membrane fractions were analyzed, PV samples displayed a threefold higher activity than the normal samples. After separation on an anion-exchange Mono-Q column, both cytosolic fractions resolved a major activity peak eluted at 0.19 mol/L NaCl and correlating with anti–SHP-1 immunoreactivity (Fig 4A; see also Fig 2). This suggests that SHP-1 is a major cytosolic PTP activity in the cells, and its activity appears to be normal in PV ECFC. After separation of the membrane extracts on the mono-Q column, samples from both normal and PV cells gave rise to a major activity peak eluted at 0.31 mol/L NaCl, but the one from PV ECFC showed at least threefold more activity than the one from normal ECFC (Fig 4B). Collectively, SHP-1 represents the major cytosolic PTP activity in the erythroid progenitor cells and its activity is normal, whereas E-PTP represents the major PTP activity in the membrane fractions and its activity is elevated in PV erythroid progenitor cells. We thus identified a major membrane associated-PTP that is hyperactive in PV ECFC.
Mono-Q-Sepharose profiles of cytosolic (A) and membrane (B) fractions from normal (•) and PV (○) ECFC. Day-6 ECFC were collected and lysed with a Dounce homogenizer in buffer A without Triton X-100. Cytosolic and membrane fractions were obtained by ultracentrifugation at 100,000g for 60 minutes as described in Materials and Methods. Approximately 1 mg samples were loaded on the mono-Q column. PTP activity was measured with 32P-labeled ENDYINAL and the data are expressed as cpm obtained from 2 μL of each 0.5-mL fraction eluted from the column. A representative figure from three experiments with similar results is shown. Each experiment was performed with blood samples from different normal volunteers and PV patients.
Mono-Q-Sepharose profiles of cytosolic (A) and membrane (B) fractions from normal (•) and PV (○) ECFC. Day-6 ECFC were collected and lysed with a Dounce homogenizer in buffer A without Triton X-100. Cytosolic and membrane fractions were obtained by ultracentrifugation at 100,000g for 60 minutes as described in Materials and Methods. Approximately 1 mg samples were loaded on the mono-Q column. PTP activity was measured with 32P-labeled ENDYINAL and the data are expressed as cpm obtained from 2 μL of each 0.5-mL fraction eluted from the column. A representative figure from three experiments with similar results is shown. Each experiment was performed with blood samples from different normal volunteers and PV patients.
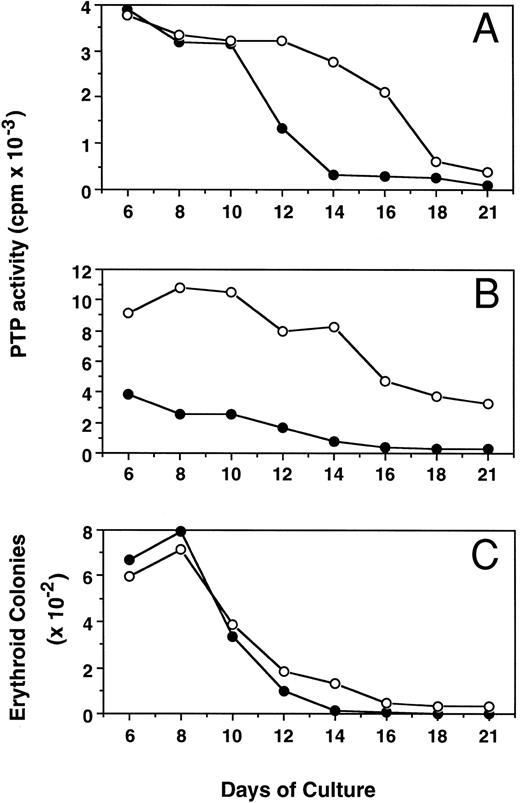
Kinetic changes of PTP activity during erythroid progenitor proliferation and differentiation.Figure 5 shows the alterations of PTP activity in cytosol (mainly SHP-1, Fig 5A) and membrane (mainly E-PTP, Fig 5B) fractions during proliferation and differentiation of both PV and normal erythroid progenitor cells in suspension cultures. The ECFC PTP activity was stable between days 6 and 8, when most of the cells possessed the capacity for forming erythroid colonies. Along with the differentiation of these cells, as evidenced by an increased number of more mature erythroblasts29 and decreased colony-forming ability from day 8 to day 21 of culture, PTP activities in both cytosol and membrane fractions of normal ECFC rapidly declined and little activity was detected by day 21. Interestingly, PTP activities in the cytosol and membrane fractions of PV cells also declined but at a slower rate, and about 40% of membrane PTP activity of PV cells was still maintained by day 21. The slower decrease in PTP activity of PV cells correlates with an increased number of PV ECFC still present at day 14 and thereafter, when essentially none of the normal cells could still form colonies (Fig 5C). When mature erythrocytes from both PV and normal peripheral blood were examined, little PTP activity could be detected and no difference was found between normal and PV cells (data not shown). The present results indicate that E-PTP is predominately present in early erythroid progenitor cells. The enhanced presence of more immature PV erythroid cells observed in the suspension cultures might be due to an increased E-PTP activity of the PV cells or vice versa.
Kinetic changes of PTP activities in cytosolic (A) and membrane (B) fractions and erythroid colony number (C) from normal (•) and PV (○) ECFC. Cells were collected at the indicated periods of suspension culture. Preparation of cytosolic and membrane extracts and assays of colony formation were performed as described in Materials and Methods. Cell extracts containing 2.0 μg total proteins were used to measure PTP activity with 32P-labeled ENDYINASL, and 1,000 cells were analyzed for colony formation in plasma clot cultures.
Kinetic changes of PTP activities in cytosolic (A) and membrane (B) fractions and erythroid colony number (C) from normal (•) and PV (○) ECFC. Cells were collected at the indicated periods of suspension culture. Preparation of cytosolic and membrane extracts and assays of colony formation were performed as described in Materials and Methods. Cell extracts containing 2.0 μg total proteins were used to measure PTP activity with 32P-labeled ENDYINASL, and 1,000 cells were analyzed for colony formation in plasma clot cultures.
DISCUSSION
We previously reported that orthovanadate, a PTP inhibitor, mimics the effect of SCF on highly purified normal human burst-forming units-erythroid (BFU-E) in vitro, but not the effect of IL-3 or EPO.35 Vanadate enhanced the number and size of the BFU-E and could be acting to potentiate the kinase activity of SCF, or another protein kinase, by inhibiting a PTP. When we performed similar experiments with PV BFU-E, this effect was greatly reduced.25 EPO and SCF rapidly induced tyrosine phosphorylation of the EPO and SCF receptors in normal ECFC and this effect was greatly enhanced by vanadate, but little enhancement of tyrosine phosphorylation was evident in PV cells.25 These results suggested that PV patients might have an abnormal PTP activity that, possibly, could be responsible for the increased cell proliferation. In the present study, we have identified an increased PTP activity in the erythroid progenitors from PV blood. This abnormal activity is attributed to a major PTP (E-PTP) partitioned in the membrane fraction of ECFC. Because tyrosine phosphorylation is a central event in the signal transduction of various hematopoietic growth factors that control proliferation and differentiation of hematopoietic stem/progenitor cells,8,9 13-15 the increased E-PTP activity that disrupts the phosphorylation and dephosphorylation balance may have profound effects on cell behavior. Thus, increased activity of E-PTP could be responsible for the pathogenesis of the PV disorder.
PTPs represent a family of highly diverse enzymes that have positive as well as negative roles in signal transduction. In hematopoietic cells, three major PTPs have been extensively studied. These are SHP-1, SHP-2, and CD45. SHP-1, an SH2 domain-containing enzyme predominantly expressed in hematopoietic cells, plays a crucial role in hematopoiesis because mutation of the SHP-1 gene in motheaten and viable motheaten mice produces a high proliferation of hematopoietic cells and a marked splenomegaly with an enhanced number of ECFC, as well as a severe autoimmune, immunodeficiency syndrome.36 Furthermore, SHP-1 negatively regulates signal transduction by EPO, IL-3, B-cell antigen, interferon-α/β, interferon-γ, and SCF.16-22 In contrast, SHP-2, a widely distributed SH2 domain-containing PTP, has a positive role in growth factor-stimulated MAP kinase activation in a number of nonhematopoietic cells.14 In hematopoietic cells, by specifically associating with the EPO receptor, c-Kit, and the IL-6 receptor subunit gp130, SHP-2 is believed to play a similar positive role in the mitogenic signaling of these receptors.23,24,37 CD45, which is specifically expressed in hematopoietic cells, has been shown to be required for T-cell antigen receptor signaling by activating Src family tyrosine kinases.38 Although these three enzymes were potential candidates for the PV abnormality, our study showed that all of them appeared normal in PV ECFC. Instead, we identified a potentially novel membrane or membrane-associated E-PTP that exhibited hyperactivity in PV ECFC. Considering the hypersensitivity of PV erythroid progenitor cells to a wide variety of growth factors, we postulate that E-PTP plays a positive role in enhancing hematopoietic cell proliferation and/or survival. Supporting this view is the fact that, along with the differentiation and maturation of ECFC, E-PTP activity in the normal ECFC declined much faster than that in the PV cells and the PV ECFC with higher PTP activity maintained their colony-forming capability over a longer period of time in suspension culture. This seems to indicate that PV ECFC, by possessing a higher level of E-PTP activity, may have a higher capability to proliferate and/or survive, thus producing many more erythrocytes upon maturation and producing the erythroid hyperplasia in PV patients. We assume that a similar defect in the myeloid and megakaryocyte lineages would contribute to the trilineage hyperplasia.
The precise mechanism by which PTPs regulate cell signaling has not been well understood. CD45 has a positive role by activating Src family protein kinases. It is not known whether E-PTP functions in a similar way. The absence of tyrosine phosphorylation of the 160-kD protein in PV ECFC may indicate that this protein is the physiologic substrate of E-PTP. Presumably, it might stay in an inactive state when phosphorylated and would positively regulate cell proliferation and/or survival when dephosphorylated.
Several possible causes exist for increased activity of E-PTP in PV ECFC. First, the expression level of the enzyme might be elevated. Second, the enzyme might be structurally abnormal due to alternate mRNA splicing or disruption of its gene by mutation, insertion, or deletion. Third, the enzyme might be upregulated by an abnormal activator in PV ECFC, or the PV cells might lack an inhibitor of the enzyme. Similarities between the normal and PV E-PTP in subcellular localization, separation profiles on Mono-Q and S12 columns and sensitivities towards PTP inhibitors most likely support the first possibility, but the other possibilities cannot be ruled out and isolation of the enzyme is required to definitively clarify this situation.
ACKNOWLEDGMENT
The authors thank Ella Stitt, Judy Luna, Amanda Hodges, and Zhongjia Tan for their excellent technical assistance; Drs Chun-Hua Dai and Min You for helpful discussions; and Dr Harriet Gilbert for her generous provision of some of the PV blood.
Supported by a Veteran Health Administration Merit Review Grant (to S.B.K.), by Grants No. DK-15555 and 2 T32-DK07186 from the National Institutes of Health (to S.B.K.), by Markey Trust Funds (to Z.Z.), by the International Human Frontier Sciences Programs (to Z.Z.), and by an institutional research grant from the American Cancer Society (to Z.Z.). X.S. is an Ortho Biotech Hematology Fellow.
Address reprint request to Sanford B. Krantz, MD, or Zhizhuang Zhao, PhD, Department of Medicine/Hematology, Vanderbilt University Medical Center, MRBII, Nashville, TN 37232-6305.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal