Abstract
Although the use of cytokine-mobilized peripheral blood stem cells has gained a significant momentum in clinical transplantation, the mobilization schemes practiced are guided by a great deal of empiricism. The mechanism(s) by which cytokines or chemokines, alone or in combination, bring about redistribution of stem/progenitor cells from bone marrow to peripheral blood are poorly understood. Likewise the fate of mobilized stem/progenitor cells and their biological properties are incompletely defined. One of the leading hypotheses to explain the mechanism of cytokine-induced mobilization encompasses the view that cytokines disrupt, directly or indirectly, cytoadhesive interactions of stem/progenitor cells with their bone marrow stroma. Compatible with this view are changes in the expression and/or function of several cytoadhesion molecules, especially integrins, postmobilization, and extensive in vitro experimentation supporting the concept of cytokine/integrin interactions. To provide a further insight on the cytokine/integrin interplay in vivo, we have combined cytokine treatments with anti-integrin treatments for mobilization in primates and mice. We found that anti-VLA4 treatment combined with either granulocyte colony-stimulating factor (G-CSF ) treatment or kit ligand treatment leads to significant enhancement of mobilization efficiency (fivefold to eightfold) well above the levels produced by either cytokine alone or anti-VLA4 treatment alone. Similar enhancement was seen when combinations of cytokines, ie, G-CSF plus kit ligand or G-CSF plus Flt3-ligand were used with anti-VLA4 in primates and mice. Furthermore, when anti-VLA4 was given in 5-Fluorouracil–treated primates, significant numbers of progenitor cells were circulating for several days during the recovery period only in the anti-VLA4 treated animals. These data suggest that (1) the effect of anti-VLA4 on mobilization, when used alone, is unlikely to be mediated by secondary cytokine elaboration in vivo; (2) three different cytokines and their combinations do not appear to influence the in vivo responsiveness to anti-VLA4 in coadministration schemes; (3) even if cytokine treatments on their own exert downmodulation of VLA4 function, the target progenitor cells influenced by anti-VLA4 or by cytokines may not necessarily overlap; and (4) augmentation of mobilization in cytokine/anti-VLA4 treatments is most likely caused by an amplification of the pool of target cells on which anti-VLA4 exerts its effects. Because cytokines or anti-VLA4 are each capable of mobilizing long-term repopulating cells and because we show with the present studies that anti-VLA4 in an autologous bone marrow cell transplantation setting does not cause any delay in engraftment, the combination of cytokine/anti-integrin treatment enhancing mobilization may have a clinical use.
IN STEADY STATE hematopoiesis only small numbers of hematopoietic progenitors circulate in peripheral blood.1,2 Administration of cytokines, such as granulocyte colony-stimulating factor (G-CSF ), kit ligand (KL), interleukin-1 (IL-1), IL-7, IL-8, IL-11, or Flt3 ligand (FL) and cytotoxic drugs, such as cyclophosphamide, is associated with striking increases in the number of circulating hematopoietic progenitors in mice, primates, and humans.3-7 In addition to mobilizing committed progenitors, these agents increase the concentration of long-term repopulating cells in the peripheral blood. As a consequence, peripheral blood has become an increasingly important source of hematopoietic progenitors for use in both autologous and allogeneic stem cell transplantation.8
The mechanism(s) by which cytokines/chemokines or cytotoxic drugs cause progenitor mobilization remain poorly understood. Although an increase in the overall size of the progenitor pool and in the survival of circulating progenitors may contribute to this process, it is also clear that dramatic increases in the number of circulating progenitors can be achieved in the absence of any increase in the size of the progenitor pool. For example, IL-1 or IL-8 cause progenitor mobilization within minutes of administration before any increase in overall progenitor number has occurred.9 10 Thus, a major underlying event in progenitor cell increases in peripheral blood applicable to all treatments is a change in the distribution of hematopoietic progenitors between the bone marrow and peripheral blood.
Hematopoietic progenitors and stem cells in bone marrow are in close proximity with hematopoietic stromal cells and their extracellular matrix. Interactions between these cells and stromal cells are important not only in retaining them within the marrow but for their subsequent proliferative expansion and differentiation.11-13 Several cytoadhesion molecules of the integrin or selectin family or CD44 and certain chemokines are considered to be instrumental in the interactions between hematopoietic cells and stromal cells.14-16 As this association is lost during the stem/progenitor exportation from the bone marrow, one of the leading hypotheses advanced to explain progenitor mobilization is that there are alterations in the cytoadhesive interactions of progenitor/stem cells with the bone marrow microenvironment.3 Direct in vivo evidence implicating the involvement of cytoadhesion molecules in mobilization was provided by our in vivo treatments with antibodies to VLA4 in primates17 or mice,18 which resulted in peripheralization of progenitors. In vitro experiments have also shown that cytokines are able to modulate the functional activity of integrins in certain cell populations.19-24 Because of these data and direct studies with mobilized progenitor cells, alterations in the expression and/or functional status of integrins after administration of cytokines have been embraced as the main mechanism underlying progenitor mobilization induced by cytokines.25-28 However, it is likely that additional mechanisms exerted on progenitor cells and/or stromal cells cooperate for these effects, but these remain undefined.
To provide further insights into the mechanism(s) of cytokine-induced mobilization as well as that of anti-VLA4, we have used in the present studies combinations of cytokines and anti-VLA4 treatment and studied the consequences in progenitor mobilization. We have found that concurrent blockade of VLA4-mediated progenitor adhesion achieved by administration of anti-VLA4 could augment the mobilization of hematopoietic progenitors yielded by G-CSF, KL, or FL and their combinations. We speculate that this augmentation is achieved by the action of anti-VLA4 on an expanded pool of target cells induced by cytokines. Further experiments are in progress to secure these speculations. Whatever the mechanism, the significant augmentation achieved could be exploited for clinical purposes to boost mobilization in certain cases of transplantation. This statement is supported by the fact that anti-VLA4 alone is capable of mobilizing long-term repopulating cells and that ex vivo anti-VLA4–treated cells do not compromise their engraftment in vivo, as shown in the present studies.
MATERIALS AND METHODS
Studies in Mice
Mobilization protocols. B6D2F1 mice 3 to 4 months of age were used in all experiments. These mice were bred and maintained in the University of Washington Comparative Medicine facility (Seattle, WA). Mice were treated with G-CSF (Filgrastim Neupogen; Amgen, Thousand Oaks, CA), 100 μg/kg subcutaneously twice daily for 3 days, or with KL (nonpegylated, a generous gift from Amgen), 100 μg/kg/d subcutaneously for 7 days. Another group of mice and controls were treated with a combination of G-CSF, 100 μg/kg/d for 3 days plus FL (CHO-derived, generously provided by Immunex, Seattle, WA), 200 μg/kg/d for 3 days. Cytokine doses were chosen to provide near optimum responses for the period given.7 29-31 All mice were sacrificed the day after the last injection and blood was drawn for assessing the number of clonogenic progenitors (CFU-C). Controls were either injected with phosphate-buffered saline (PBS) plus 1% bovine serum albumin (BSA) or with isotype control IgG, if available. Apart from cytokine treatments, another group of mice was given an endotoxin-free anti-VLA4 antibody (PS/2, a rat IgG2a anti-murine α4 antibody, generously supplied by Dr R.R. Lobb, Biogen, Cambridge, MA) at 2 mg/kg/d for 3 days and sacrificed the next day. Combinations of cytokine plus anti-VLA4 treatments were also done, which included G-CSF plus anti-VLA4, KL plus anti-VLA4, or G-CSF plus FL plus anti-VLA4 at the doses indicated previously. All animal groups were again sacrificed the day after the last treatment and blood was collected.
Colony-forming unit-spleen (CFU-S) assays. The number of CFU-S12 present in the blood of treated animals was assessed by injection of an aliquot of test blood into the tail veins of irradiated secondary recipients.18 Mice used as secondary recipients received 850 cGy irradiation. This level of irradiation was chosen because it was associated with the rate of less than 0.1 CFU-S12 per spleen in a nontransplanted irradiated control group. Mice were sacrificed 12 days later and their spleens excised and suspended in Bouin's medium for 10 minutes followed by 10% neutral buffered formalin. The numbers of macroscopically visible surface colonies (CFU-S12) were counted by the same observer for all experiments.
Assays for radioprotective cells and long-term repopulating cells. The presence of radioprotective cells in the peripheral blood of mice was studied by comparing the survival of recipient preirradiated mice transplanted with peripheral blood mononuclear cells from mice treated with either PBS plus 1% BSA or with anti-VLA4 antibody. A dose of irradiation (1,050 cGy) previously shown to result in 100% mortality by day 16 was chosen. Irradiation was delivered by a dual source 137Cs Irradiator (GammaCell 40; Nordion International Inc, Ontario, Canada) with a dose of between 125 and 130 cGy/min. Control-irradiated mice were included in all experiments. Detection of long-term repopulating cells in the peripheral blood of mice treated with anti-VLA4 antibody was also determined by transplantation of blood mononuclear cells from male mice treated with antibodies to VLA4 for 3 days into lethally irradiated (1,050 rads) female mice. The presence of donor-derived neutrophils in the peripheral blood recipients was evaluated 8 months after transplantation using fluorescent in situ hybridization with a probe specific for the Y chromosome sequence.32 A minimum of 200 cells were evaluated for the presence of Y-specific hybridization.
CFU-C assays. CFU-C assays were performed using a methyl cellulose mixture consisting of 1.2% (wt/vol) methyl cellulose (Fisher Scientific, Fairlawn, NJ), 30% fetal bovine serum (Intergen, Purchase, NY), 1% BSA, 0.1 mmol/L 2-mercaptoethanol, 5 U/mL recombinant erythropoietin (Genetics Institute, Cambridge, MA), 10% (vol/vol) 5× concentrated WEHI-3 conditioned medium, 5% (vol/vol) pokeweed-mitogen-stimulated spleen-cell–conditioned medium, and 50 ng/mL recombinant murine stem cell factor (SCF; Peprotech, Rocky Hill, NJ), in Iscove's modified Dulbecco's medium (IMDM). After mobilization treatments, the mice were sacrificed and mononuclear cells from 0.25 to 1 mL of peripheral blood from each mouse were plated into duplicate plates. Cultures were maintained in a high humidity 37°C 5% CO2 /95% air incubator. Colonies incubated for 8 to 10 days were counted on the basis of morphological criteria using a dissecting microscope, and all colonies, ie, granulocyte macrophage colony-forming units (CFU-GM) or burst-forming units-erythroid (BFUe) were pooled and reported as CFU-C.
Studies in Nonhuman Primates
Mobilization protocols. Studies were done in juvenile baboons (Papio Cynocephalus ). Animals were housed and cared for in our accredited Regional Primate Center and approved protocols were used. Mobilization treatments included the use of single cytokines or a combination of cytokines in a sequential or concurrent fashion. A total of 8 animals have been previously treated with G-CSF alone at 100 μg/kg/d for 5 days.33 Four animals were treated concurrently with G-CSF (100 μg/kg/d) and KL (25 μg/kg/d) for 5 days and one animal with G-CSF (100 μg/kg/d) plus KL (100 μg/kg/d for 5 days). Doses for G-CSF and KL were adopted from previous primate studies.33-35 Two other animals were given in addition to G-CSF plus KL, injections of anti-VLA4 (1 mg/kg/d, HP 1/2 monoclonal anti-human α4 antibody, protein A purified and endotoxin-free, a gift from Dr R.R. Lobb, Biogen) either concurrently with cytokine treatment or sequentially. An additional pair of animals received G-CSF 30 μg/kg alone or followed by two injections of the humanized anti-VLA4. Blood was drawn before treatment, daily during treatment, and a few days after discontinuation of treatments. Complete blood count was determined (hematocrit, white blood cells, and platelets), and clonogenic assays from either peripheral blood or bone marrow mononuclear cells were set up in methylcellulose, as previously described,17 for assessing BFUe, CFU-GM, colony-forming unit-megakaryocyte (CFU-Mk), or macroscopically visible colonies (high proliferative potential [HPP], >0.5 mm in diameter of compact colony growth). Briefly, for clonogenic assays, a mixture consisting of 1.2% methyl cellulose (Fisher Scientific), 50% fetal bovine serum (Intergen), 1% BSA, 0.1 mmol/L 2-mercaptoethanol, 2 U/mL recombinant erythropoietin (Genetics Institute), 50 ng/mL KL/stem cell factor (Amgen), and 50 U/mL gibbon IL-3 kindly provided by Dr K. Kaushansky, University of Washington, was used. Blood or bone marrow was collected in preservative-free heparin and the mononuclear cells plated in triplicate at 100,000 and 500,000 per mL. After incubation at 37°C with 5% CO2 at high humidity, BFUe and granulocyte/macrophage colony-forming units (CFU-GM) were counted on days 12 to 14 on the basis of morphological criteria observed with a dissecting microscope. Macroscopically visible compact colonies (HPP, mixed or pure) that were over 0.5 to 1 mm in diameter were counted separately at day 21.
5-Fluorouracil (5FU) treatments. Two baboons were given 5FU as a single intravenous injection of 100 mg/kg and blood cell counts and clonogenic circulating progenitors were assessed for 2 weeks post-5FU injection. Two other animals similarly treated with 5FU received four consecutive injections of anti-VLA4 (1 mg/kg) at days 5 to 8 post-5FU. The same parameters, blood counts, and clonogenic progenitors in the peripheral blood were monitored as in control animals. Before treatment and at day 11, bone marrow samples were aspirated from one 5FU control and one 5FU plus anti-VLA4–treated animal to assess nucleated cell counts and progenitor cell content per milliliter of bone marrow.
Transplantation of autologous bone marrow treated ex vivo with anti-VLA4. Two baboons weighing 7.9 and 8.2 kg were transplanted, one with 1.9 × 109 (2.4 × 108 cells/kg), the other with 1.4 × 109 bone marrow nucleated cells (1.71 × 108 cells/kg). Buffy-coated bone marrow samples were subjected to red blood cell lysis and the cells were incubated with anti-VLA4 antibody at 300 μg/mL of bone marrow buffy coat cells in a total volume of 50 mL. Cells were infused intravenously without washing excess antibody in a total volume of 50 mL. Both recipient animals were subjected to prior irradiation with 1,020 cGy TBI. During the early posttransplant period, recipients were given antibiotics and whole blood (if necessary) from matched healthy donors.
Cell Cycle Studies
To provide estimates on the number of cycling cells present in bone marrow or peripheral blood before or after anti-VLA4 or G-CSF treatments, we used the Ara-C suicide method as described by Cannistra et al.36
RESULTS
Anti-VLA4 Antibody Mobilizes CFU-S and Long-Term Repopulating Cells
In previous studies we have shown that antibodies to VLA4 increased the numbers of clonogenic progenitors in peripheral blood17 presumably by displacing them from bone marrow. To study whether more primitive hematopoietic progenitors or stem cells are also mobilized, we assessed the effect of anti-VLA4 antibody treatment on the number of circulating CFU-S, radioprotective cells, and long-term repopulating cells. Treatment with anti-VLA4 for 3 consecutive days increased the number of circulating CFU-S from ∼0.2/mL of blood (1 CFU-S in six control spleens) to 53.3 ± 8.25 CFU-S/mL in the group of six mice treated with anti-VLA4. Recipient mice were injected with 150 μL of blood from VLA4 treated but 1 mL from PBS/BSA-treated animals. We next studied whether the anti-VLA4 treatment mobilized cells with radioprotective capacity. Survival of lethally irradiated mice transplanted with mononuclear cells isolated from peripheral blood of mice treated with either anti-VLA4 or PBS/BSA was compared. All mice (14 of 14) transplanted with mononuclear cells (5 × 105 per mouse) from the anti-VLA4–treated animals survived 30 days after transplantation, whereas only 14% of mice (2 of 14) who received mononuclear cells from PBS/BSA-treated animals were alive 30 days after transplantation. All mice that received radiation alone without donor blood died. Eight months after the transplantation of mononuclear cells from blood harvested from mice treated with three doses of anti-VLA4, 12 mice were alive and 9 of them were sacrificed to assess the level of donor cells in the peripheral blood using a Y chromosome–specific probe.32 All mice studied showed evidence of engraftment with donor long-term reconstituted stem cells and the percentage of Y chromosome–positive interphase nuclei were from 70% to 90%. Chimerism was not assayed on mice transplanted with mononuclear cells from PBS/BSA-treated mice, because no mice survived long term.
Effect of Anti-VLA4 Treatment on Progenitor Proliferative Status
To test whether anti-VLA4 treatment per se exerted any influence on proliferation of progenitors as evaluated by our in vitro assays or in vivo (1) we performed clonogenic in vitro assays in the presence or absence of the anti-VLA4 antibody or control antibody (anti-CD18); (2) we assessed bone marrow cellularity before and after 3-day antibody treatment in primates, or the cellularity per femur in a group of mice treated with anti-VLA4 (3-day treatment) compared with a vehicle-treated control group; and (3) we assessed cell cycling changes, post–anti-VLA4 treatment, and compared them with pretreatment values. In three independent experiments using mononuclear cells from bone marrow or peripheral blood or CD34+ cells we found no evidence that the addition of anti-VLA4 or anti-CD18 antibody influenced the number of the clonogenic progenitors or the size of colonies in vitro (data not shown). Regarding bone marrow cellularity, a group of 5 mice treated with anti-VLA4 or 10 vehicle-treated controls had 21.3 ± 1.4 × 106 nucleated cells or 19.4 ± 0.5 × 106 per femur, respectively, and a total progenitor content of 56,818 ± 5,261 or 58,200 ± 3,000 per femur, respectively. In the two primates treated for 3 days with anti-VLA4 (1 mg/kg/d) we aspirated bone marrow before treatment and after the 3-day treatment. Before treatment, bone marrow total progenitor content was 17,208 (BFUe = 2,677) and 8,650 (BFUe = 3,554) per mL of aspirated marrow. After treatment it was 239,496 (BFUe = 81,305) and 17,484 (BFUe = 4,141) for the two animals, respectively. Thus, a significant increase both in nucleated cell number and progenitor content was noted after treatment. Although a sampling error could be a factor, we do not think that this increment is simply explained by peripheral blood contamination of a posttreatment bone marrow sample. An independent assessment of CFUe content showed 6,604 ± 357 CFUe/mL of bone marrow before treatment, at a time when none was seen in peripheral blood and 561,181 ± 147,411 CFUe/mL bone marrow after treatment, when only very rare CFUe were present in blood. However, given the absence of any effect of VLA4 on the proliferative status of progenitors and of cellularity changes in mice in which assessment of cellularity is more readily quantifiable, we believe that the increase in cellularity post–anti-VLA4 treatment reflects easiness of bone marrow aspiration rather than a proliferative change, similar to a situation concerning bone marrow changes post–anti-vascular cell adhesion molecule-1 (VCAM-1) treatment.37 Finally, assessment of cell cycling status of progenitors present in bone marrow or blood at baseline and after anti-VLA4 treatment showed no significant changes in bone marrow before versus after treatment. Cycling progenitors were in low proportion in blood before treatment compared with bone marrow (bone marrow suicide rate, 39% [298 of 490] and 31% [93 of 136]; peripheral blood, 15.6% [1.4 of 1.7] and 17.1% [5.6 of 6.8]). After treatment lower rates were observed but the differences were not statistically significant from pretreatment values (bone marrow, 19.4% [158 of 196] and 17.8% [111 of 135]; peripheral blood, 0% [26 of 21 and 13 of 8 for the two animals]).
Cytokine-Induced Mobilization Is Enhanced by Concomitant Anti-VLA4 Treatment
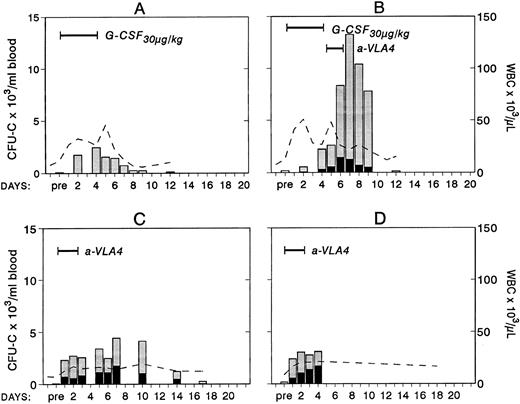
To study whether treatment with anti-VLA4 antibody alters the efficacy of G-CSF–induced mobilization, mice were treated simultaneously with G-CSF at 200 μg/kg (100 μg/kg twice daily) and either anti-VLA4 or PBS/BSA for 3 consecutive days (single daily injections). On the fourth day peripheral blood was collected by cardiac puncture and the numbers of CFU-C measured. Simultaneous treatment with G-CSF and anti-VLA4 increased significantly the number of circulating CFU-C above the level observed with G-CSF alone (Fig 1). G-CSF plus PBS/BSA treatments (5 mice) yielded 1,240 ± 842 CFU-C/mL. G-CSF plus anti-VLA4 yielded 5,012 ± 1,031/mL of blood (P < .0002). Likewise, anti-VLA4 augmented G-CSF–induced mobilization of CFU-S12 into blood as 5 mice treated with G-CSF plus PBS/BSA had 160 CFU-S12 ± 3.1/mL of blood, whereas mice treated with G-CSF plus anti-VLA4 had 664 CFU-S12 ± 39/mL (Fig 1; P < .0002). Additional limited data from primate G-CSF and anti-VLA4 treatments are in accord with the murine data. Eight baboons treated previously with G-CSF (100 μg/kg/d for 5 days) had an average peak of 8,405 ± 3,024/mL mobilized CFU-C between days 5 and 7.33 One animal treated with 30 μg/kg/d G-CSF showed 2,579 ± 134/mL at peak time, whereas another animal treated also with 30 μg/kg G-CSF followed by anti-VLA4 for the next 2 days had a peak level of 13,255 ± 515/mL after discontinuation of G-CSF treatment (Fig 2). Although only one animal each was treated with this scheme, which may not be an optimal one, it is important to note that at a time when a decline in circulating progenitors is expected post–G-CSF treatment, significantly high numbers of CFU-C were seen (at days 7 to 9 CFU-C/mL were 13,255 to 7,800/mL) higher than or similar to the average peak values of CFU-C noted in eight animals treated with higher doses of G-CSF alone.33 To test whether cooperativity in G-CSF+ anti-VLA4–induced mobilization concerned only this particular cytokine, we combined in subsequent experiments KL-induced mobilization with concurrent anti-VLA4 treatment. Control mice (five mice) were treated with KL (100 μg/kg) for 7 days and received in addition PBS/BSA for 7 days. Another group of five mice received KL in the same dose and anti-VLA4 (2 mg/kg/d) on the last 3 days of KL treatment. Because the peak of KL-induced mobilization is slightly longer than the G-CSF,31 the mice were treated for 7 days to ensure that peak values were not missed. On the eighth day the mice were sacrificed, peripheral blood was obtained by cardiac puncture, and the numbers of CFU-C and CFU-S12 were assessed. In the mice receiving combined KL and anti-VLA4 treatment, ninefold as many circulating CFU-C were present compared with mice treated with only KL and PBS/BSA (664 ± 133 in controls; 6,132 ± 812 in KL plus anti-VLA4; P < .003). A difference of similar magnitude was observed when CFU-S12 were evaluated from the two groups of animals (eight mice with KL, 45 ± 10.5 CFU-S/mL; eight mice with KL plus anti-VLA4, 359 ± 32 CFU-S/mL; P < .000015).
Four groups of mice (5 each) were treated with either G-CSF (200 μg/kg/d or KL (100 μg/kg/d) for 3 days or with combinations of G-CSF plus anti-VLA4 or KL plus anti-VLA4 again for 3 days; anti-VLA4 was given intravenously at 1 mg/kg/d). The combination treatments led to significant enhancement of mobilization of both CFU-C and CFU-S12 compared with cytokine-only–treated controls or anti-VLA4 alone–treated controls (anti-VLA4 alone gives 200 to 900 CFU-C/mL depending on the strain of mice, see also Fig 4).
Four groups of mice (5 each) were treated with either G-CSF (200 μg/kg/d or KL (100 μg/kg/d) for 3 days or with combinations of G-CSF plus anti-VLA4 or KL plus anti-VLA4 again for 3 days; anti-VLA4 was given intravenously at 1 mg/kg/d). The combination treatments led to significant enhancement of mobilization of both CFU-C and CFU-S12 compared with cytokine-only–treated controls or anti-VLA4 alone–treated controls (anti-VLA4 alone gives 200 to 900 CFU-C/mL depending on the strain of mice, see also Fig 4).
Changes in white blood cells (- -) and in circulating clonogenic progenitors (CFU-C/mL blood) in four treated nonhuman primates. Animal A was given G-CSF (30 μg/kg/d for 5 days) and animal B was given G-CSF (30 μg/kg/d for 5 days) followed by a humanized anti-VLA4 antibody (1 mg/kg/d for 2 days). Animals C and D were given only the humanized anti-VLA4 (1 mg/kg/d for 3 days) BFUe (▪) and nonerythroid () colonies.
Changes in white blood cells (- -) and in circulating clonogenic progenitors (CFU-C/mL blood) in four treated nonhuman primates. Animal A was given G-CSF (30 μg/kg/d for 5 days) and animal B was given G-CSF (30 μg/kg/d for 5 days) followed by a humanized anti-VLA4 antibody (1 mg/kg/d for 2 days). Animals C and D were given only the humanized anti-VLA4 (1 mg/kg/d for 3 days) BFUe (▪) and nonerythroid () colonies.
Mobilization Induced by Combinations of Cytokines Is Also Augmented by Anti-VLA4 Treatment
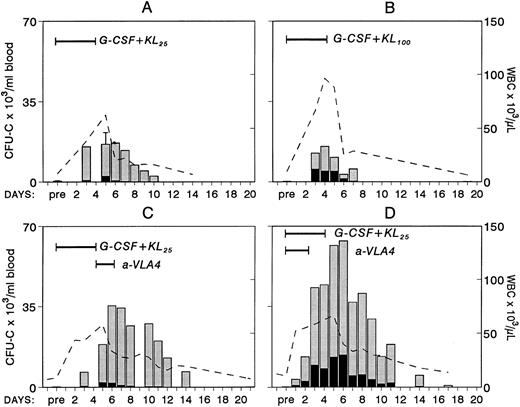
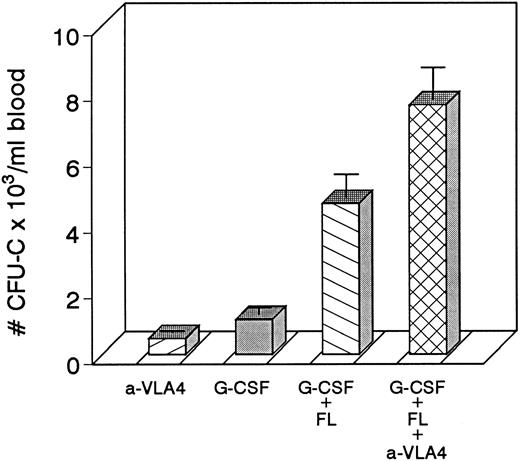
Because combinations of cytokines synergistically induce mobilization of progenitors at higher levels, we tested whether the most successful combination treatments, ie, G-CSF plus KL31,34-35 or G-CSF plus FL,33 when combined with concurrent treatment with anti-VLA4 were able to further enhance optimum mobilization. The G-CSF plus KL combination was given to four baboons at a dosage of 100 μg/kg/d G-CSF plus 25 μg/kg/d KL for 5 days, and one animal was treated with 100 μg/kg/d G-CSF and 100 μg/kg/d KL both for 5 days. G-CSF plus FL combinations were tested in mice. Control groups of mice with G-CSF alone and G-CSF plus FL were also studied. As seen in Fig 3, anti-VLA4 treatment significantly enhanced mobilization induced by G plus KL in baboons or by G plus FL treatments in mice (Fig 4). Concurrent anti-VLA4 treatments with cytokines rather than sequential seemed to be more effective (Fig 3C and D).
Changes in white blood cells (- -) and in circulating clonogenic progenitors (CFU-C/mL blood) in nonhuman primates treated with combinations of G-CSF and KL. In panel A, results from four animals treated with G-CSF (100 μg/kg/d for 5 days) and KL (25 μg/kg/d for 5 days) are shown. (Pretreatment and day-5 values were available from all four animals and only these values have error bars.) One animal (B) was treated with the same dose of G-CSF but with KL 100 μg/kg/d for 5 days. The animal in panel C was given anti-VLA4 antibody (1 mg/kg/d) for 2 days after G-CSF plus KL treatment (like animal A in Fig 2), whereas the animal in panel D was given G-CSF plus KL (like animal A) but for the first 3 days of treatment was also given anti-VLA4.
Changes in white blood cells (- -) and in circulating clonogenic progenitors (CFU-C/mL blood) in nonhuman primates treated with combinations of G-CSF and KL. In panel A, results from four animals treated with G-CSF (100 μg/kg/d for 5 days) and KL (25 μg/kg/d for 5 days) are shown. (Pretreatment and day-5 values were available from all four animals and only these values have error bars.) One animal (B) was treated with the same dose of G-CSF but with KL 100 μg/kg/d for 5 days. The animal in panel C was given anti-VLA4 antibody (1 mg/kg/d) for 2 days after G-CSF plus KL treatment (like animal A in Fig 2), whereas the animal in panel D was given G-CSF plus KL (like animal A) but for the first 3 days of treatment was also given anti-VLA4.
Four groups of mice (5 each) were treated with either anti-VLA4 only, G-CSF only, G-CSF plus FL, or with G-CSF plus FL plus anti-VLA4 at doses indicated in the Methods. Each treatment was given for 3 days. Significant enhancement of mobilization was seen in the last group of mice.
Four groups of mice (5 each) were treated with either anti-VLA4 only, G-CSF only, G-CSF plus FL, or with G-CSF plus FL plus anti-VLA4 at doses indicated in the Methods. Each treatment was given for 3 days. Significant enhancement of mobilization was seen in the last group of mice.
Post-5FU Mobilization in Primates and Anti-VLA4 Treatment
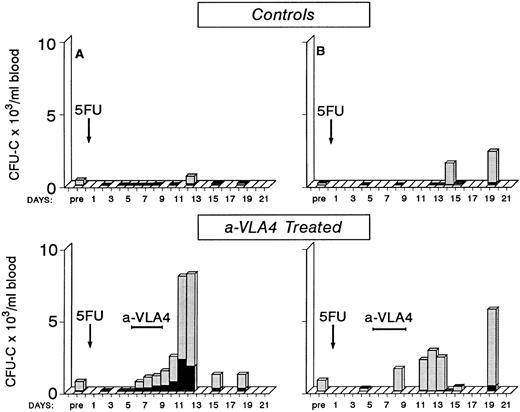
Early observations have suggested that mobilization of progenitors postcytotoxic chemotherapy is observed during the recovery phase4 38-41 and mobilization efficiency varies with different chemotherapy agents. Whether the cytotoxic treatment damages progenitor/stromal cell relationships directly or whether indirect effects (ie, an increase in cytokines) are responsible for this mobilization is unclear. To test whether anti-VLA4 could enhance not only cytokine-induced mobilization but also the postchemotherapy-induced mobilization, we treated two baboons with 5FU (single injection of 100 mg/kg 5FU) and followed their blood counts and circulating progenitors for approximately 3 weeks post-5FU. One animal showed a significant number of circulating progenitors at day 14 and 19 (10-fold to 20-fold above pretreatment values) after several days of 0 to 20 CFU-C/mL of blood; the other animal showed increased numbers at day 12 (Fig 5). Two other primates were treated with the same dose of 5FU but at days 5 to 8 were given three injections of anti-VLA4 (1 mg/kg/d). As seen in Fig 5, in these two animals there are several days with significant output of CFU-C during and after anti-VLA4 treatment in contrast to control 5FU animals. Peak levels of 5,667 CFU-C/mL of blood and 8,020 CFU-C/mL were observed compared with 635/mL and 2,331/mL in the two 5FU controls. In addition to CFU-C, macroscopically visible colonies of >0.5 mm in diameter of compact growth (HPP) were seen in all 5FU-treated animals. Peak HPP levels in two control animals were 118 ± 11 and 116 ± 6 at days 12 and 14, respectively. Peak levels in the two anti-VLA4–treated animals were 433 ± 96 and 932 ± 179 at days 13 and 11, respectively. Bone marrow was aspirated from one control 5FU and one 5FU plus anti-VLA4–treated animal once before treatment and on three occasions posttreatment. In the latter animal there was a sharp increase in CFU-C and HPP/mL of bone marrow aspirate between days 8 and 11 (from 2,921 ± 152 to 24,990 ± 1,225 CFU-C/mL and from 267 ± 18 to 2,254 HPP/mL). Concomitantly, CFU-C (see Fig 5) and HPP values in peripheral blood were increased between days 8 to 12. Similar observations were made in the control animal (data not shown). These data suggest that there is no significant delay between bone marrow progenitor recovery and their exportation to peripheral blood.
Four baboons were given a single injection of 5FU (100 mg/kg) and blood counts and circulating clonogenic progenitors were monitored for approximately 3 weeks posttreatment. Two of the animals received between days 5 to 8 a total of four injections of anti-VLA4. Note that in the animals receiving anti-VLA4 there are several days with a significant number of circulating progenitors in contrast with control 5FU animals.
Four baboons were given a single injection of 5FU (100 mg/kg) and blood counts and circulating clonogenic progenitors were monitored for approximately 3 weeks posttreatment. Two of the animals received between days 5 to 8 a total of four injections of anti-VLA4. Note that in the animals receiving anti-VLA4 there are several days with a significant number of circulating progenitors in contrast with control 5FU animals.
Transplantation of Ex Vivo Anti-VLA4–Treated Autologous Primate Bone Marrow Cells
As shown previously, anti-VLA4 treatment induces mobilization not only of committed clonogenic progenitors but of long-term repopulating cells and enhances mobilization induced by a single cytokine or by a combination of cytokines. In view of our previous data suggesting an impairment of anti-VLA4 on lodgement of intravenously infused cells in bone marrow, we needed to ascertain whether anti-VLA4 treatment compromised engraftment of properly conditioned recipients by interfering with homing.18 Therefore, we transplanted two baboons shortly after lethal irradiation with autologous bone marrow cells treated in vitro with anti-VLA4 and infused without washing off the excess antibody. The kinetics of total white blood cell count recovery as well as of platelet recovery are shown in Fig 6. White blood cells were over 1,000 by day 15 and platelets were over 100,000 by day 18. This recovery rate appears to be comparable with previously observed rates with autologous bone marrow transplantation.35 In addition to white blood cell counts, circulating clonogenic progenitors 24 to 48 hours after transplantation and for the next 10 days were also assessed to see whether a large circulating pool of progenitors was present because of the administration of anti-VLA4. The data show that for the first 3 days, significant numbers of CFU-C were present in the peripheral blood of the irradiated recipients. Similar early data are not available for other transplanted animals for comparison. However, recent data from human transplants (with mobilized blood) showing virtual clearance of injected CD34+ progenitors by 8 hours postinfusion (John Di Persio, personal communication) would suggest that the levels observed are likely higher than expected. In the murine model <0.5% of injected CFU-C are expected to remain in the peripheral blood by 48 hours (Papayannopoulou et al18 and unpublished data). In the present experiments we estimated (based on the total number of infused CFU-C) that 5% and 7% of injected CFU-C were in circulation. However, it is not clear whether the murine data are applicable to primate data because a significant seeding is occurring in the spleen of mice, which is not expected to occur in the spleen of primates. If indeed there is a delayed homing for the first few days because of excess anti-VLA4 antibody in circulation, this will imply that our prior data on anti-VLA4 effects on murine bone marrow homing are applicable to primates and possibly humans. However, despite an initial delay it is important to emphasize that engraftment was not ultimately compromised in this setting.
Autologous bone marrow transplantation in two baboons. Before infusion, bone marrow cells were treated with anti-VLA4 antibody, the cells were infused without washing, and blood samples were taken at various points thereafter to evaluate blood counts and circulating clonogenic progenitors. Note that prompt engraftment occurred in both animals compared with historical controls. (One of the animals later died from a presumed viral infection, whereas the other animal remained healthy beyond 2 years from transplantation.)
Autologous bone marrow transplantation in two baboons. Before infusion, bone marrow cells were treated with anti-VLA4 antibody, the cells were infused without washing, and blood samples were taken at various points thereafter to evaluate blood counts and circulating clonogenic progenitors. Note that prompt engraftment occurred in both animals compared with historical controls. (One of the animals later died from a presumed viral infection, whereas the other animal remained healthy beyond 2 years from transplantation.)
DISCUSSION
In previous studies we have shown that treatment of primates17 or mice18 with function blocking antibodies to VLA4 can induce peripheralization of hematopoietic progenitor cells. A nonfunction blocking anti-VLA4 antibody (B5G10) was without effect,42 and the half-life of the antibodies used correlated with the duration of the mobilizing effect. Thus, primates treated with a humanized antibody having a longer half-life in circulation showed a longer lasting effect (Fig 2 and Dr R.R. Lobb, personal communication). Furthermore, in the present study we show that not only committed progenitors are mobilized by these treatments but also long-term repopulating cells. This is in accord with previous observations showing that CFU-S (and very likely, long-term repopulating cells) express VLA4 integrins.43 It is important to emphasize that late progenitor cells, like CFUe, or precursor cells at different levels of maturation, despite the fact that they all express VLA4 integrins on their surface,44 are not mobilized by these treatments. Only rare CFUe and a small number (up to 1,000/mL) of nucleated red blood cells were present in blood (Fig 7), implying that the integrins in these later cells are either constitutively nonfunctional or differently regulated than the ones in progenitor cells. We found no evidence that anti-VLA4–induced mobilization is secondary to effects on progenitor proliferation. Instead, we think that the antibody's adhesion-blocking property is directly involved in the mobilization process, especially when administration of a nonfunction blocking antibody (B5G10) did not have a mobilizing effect.42 Whether adhesion blockade per se and/or de-adhesion are sufficient for mobilization, or whether a sequence of events, which includes stimulation of migration secondary to inside-out signaling, is also triggered by antibody treatment and is responsible for mobilization is unclear. Furthermore, because anti–VCAM-1 antibody also induces mobilization, whereas a fibronectin connecting segment-1 competitor peptide does not,45 VCAM-1 rather than fibronectin is the likely partner in vivo for VLA4 on progenitors. In addition, our data with anti–VCAM-1 provide a direct demonstration that effects on stromal cells can cause progenitor mobilization.
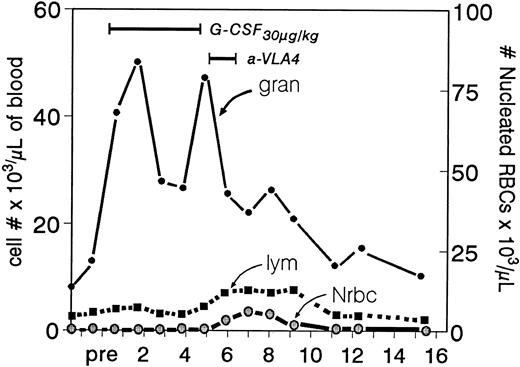
Ranges in peripheral white blood cells (granulocytes and lymphocytes) after the sequential use of G-CSF and anti-VLA4 (animal B in Fig 2). Note that during the anti-VLA4 treatment despite the expected significant drop in granulocytes (after cessation of G-CSF ) there is a small peak in lymphocytes (the characteristic response in anti-VLA4 treatment) and an even smaller peak of nucleated red blood cells. The latter is virtually unmeasurable in treatments with anti-VLA4 alone.
Ranges in peripheral white blood cells (granulocytes and lymphocytes) after the sequential use of G-CSF and anti-VLA4 (animal B in Fig 2). Note that during the anti-VLA4 treatment despite the expected significant drop in granulocytes (after cessation of G-CSF ) there is a small peak in lymphocytes (the characteristic response in anti-VLA4 treatment) and an even smaller peak of nucleated red blood cells. The latter is virtually unmeasurable in treatments with anti-VLA4 alone.
The central observation in the present paper is that mobilization, induced either by a single cytokine or a combination of cytokines, can be enhanced by concurrent treatment with anti-VLA4 integrin. This observation is of particular interest, because modulation of integrin function has been considered one of the leading mechanisms in cytokine-induced mobilization.3 Indeed, a great deal of attention has been given to the expression of cytoadhesion molecules on mobilized progenitor cells. Thus, β1 and β2 integrins have been tested (mostly post–G-CSF mobilization) and found to be at lower levels of expression in mobilized cells compared with steady-state bone marrow CD34+ cells (To et al3 and references therein). The functional status of integrins in mobilized cells has been tested on fewer occasions, and there is disagreement among investigators.24,46,47 Whether a decrease in expression of the integrins or a decrease in their functional activity is a direct primary event or a secondary event induced by G-CSF is not clear. It is not known whether cells mobilized with other cytokines, ie, FL, KL, IL-7, or IL-8, display the same functional characteristics as G-CSF–mobilized cells. In addition to decreased expression and/or function of integrins in progenitor cells (CD34+ cells), a consistent decrease in the expression of c-kit in mobilized CD34+ cells by a variety of mobilization schemes25,26,48 has been noted. Certainly, modulation of integrin function by cytokines, especially KL, has been clearly shown in vitro using cell lines as target cells.19,21 These in vitro observations are intriguing, but a testable in vivo approach for these concepts has yet to be formulated. For example, it was suggested that the decrease of c-kit in mobilized cells is caused by an initial increased expression of KL within the marrow microenvironment, which subsequently causes downmodulation of c-kit, leading concurrently to increased adhesion and migration of progenitor cells.26,48 Certainly there is a complex association between adhesion and migration,49 and continuous administration of cytokines may cause a series of dynamic changes in vivo affecting both stromal cells and/or progenitor cells, but these are difficult to follow in vivo. Our knowledge of mechanisms in general, which influence both ingress and egress of hematopoietic progenitors or more mature cells from the bone marrow, is extremely limited at present.
Within the context of the above considerations, What is the cooperativity between cytokines and abrogation of integrin function telling us? If one accepts that postcytokine-induced mobilization is mediated through downmodulation of integrin function, then anti-integrin antibody treatment could provide a more complete abrogation of cytoadhesion function and thereby enhance mobilization. As cooperativity was observed with all combinations of cytokines, this thesis would imply that all cytokines tested (ie, G-CSF, KL, and FL) work through the same mechanism, ie, through β1 integrin downmodulation. However, this has not been shown experimentally for other treatments aside from G-CSF treatments. Conceivably cytokines and integrins could also synergize through other, as yet undiscovered pathways leading to mobilization. For example, a synergistic effect of cytokines and anti-integrin treatment on migration of progenitor cells from the marrow could account for the enhanced mobilization. Or one could suggest that two different mechanisms are at play and that this phenomenon is additive. According to this scenario, anti-integrin mobilizes only a fraction of cells from the bone marrow. After cytokine treatment this fraction of target cells is expanded and is affected by anti-integrin treatment. This reasoning will presume that all cytokines tested exert a proliferative expansion of progenitors in the bone marrow, a more or less expected consequence of cytokine treatment. A less exaggerated mobilization was noted in the 5FU model during the ongoing regeneration of progenitor pools likely compatible with limited progenitor content in bone marrow postchemotherapy. It is of note that anti-VLA4 did not modify the kinetics of progenitor mobilization post-5FU but only the amplitude of mobilizable progenitors (Fig 5). The additive effect of anti-VLA4 is favored by the fact that enhancement was observed with at least three different cytokines and their combinations, as well as postchemotherapy. Whatever the mechanism of cooperativity, our data do suggest that anti-VLA4 can affect cytokine-primed cells and make it unlikely that the mechanism of anti-VLA4 mobilization as a single treatment is mediated in part or in total by secondary elaboration of hematopoietic cytokines.17 That being the case, enhancement would not have been expected with the combined treatment. To understand the mechanism of cytokine and anti-integrin cooperativity, further experiments are warranted, especially using appropriate murine models.
Although the mechanism of cooperative enhancement in mobilization between cytokines and anti-integrins is at present unclear, one can propose that such an observation can be exploited for clinical purposes, especially in a subgroup of patients in whom more efficient mobilization may be needed. However, this will only be useful if anti-integrins also mobilize long-term repopulating cells and if they do not interfere with homing and engraftment of transplanted progenitors. The transplantation experiments performed in mice assured us that long-term repopulating cells are also mobilized and provide long-term engraftment. Transplantation experiments in baboons further point out that engraftment was not compromised or delayed by the infusion of anti-VLA4–treated autologous bone marrow cells. It is of interest that in the first 2 to 3 days posttransplantation, a transient delay in homing of infused progenitors may have occurred because a substantial amount of infused progenitors were circulating the first 3 days. This observation, if true, illustrates that mechanisms of homing or components of it are regulated by VLA4/VCAM-1 pathway in primates, confirming the murine data.18
ACKNOWLEDGMENT
We are grateful to Debra Glanister and Ray Colby for their help with the primate studies. We are also indebted to Dr Roy R. Lobb for the generous gift of antibodies, Dr M. Hemler for the B5G10 antibody, and to Sherri Brenner for her skillful secretarial assistance.
Supported by National Institutes of Health Grants No. HL46557, HL35191, HL54881, and RR00166.
Address reprint requests to Thalia Papayannopoulou, MD, Box 357710, Hematology, University of Washington, Seattle, WA 98195.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal