Abstract
von Willebrand factor (vWF ) in the presence of botrocetin induces p72syk activation, assessed as its autophosphorylated level and in vitro kinase assays, the transient association of p72syk with p60c-src, and the translocation of p60c-src and p54/58lyn to cytoskeletal fractions. Jararaca glycoprotein Ib-binding protein (GPIb-BP), which specifically binds to GPIb, abolished these phenomena, suggesting that they are mediated by the vWF-GPIb interaction. These tyrosine kinase-related events were not inhibited by GRGDS peptide (plus EGTA), indicating that GPIIb/IIIa is not involved in the observed responses. Shc, an adaptor protein, was also tyrosine phosphorylated by the botrocetin-vWF activation. When GPIb was immunoprecipitated with nonfunctional monoclonal antibodies (MoAbs) directed against GPIb, a kinase activity was found to associate with GPIb upon botrocetin-vWF activation. On the other hand, anti-GPIb MoAbs that inhibit the vWF-GPIb interaction did not coprecipitate a kinase activity. Because the recovery of GPIb did not differ significantly, it is suggested that the excessive presence of inhibitory anti-GPIb MoAb dissociated a kinase activity from GPIb. Phosphoamino acid analysis showed that the kinase activity was that of a tyrosine kinase. The identity of the tyrosine kinase and the mode of interaction with the cytoplasmic region of GPIb await to be determined. Our findings suggest that the tyrosine kinase associated with GPIb serves at a most proximal step in the signal transduction pathway involved in the vWF-GPIb-induced platelet activation, which leads to other tyrosine kinase-related intracellular signals.
THROMBUS FORMATION is initiated by platelet adhesion to the sites of vascular injuries. The sequence of these responses involves the exposure of subendothelial extracellular matrices and the interaction between von Willebrand factor (vWF )1,2 and a platelet membrane receptor, glycoprotein Ib (GPIb).3 Human vWF serves to bridge the constituents of subendothelium to GPIb on the membrane of circulating platelets. The role of the vWF-GPIb interaction in platelet activation is particularly important when the hemodynamic condition creates high shear stress at the sites of arterial occlusion.4 Whereas much is known about the molecular bases of the interaction between vWF and GPIb, little has been clarified about the intracellular signal transduction pathway in platelet activation induced by the vWF-GPIb interaction. The role of intracellular Ca2+ elevation as a secondary mediator has been suggested for the ristocetin-induced GPIb-vWF interaction and shear-induced platelet aggregation.5-7 Protein kinase C activation also occurs in GPIb-mediated platelet activation.8 However, as shown for other cells, these two signal transduction pathways cannot totally explain the activation mechanism involved in the vWF-GPIb interaction.
Recently, an accumulating body of evidence has suggested that protein tyrosine phosphorylation plays an important role in intracellular signal transduction, especially in cell growth, cell-cell, and cell-matrix adhesive interactions. Platelets contain high activity of tyrosine kinases. All platelet tyrosine kinases reported to date are nonreceptor types, with p60c-src being the most abundant.9,10 In addition to p60c-src, several tyrosine kinases have been identified, such as p54/58lyn, p59fyn, and p62yes, which are related to the src family, and other structurally unrelated kinases such as p72syk and p125FAK.11,12 Various agonists induce the appearance of a number of tyrosine-phosphorylated proteins, and activation of p72syk, p60c-src, and p125FAK occurs with distinct time courses. p72syk activation occurs immediately after platelet activation, whereas p125FAK is only activated when platelets are highly aggregated.13 A wide range of platelet functions, including intracellular Ca2+ mobilization and GPIIb/IIIa activation, appear to be regulated by tyrosine kinase activity. These lines of evidence suggest that protein-tyrosine phosphorylation and tyrosine kinase activation are closely related to the regulation of platelet function, ranging from the initial phase of activation to the late stage of aggregation.
On the other hand, there have been only a few reports on the involvement of protein-tyrosine phosphorylation in the GPIb-vWF interaction. Platelet activation mediated by the vWF-GPIb interaction resulted in protein-tyrosine phosphorylation of a 64-kD protein.14 Razdan et al15 and Oda et al16 have shown that shear stress causes a time-dependent appearance of tyrosine-phosphorylated proteins in human platelets. p60c-src translocates to cytoskeleton upon platelet activation induced by the GPIb-vWF interaction.17 However, to the best of our knowledge, there has been no report on the identification of specific tyrosine-phosphorylated proteins or the activation of a specific tyrosine kinase(s) induced by the vWF-GPIb interaction. In a preliminary study, we found that ristocetin, a widely used agent for facilitating the vWF-GPIb interaction, nonspecifically interferes with tyrosine kinase activity. This adverse effect of ristocetin on tyrosine kinases may be a factor that has precluded the evaluation of tyrosine kinase activity in platelet activation induced by the vWF-GPIb interaction. To circumvent this hindrance, we evaluated the effects of a GPIb-interacting agent, botrocetin. Botrocetin, purified from the Bothrops Jararaca venom, induces platelet agglutination by facilitating the binding of vWF to GPIb,18 similar to ristocetin, and the site of action at the vWF molecule has also been determined.19
In the present study, we present several lines of evidence to suggest that tyrosine kinases are involved in the GPIb-vWF–induced intracellular signals and that a tyrosine kinase activity associates with GPIb upon the vWF-GPIb interaction, which can be coimmunoprecipitated with nonfunctional anti-GPIb MoAbs, but not by inhibitory anti-GPIb MoAbs.
MATERIALS AND METHODS
Materials. Monoclonal antibodies (MoAbs) against p72syk , p54/58lyn, and leupeptin were obtained from Wako Pure Chemical Industries, Ltd (Tokyo, Japan). Anti-p60c-src MoAb (GD11), anti-phosphotyrosine MoAb (4G10), and anti-p42mapk MoAb were purchased from Upstate Biotechnology, Inc (Lake Placid, NY). Gly-Arg-Gly-Asp-Ser (GRGDS) peptide was obtained from Peptide Institute (Osaka, Japan). Anti-Shc polyclonal Ab was obtained from Transduction Laboratories (Lexington, KY). Acetylsalicylic acid, enolase, bovine serum albumin (BSA), prostaglandin I2 (PGI2 ), phenylmethylsulfonyl fluoride (PMSF ), sodium orthovanadate, and Triton X-100 were from Sigma (St Louis, MO). Protein A-Sepharose was obtained from Pharmacia Japan (Tokyo, Japan). Peroxidase-conjugated goat antimouse Ig (IgG) was from Cappel Organ Teknika Co (Durham, NY). vWF was purified as previously described.20 The crude venom of Bothrops jararaca was purchased from Sigma, and the “two-chain” botrocetin and jararaca GPIb-BP were highly purified, based on the method of Fujimura et al.21,22 A nonfunctional anti-GPIb MoAb, WGA-3, and an inhibitory anti-GPIb MoAb, GUR83-35, which interferes with the binding of vWF to GPIb, were provided from Dr M. Handa (Keio University, Tokyo, Japan). Both antibodies reacted with the NH2-terminal 45-kD trypsin fragment of GPIbα subunit as determined by the previously described method using solid-phase immunoisolation technique.23 Another nonfunctional anti-GPIb MoAb, GS-70, which also reacts with GPIbα (unpublished data), was a generous gift from Dr N. Yamamoto (Tokyo Metropolitan Institute of Medical Science, Tokyo, Japan). NNKY5-5, an anti-GPIb MoAb that inhibits the binding of vWF to GPIb, was provided by Dr S. Nomura (Kansai Medical University, Osaka, Japan).
Platelet preparation. Venous blood from healthy drug-free volunteers was collected into a tube containing acid-citrate-dextrose. Platelet-rich plasma obtained after centrifugation of whole blood at 160g for 10 minutes was incubated with 1 mmol/L acetylsalicylic acid (ASA) for 30 minutes to exclude the secondary effects of thromboxane A2 (TXA2 ), unless otherwise stated. The platelets were washed and resuspended in HEPES buffer containing 138 mmol/L NaCl, 3.3 mmol/L NaH2PO4 , 2.9 mmol/L KCl, 1.0 mmol/L MgCl2 , 1 mg/mL of glucose, and 20 mmol/L HEPES (pH 7.4) at a concentration of 109 cells/mL. Thirty minutes before experiments were performed, the platelet suspension was supplemented with 1 mmol/L CaCl2 , unless otherwise stated.
Platelet aggregation. Washed platelets were activated with 10 μg/mL of vWF and 3 μg/mL of botrocetin under continuous stirring at 1,000 rpm for the indicated periods in an AA-100 platelet aggregation analyzer (Sysmex, Kobe, Japan). The instrument was calibrated with a platelet suspension for zero light transmission and with buffer for 100% transmission.
Identification of phosphotyrosine-containing proteins by immunoblotting. After platelet activation, reactions were terminated by adding Laemmli sodium dodecyl sulfate (SDS) reducing buffer24 plus 10 mmol/L NaVO4 , 10 mmol/L ethylenediamine tetraacetic acid (EDTA), and 1 mmol/L PMSF, followed by boiling for 3 minutes. Platelet proteins were separated by 8% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and electrophoretically transferred onto Clear Blot Membrane-P (Atto, Tokyo, Japan). The membranes were blocked with 1% BSA in phosphate-buffered saline (PBS). After extensive washing with PBS containing 0.1% Tween 80, the immunoblots were incubated for 2 hours with the indicated antibody. Antibody binding was detected using peroxidase-conjugated goat antimouse IgG and visualized with ECL chemiluminescence reaction reagents (Amersham, UK) and Konica X-ray film (JX 8 × 10; Konica Co, Tokyo, Japan).
Isolation of cytoskeletal fractions and immunoprecipitation kinase assay. After platelets were activated with vWF-botrocetin for the indicated period, reactions were terminated with an equal volume of ice-cold lysis buffer (2% Triton X-100, 100 mmol/L Tris/HCl, pH 7.5, 50 mmol/L NaCl, 5 mmol/L EDTA, 2 mmol/L vanadate, 1 mmol/L PMSF, and 100 μg/mL of leupeptin). The lysate was sonicated and centrifuged at 16,000g for 5 minutes. The pellet included Triton X-100-insoluble, cytoskeletal fractions. Finally, the Triton X-insoluble pellet was resuspended in Laemmli buffer25 and boiled for 3 minutes. Proteins present in this fraction were detected by Western blotting using MoAbs against various tyrosine kinases described above.
The supernatant was precleared with protein A sepharose beads for 30 minutes at 4°C and then mixed with specific antityrosine kinase MoAb or anti-GPIb MoAb. The mixture was rotated for 2 hours at 4°C and, after the addition of protein A sepharose beads, further rotated for 2 hours. The sepharose beads were washed three times with lysis buffer. The sample was then split into two portions. One was used for immunoblotting as described elsewhere, and the other was processed further for an in vitro kinase assay. The in vitro kinase assay was performed as previously described.26 The beads were washed once with HEPES buffer (10 mmol/L HEPES/NaOH, 1 mmol/L vanadate, pH 8.0) and then incubated with 25 μL of kinase reaction buffer (300 mmol/L HEPES/NaOH, 15 mmol/L MnCl2 , 150 mmol/L MgCl2 , pH 8.0) with 10 μg of acid-treated enolase. The reaction was initiated by the addition of 2 μmol ATP (10 μCi of [γ-32P]ATP). After 10 minutes at 20°C, reactions were stopped by the addition of Laemmli buffer and then subjected to boiling for 3 minutes. The proteins were separated under reducing conditions by 8% SDS-PAGE and transferred onto Clear Blot Membrane P. The membrane was treated with 1 mol/L KOH for 60 minutes, dried, and quantified with a BAS-2000 phosphor-image analyzer (Fuji Film, Tokyo, Japan).
Phosphoamino acid analysis. After the in vitro kinase reactions described above, the samples were applied on 8% SDS-PAGE. The 32P-labeled proteins corresponding to phosphorylated enolase and the phosphorylated 60-kD protein were excised from polyacrylamide gels and homogenized in 100 mmol/L NH4HCO3 containing 0.2% SDS, 5% 2-mercaptoethanol, and 20 μg/mL of ribonuclease A.27 The homogenate was incubated for 5 minutes in boiling water and extracted overnight at 37°C. Proteins were precipitated with 20% trichloroacetic acid. After washing in ice-cold aceton and drying, the proteins were dissolved in 5.7 mol/L HCl and hydrolyzed for 1 hour at 110°C. Supernatants were concentrated by centrifugation, mixed with carrier phosphoamino acids, and analyzed by two-dimensional electrophoresis (pH 1.9 followed by pH 3.5). After autoradiography, the 32P-labeled amino acids were identified by their comigration with the carrier proteins stained with ninhydrin.
Measurement of p42/44mapk activation. Measurement of mitogen-activated protein (MAP) kinase activity in platelet activation was performed as previously described.28 Briefly, platelet reactions were stopped with an equal volume of SDS sample buffer (2% SDS, 5 mmol/L EDTA, 10 mmol/L Tris, 0.5 mmol/L PMSF, pH 7.3). The samples were boiled for 5 minutes and diluted 40-fold with Tris-buffered saline (20 mmol/L Tris, 137 mmol/L NaCl, 0.1% Tween 20, pH 7.6) containing 2 mg/mL of BSA, 1 mmol/L PMSF, and 1 mmol/L EDTA. The platelet lysates were precleared with sepharose beads for 1 hour and then incubated overnight with 4 μg of anti-p42mapk MoAb and with protein A-sepharose CL-4B. The immunoprecipitates were resolved on 10% SDS-PAGE containing 0.5 mg/mL of myelin basic protein (MBP) in the gel. Proteins in gel were denatured with 6 mol/L guanidine HCl and then renatured for 16 hours at 4°C with 50 mmol/L Tris containing 5 mmol/L 2-mercaptoethanol and 0.04% Tween 20. For the kinase assay, the gels were incubated with kinase assay buffer (50 mmol/L Tris, 5 mmol/L MgCl2 , 1 mmol/L EGTA, 5 mmol/L dithiothreitol, 50 μmol/L ATP, and 200 μCi of [γ32P] ATP, pH 8.0) and washed with 50% trichloroacetic acid and 1% Na4P2O7 . Gels were then dried and the radioactivity was measured with a BAS-2000 phosphor-image analyzer.
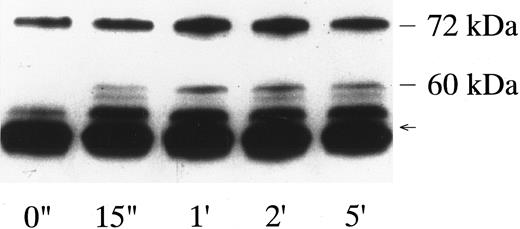
p72syk-associated tyrosine kinase activity induced by botrocetin-vWF. Platelets suspended in a buffer containing 1 mmol/L Ca2+ were activated with 3 μg/mL of botrocetin and 10 μg/mL of vWF for the indicated time intervals. Reactions were terminated with lysis buffer, and p72syk was isolated by immunoprecipitation with anti-p72syk MoAb. Immunoprecipitates were directly subjected to Western blotting using the antiphosphotyrosine MoAb, 4G10. The arrow represents the band presumably derived from IgG heavy chains. The data are representative of three experiments.
p72syk-associated tyrosine kinase activity induced by botrocetin-vWF. Platelets suspended in a buffer containing 1 mmol/L Ca2+ were activated with 3 μg/mL of botrocetin and 10 μg/mL of vWF for the indicated time intervals. Reactions were terminated with lysis buffer, and p72syk was isolated by immunoprecipitation with anti-p72syk MoAb. Immunoprecipitates were directly subjected to Western blotting using the antiphosphotyrosine MoAb, 4G10. The arrow represents the band presumably derived from IgG heavy chains. The data are representative of three experiments.
RESULTS
Platelet activation induced by the vWF-botrocetin interaction. Botrocetin, purified from the Bothrops jararaca snake venom, binds to vWF and forms an activated complex which binds to GPIb.29 The combination of 10 μg/mL of vWF and botrocetin in the concentration range between 0.3 and 20 μg/mL induced platelet agglutination/aggregation independent of extracellular Ca2+.14 Ristocetin, at concentrations ranging from 0.3 to 2.0 mg/mL, also induced platelet agglutination/aggregation in the presence of vWF.5,30 The magnitude of platelet aggregation induced by botrocetin, assessed as changes in light transmission, reached the maximum level at a concentration of 1 μg/mL, but it was less than that of 1 mg/mL of ristocetin. In experiments thereafter, the combination of 10 μg/mL of vWF and 3 μg/mL of botrocetin was used to activate platelets. Because vWF-botrocetin–mediated platelet agglutination/aggregation was accompanied by the appearance of the 64-kD tyrosine-phosphorylated protein,14 we then evaluated the changes in tyrosine kinase activity.
Tyrosine kinase activation induced by the vWF-botrocetin interaction. Botrocetin per se up to 20 μg/mL had no measurable effects on the tyrosine kinase activity, assessed as in vitro kinase assays of immunoprecipitated preparations of p72syk or p60c-src, whereas ristocetin, at concentrations of as low as 0.3 mg/mL, inhibited in vitro kinase assays of these tyrosine kinases (data not shown). Similarly, when platelets were activated by the combination of ristocetin and vWF, in vitro kinase assays of p72syk or p60c-src showed no kinase activity, and there were no changes related to platelet activation. These findings suggest that ristocetin nonspecifically interacts with tyrosine kinases, precluding the kinase activity measurement. Thus, in experiments thereafter, botrocetin, but not ristocetin, was used to facilitate the binding of vWF to GPIb.
When platelets were activated by the combination of 10 μg/mL vWF and 3 μg/mL of botrocetin, lysed, and immunoprecipitated with the corresponding antibodies, there was no measurable increase in the tyrosine kinase activity of p60c-src, p54/58lyn, or p125FAK (data not shown). On the other hand, vWF-botrocetin stimulation induced a twofold to threefold increase in the level of p72syk autophosphorylation, which peaked 15 to 60 seconds after stimulation and subsided to lower levels thereafter (Fig 1). Concomitant with the change in p72syk autophosphorylation, in vitro kinase assays showed that the tyrosine kinase activity, assessed by acid-treated enolase phosphorylation, transiently increased. Western blotting using antiphosphotyrosine MoAb, 4G10, also showed a transient appearance of 60-kD tyrosine-phosphorylated protein, which coprecipitated with anti-p72syk MoAb (Fig 1). Western blotting using anti-p60c-src MoAb showed that the 60-kD band was p60c-src (Fig 2), suggesting that p60c-src transiently associates with p72syk upon vWF-botrocetin–induced platelet activation. Anti-p59fyn MoAb or anti-p54/58lyn MoAb did not coprecipitate p60c-src (data not shown), suggesting that p60c-src specifically associates with p72syk upon vWF-botrocetin–induced activation. These phenomena were not observed when platelets were stimulated by botrocetin alone (data not shown).
Association of p60c-src with p72syk induced by botrocetin-vWF. Platelets were activated with 3 μg/mL of botrocetin and 10 μg/mL of vWF, and p72syk-associated proteins were isolated by immunoprecipitation with anti-p72syk MoAb. The sample was applied to SDS-PAGE and Western blotting using anti-p60c-src MoAb. The data are representative of three experiments.
Association of p60c-src with p72syk induced by botrocetin-vWF. Platelets were activated with 3 μg/mL of botrocetin and 10 μg/mL of vWF, and p72syk-associated proteins were isolated by immunoprecipitation with anti-p72syk MoAb. The sample was applied to SDS-PAGE and Western blotting using anti-p60c-src MoAb. The data are representative of three experiments.
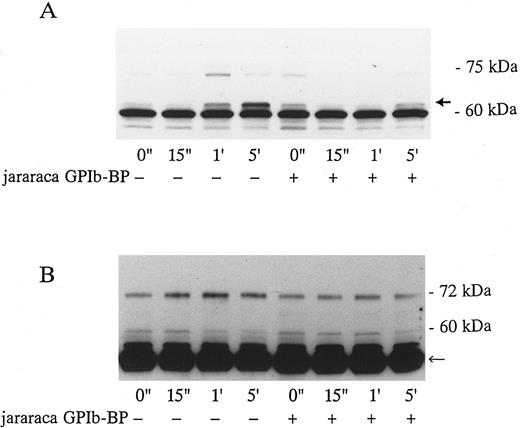
Effects of Jararaca GPIb-BP and GRGDS peptide on protein-tyrosine phosphorylation (PTP) and p72syk associated tyrosine kinase activity induced by vWF-botrocetin. Jararaca GPIb-BP is a GPIb-binding protein that was isolated from the snake venom of Bothrops jararaca.22 Jararaca GPIb-BP itself induces neither platelet aggregation nor serotonin release from platelets, and it completely inhibits vWF binding to GPIb in the presence of ristocetin or botrocetin.22 We confirmed that jararaca GPIb-BP by itself induces neither protein-tyrosine phosphorylation (PTP) nor p72syk activation and that it completely inhibits platelet agglutination/aggregation induced by the combination of vWF and botrocetin (data not shown). The effects of jararaca GPIb-BP were then evaluated on vWF-botrocetin–induced PTP and p72syk activation. vWF-botrocetin induced the appearance of 64-kD PTP 1 minute after stimulation.14 Occasionally, a faint 75-kD band of PTP was observed, but this was not always reproducible. Treatment of platelets with 10 μg/mL of jararaca GPIb-BP for 10 minutes completely abrogated the appearance of 64-kD PTP (Fig 3A). It also inhibited p72syk-associated tyrosine kinase activity induced by vWF-botrocetin (Fig 3B). These findings suggest that protein-tyrosine phosphorylation and p72syk activation induced by vWF-botrocetin is specifically mediated through the interaction between GPIb and vWF, which can be blocked by the binding of jararaca GPIb-BP to GPIb. It may be argued that vWF promotes platelet aggregate formation by binding to GPIIb/IIIa of activated platelets. Hence, we examined the effects of functional blockade of GPIIb/IIIa on p72syk activation. Pretreatment of platelets with 200 μmol/L GRGDS peptide plus 1 mmol/L EGTA, which blocks vWF binding to GPIIb/IIIa and fibrinogen binding to GPIIb/IIIa, completely inhibited thrombin-induced platelet aggregation, but had minimal effects on aggregation stimulated by vWF-botrocetin (data not shown). Under the same conditions, p72syk activation was not affected by 200 μmol/L GRGDS peptide (Fig 4). We had already shown that 64-kD PTP induced by vWF-botrocetin was not affected by GRGDS peptide.14 These observations suggest that p72syk activation induced by vWF-botrocetin is specifically mediated through the vWF-GPIb interaction.
Effects of jararaca GPIb-BP on PTP of whole cell lysates (A) and p72syk activation (B) induced by botrocetin-vWF. Platelets were incubated with or without 10 μg/mL of jararaca GPIb-BP for 5 minutes and then activated with 3 μg/mL of botrocetin and 10 μg/mL of vWF for the indicated time intervals. For PTP analysis, reactions were terminated with Laemmli SDS buffer, and samples were Western-blotted using antiphosphotyrosine MoAb, 4G10. For analysis of p72syk autophosphorylation, reactions were terminated by adding lysis buffer. p72syk was immunoprecipitated with anti-p72syk MoAb, and the sample was Western-blotted using antiphosphotyrosine MoAb, 4G10. (A) PTP in whole cell lysates. The arrowhead indicates 64-kD PTP. (B) p72syk autophosphorylation. The arrowhead represents the band presumably from IgG heavy chains. The data are representative of three experiments.
Effects of jararaca GPIb-BP on PTP of whole cell lysates (A) and p72syk activation (B) induced by botrocetin-vWF. Platelets were incubated with or without 10 μg/mL of jararaca GPIb-BP for 5 minutes and then activated with 3 μg/mL of botrocetin and 10 μg/mL of vWF for the indicated time intervals. For PTP analysis, reactions were terminated with Laemmli SDS buffer, and samples were Western-blotted using antiphosphotyrosine MoAb, 4G10. For analysis of p72syk autophosphorylation, reactions were terminated by adding lysis buffer. p72syk was immunoprecipitated with anti-p72syk MoAb, and the sample was Western-blotted using antiphosphotyrosine MoAb, 4G10. (A) PTP in whole cell lysates. The arrowhead indicates 64-kD PTP. (B) p72syk autophosphorylation. The arrowhead represents the band presumably from IgG heavy chains. The data are representative of three experiments.
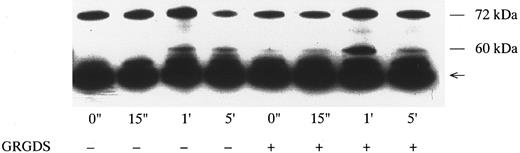
Effects of GRGDS peptide plus EGTA on p72syk-associated tyrosine kinase activity induced by botrocetin-vWF. Platelets were incubated with or without 200 μmol/L GRGDS peptide plus 1 mmol/L EGTA (instead of Ca2+) for 5 minutes and then activated with 3 μg/mL of botrocetin and 10 μg/mL of vWF for the indicated time intervals. For analysis of p72syk autophosphorylation, reactions were terminated by adding lysis buffer. p72syk was immunoprecipitated with anti-p72syk MoAb, and the sample was Western-blotted using antiphosphotyrosine MoAb, 4G10. The arrowhead represents the band presumably derived from IgG heavy chains. The data are representative of three experiments.
Effects of GRGDS peptide plus EGTA on p72syk-associated tyrosine kinase activity induced by botrocetin-vWF. Platelets were incubated with or without 200 μmol/L GRGDS peptide plus 1 mmol/L EGTA (instead of Ca2+) for 5 minutes and then activated with 3 μg/mL of botrocetin and 10 μg/mL of vWF for the indicated time intervals. For analysis of p72syk autophosphorylation, reactions were terminated by adding lysis buffer. p72syk was immunoprecipitated with anti-p72syk MoAb, and the sample was Western-blotted using antiphosphotyrosine MoAb, 4G10. The arrowhead represents the band presumably derived from IgG heavy chains. The data are representative of three experiments.
Tyrosine phosphorylation of Shc induced by vWF-botrocetin. Tyrosine phosphorylation of the 64-kD protein and p72syk activation induced by vWF-botrocetin suggests that tyrosine phosphorylation of signaling proteins is involved in the signal transduction pathway. Shc, which has an SH2 domain, serves as an adaptor between tyrosine phosphorylated proteins and their substrates, and its phosphorylation appears to play an important role in signal transduction in various cells.31-33 Thus, we sought to determine whether Shc is also tyrosine phosphorylated during platelet activation induced by the vWF-GPIb interaction. Immunoprecipitation with an anti-Shc antibody, followed by Western blotting with an antiphosphotyrosine MoAb, showed that Shc was heavily tyrosine-phosphorylated in platelets activated by vWF-botrocetin (Fig 5). In contrast, Shc tyrosine phosphorylation was only minimal in thrombin-activated platelets (data not shown). These findings suggest that vWF-GPIb–mediated platelet activation uses an Shc-related signal transduction pathway distinct from that of thrombin activation.
Time course of Shc tyrosine phosphorylation induced by botrocetin-vWF. Platelets were activated with 3 μg/mL of botrocetin and 10 μg/mL of vWF, and reactions were terminated by adding lysis buffer after the indicated time intervals. Shc was immunoprecipitated with anti-Shc polyclonal Ab. The sample was then Western-blotted using the antiphosphotyrosine MoAb, 4G10. The data are representative of three experiments.
Time course of Shc tyrosine phosphorylation induced by botrocetin-vWF. Platelets were activated with 3 μg/mL of botrocetin and 10 μg/mL of vWF, and reactions were terminated by adding lysis buffer after the indicated time intervals. Shc was immunoprecipitated with anti-Shc polyclonal Ab. The sample was then Western-blotted using the antiphosphotyrosine MoAb, 4G10. The data are representative of three experiments.
Failure of vWF-GPIb to activate MAP kinase. The sequential kinase cascade leading to the activation of MAP kinase lies downstream of Shc, Ras, and Sos. Platelets contain two isoforms of MAP kinase, 42mapk and p44mapk, and MAP kinase is activated in collagen- or thrombin-stimulated platelets.28 34 Because Shc is tyrosine-phosphorylated by the vWF-GPIb interaction, we investigated MAP kinase activation stimulated by botrocetin-vWF. There was little p42/44mapk activation in vWF-GPIb–stimulated platelets, whereas p42/44mapk was markedly activated in thrombin-induced platelet activation (Fig 6), suggesting that p42/44mapk activation does not contribute to the vWF-GPIb–induced signal transduction pathways.
Measurement of p42mapk and p44mapk induced by botrocetin-vWF. Platelets (109/mL) were stimulated with 1 U/mL of thrombin or 3 μg/mL of botrocetin and 10 μg/mL of vWF for the indicated time intervals, and reactions were terminated with an equal volume of SDS sample buffer. Samples were boiled for 5 minutes and diluted 40-fold with Tris-buffered saline. p42mapk was immunoprecipitated using anti-p42mapk MoAb and the immunoprecipitates were subjected to electrophoresis on 10% SDS-PAGE gels containing 0.5 mg/mL of MBP. The MAP kinase in gel was then renatured, and the gels were incubated with the kinase assay buffer containing [γ-32P]ATP. The autoradiograph shows the band of renatured p42mapk activity. The data are representative of three experiments.
Measurement of p42mapk and p44mapk induced by botrocetin-vWF. Platelets (109/mL) were stimulated with 1 U/mL of thrombin or 3 μg/mL of botrocetin and 10 μg/mL of vWF for the indicated time intervals, and reactions were terminated with an equal volume of SDS sample buffer. Samples were boiled for 5 minutes and diluted 40-fold with Tris-buffered saline. p42mapk was immunoprecipitated using anti-p42mapk MoAb and the immunoprecipitates were subjected to electrophoresis on 10% SDS-PAGE gels containing 0.5 mg/mL of MBP. The MAP kinase in gel was then renatured, and the gels were incubated with the kinase assay buffer containing [γ-32P]ATP. The autoradiograph shows the band of renatured p42mapk activity. The data are representative of three experiments.
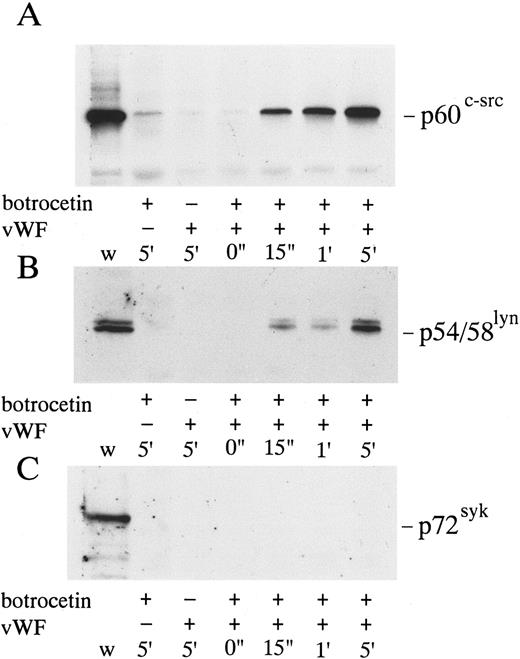
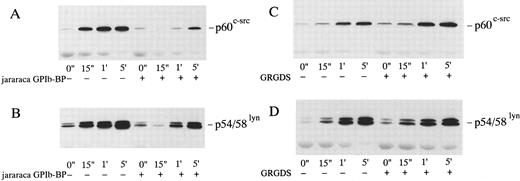
Association of p60c-src and p54/58lyn with Triton X-100–insoluble cytoskeletal fractions in platelets activated by vWF-botrocetin. Tyrosine kinases such as p60c-src and p72syk translocate to cytoskeletons upon platelet activation induced by various agonists.35-37 We therefore assessed the translocation of tyrosine kinases, p72syk, p60c-src, and p54/58lyn, to cytoskeletons during platelet activation induced by vWF-botrocetin. The addition of vWF or botrocetin alone did not cause the translocation of p72syk, p60c-src, and p54/58lyn to cytoskeletal fractions (Fig 7). vWF-botrocetin stimulation induced an 8.3- ± 0.8-fold (n = 3) and a 9.2- ± 1.1-fold (n = 3) increase of p60c-src and of p54/58lyn, respectively, associated with Triton X-100–insoluble cytoskeletal fractions, as compared with the resting state (Fig 7). Translocation of p60c-src and p54/58lyn to cytoskeletal fractions was delayed and diminished by 10 μg/mL of jararaca GPIb-BP (Fig 8A and B), but was not abolished by 200 μmol/L GRGDS peptide plus 1 mmol/L EGTA (Fig 8C and D). These findings suggest that translocation of tyrosine kinases to cytoskeletons induced by botrocetin-vWF is specifically mediated through the interaction between GPIb and vWF. In contrast, p72syk translocation to the Triton-X–insoluble cytoskeletons was not detectable (Fig 7).
Cytoskeletal association of p60c-src, p54/58lyn, and p72syk induced by botrocetin-vWF. Platelets were activated with 3 μg/mL of botrocetin and 10 μg/mL of vWF for the indicated time intervals. Reactions were then stopped with lysis buffer, and Triton-X–insoluble fractions were harvested by centrifugation at 16,000g for 5 minutes. The pellets were solubilized with Laemmli buffer. Cytoskeletal association of tyrosine kinases is detected by Western blotting using anti-p60c-src (A), anti-p54/58lyn (B), and anti-p72syk (C) MoAbs. w, whole cell lysates.
Cytoskeletal association of p60c-src, p54/58lyn, and p72syk induced by botrocetin-vWF. Platelets were activated with 3 μg/mL of botrocetin and 10 μg/mL of vWF for the indicated time intervals. Reactions were then stopped with lysis buffer, and Triton-X–insoluble fractions were harvested by centrifugation at 16,000g for 5 minutes. The pellets were solubilized with Laemmli buffer. Cytoskeletal association of tyrosine kinases is detected by Western blotting using anti-p60c-src (A), anti-p54/58lyn (B), and anti-p72syk (C) MoAbs. w, whole cell lysates.
Effects of jararaca GPIb-BP or GRGDS peptide plus EGTA on the cytoskeletal association of p60c-src and p54/58lyn induced by botrocetin-vWF. Platelets were incubated with or without 10 μg/mL of jararaca GPIb-BP (A and B) or with or without 200 μmol/L GRGDS peptide plus 1 mmol/L EGTA (instead of Ca2+) (C and D) for 5 minutes and then activated with 3 μg/mL of botrocetin and 10 μg/mL of vWF for the indicated time intervals. Cytoskeletal fractions were harvested as described in the legend for Fig 7. Immunoblot analysis of cytoskeletal fractions were performed with anti-p60c-src (A and C) and anti-p54/58lyn (B and D) MoAbs.
Effects of jararaca GPIb-BP or GRGDS peptide plus EGTA on the cytoskeletal association of p60c-src and p54/58lyn induced by botrocetin-vWF. Platelets were incubated with or without 10 μg/mL of jararaca GPIb-BP (A and B) or with or without 200 μmol/L GRGDS peptide plus 1 mmol/L EGTA (instead of Ca2+) (C and D) for 5 minutes and then activated with 3 μg/mL of botrocetin and 10 μg/mL of vWF for the indicated time intervals. Cytoskeletal fractions were harvested as described in the legend for Fig 7. Immunoblot analysis of cytoskeletal fractions were performed with anti-p60c-src (A and C) and anti-p54/58lyn (B and D) MoAbs.
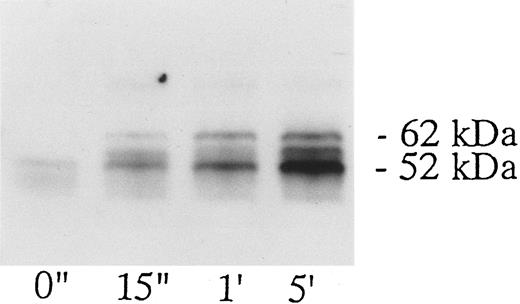
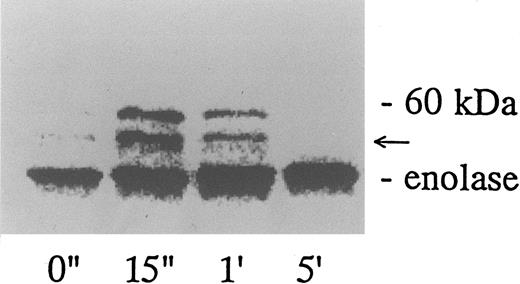
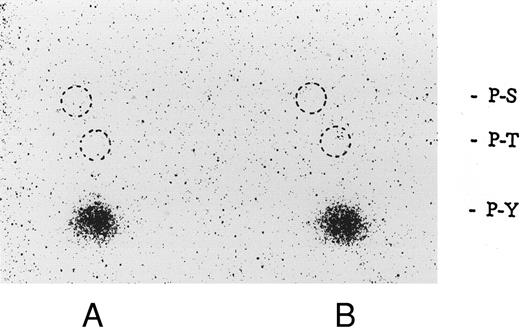
Association of tyrosine kinase(s) with GPIb. Signal transduction pathways involving some membrane glycoproteins are mediated by the association between receptors and tyrosine kinases.38-40 We therefore sought to determine whether GPIb binds to tyrosine kinases during platelet activation induced by the combination of vWF and botrocetin. First, we used two anti-GPIb MoAbs, GUR83-35 and NNKY5-5, that inhibit vWF binding to GPIb for GPIb immunoprecipitation and found no tyrosine kinase activity associated with GPIb after platelet activation (data not shown). We then used two anti-GPIb MoAbs, WGA-3 and GS-70, both of which bind to GPIb but do not interfere with the binding of vWF to GPIb. When GPIb was immunoprecipitated with these nonfunctional anti-GPIb MoAbs after platelets were activated by the combination of vWF and botrocetin, in vitro kinase assays of the GPIb-immunoprecipitate showed that the phosphorylated levels of enolase, added as substrate, and an unidentified protein of approximately 60 kD were elevated (Fig 9). The kinase activity reached its peak 15 seconds after activation and gradually decreased after 1 minute. GPIb itself was not phosphorylated. These findings suggest that GPIb associates with protein kinase(s) upon vWF-botrocetin–platelet activation. We then asked whether the elevated kinase activity associated with GPIb was a tyrosine- or serine/threonine-kinase. Phosphoamino acid analysis of 32P-labeled enolase and the 60-kD protein showed that only the tyrosine residues were phosphorylated, suggesting that tyrosine kinase activity associated with GPIb upon the vWF-GPIb interaction (Fig 10). Western blotting of immunoprecipitated GPIb with antiphosphotyrosine MoAb showed no band of tyrosine phosphorylation, suggesting that the tyrosine kinase itself is not autophosphorylated (data not shown). Western blotting with anti-p72syk, anti-p54/58lyn, anti-p60c-src, anti-p62yes, or anti-p59Fyn MoAbs showed no cross-reactivity, and the tyrosine kinase associated with GPIb remains to be identified.
GPIb-associated kinase activity induced by botrocetin-vWF. Platelets (109 cells/mL) suspended in a buffer containing 1 mmol/L Ca2+ were activated with 3 μg/mL of botrocetin and 10 μg/mL of vWF for the indicated time intervals. Reactions were then terminated with lysis buffer, and GPIb was isolated by immunoprecipitation with anti-GPIb MoAbs, WGA3. In vitro kinase assays were performed using enolase as exogenous substrate. The proteins were separated under reducing conditions by 8% SDS-PAGE and quantified with a BAS 2000 Phosphorimager. The arrow represents the band presumably derived from IgG heavy chains. The data are representative of three experiments.
GPIb-associated kinase activity induced by botrocetin-vWF. Platelets (109 cells/mL) suspended in a buffer containing 1 mmol/L Ca2+ were activated with 3 μg/mL of botrocetin and 10 μg/mL of vWF for the indicated time intervals. Reactions were then terminated with lysis buffer, and GPIb was isolated by immunoprecipitation with anti-GPIb MoAbs, WGA3. In vitro kinase assays were performed using enolase as exogenous substrate. The proteins were separated under reducing conditions by 8% SDS-PAGE and quantified with a BAS 2000 Phosphorimager. The arrow represents the band presumably derived from IgG heavy chains. The data are representative of three experiments.
Phosphoamino acid analysis of 32P-labeled enolase and the kinase activity induced by botrocetin-vWF. After in vitro kinase reactions described in Fig 9, the samples were applied on 8% SDS-PAGE. The 32P-labeled proteins corresponding to phosphorylated enolase and 60-kD protein were excised from polyacrylamide gels. Phosphoamino acid analysis was analyzed by two-dimensional electrophoresis described in the Materials and Methods. The positions of the phosphoamino acids were determined by ninhydrin staining of standards added to each extract. P-Y, phosphotyrosine; P-T, phosphothreonine; P-S, phosphoserine. (A) Enolase; (B) 60-kD band. The data are representative of two experiments.
Phosphoamino acid analysis of 32P-labeled enolase and the kinase activity induced by botrocetin-vWF. After in vitro kinase reactions described in Fig 9, the samples were applied on 8% SDS-PAGE. The 32P-labeled proteins corresponding to phosphorylated enolase and 60-kD protein were excised from polyacrylamide gels. Phosphoamino acid analysis was analyzed by two-dimensional electrophoresis described in the Materials and Methods. The positions of the phosphoamino acids were determined by ninhydrin staining of standards added to each extract. P-Y, phosphotyrosine; P-T, phosphothreonine; P-S, phosphoserine. (A) Enolase; (B) 60-kD band. The data are representative of two experiments.
DISCUSSION
Changes in protein tyrosine phosphorylation of cell lysates and translocation of p60c-src occur upon GPIb-mediated platelet activation.5,15 17 In the present study, we have confirmed these findings and further demonstrated that several intracellular changes related to tyrosine kinases also take place in botrocetin-vWF–induced platelet activation. These include tyrosine phosphorylation of Shc (an adaptor protein); translocation of p60c-src and p54/58lyn, but not of p72syk to cytoskeletal fractions; p72syk activation; and the association between p72syk and p60c-src.
Among a number of tyrosine kinases present in platelets, three tyrosine kinases, p72syk, p60c-src, and p125FAK, are known to increase the activity upon platelet activation induced by various agonists.12,13 Based on the level of p72syk autophosphorylation and in vitro kinase assays, we found that botrocetin-vWF promoted an increase in the level of p72syk activity, whereas changes in p60c-src or p125FAK activity were not observed. Compared with collagen or thrombin stimulation, which causes a 10-fold increase in the p72syk activity,37,41 the effect of botrocetin-vWF was limited, amounting to a twofold to threefold increase at most. Jararaca GPIb-binding protein, which inhibits the binding of vWF to GPIb, abrogated the increase in p72syk activity and the translocation of p60c-src and p54/58lyn to cytoskeletal fractions induced by botrocetin-vWF, suggesting that p72syk activation is caused specifically by the GPIb-vWF interaction. Furthermore, those tyrosine kinase-related events induced by vWF-botrocetin appear to occur independently of vWF and fibrinogen binding to GPIIb/IIIa because the responses were not affected by GRGDS plus EGTA. This finding is consistent with the report by Jackson et al17 that pretreatment of RGDS did not alter the vWF-stimulated activation. To the best of our knowledge, our report is the first to show changes in tyrosine kinase activity mediated by the GPIb-vWF interaction. Our success may be attributed to the use of botrocetin, instead of ristocetin, for facilitating the association between GPIb and vWF. Ristocetin, a glycopeptide antibiotic rich in cationic charges, appears to interfere with the p72syk activity, rendering the detection of its changes impossible. We have also found that p72syk associates with p60c-src upon botrocetin-vWF stimulation. The interaction between p72syk and p60c-src has been reported with Fc receptor-mediated platelet activation,42 and we have recently found that the SH2 domain of p72syk is responsible for their association (unpublished data). It is likely that tyrosine phosphorylated p60c-src is associated with p72syk. However, as discussed above, there is no measurable increase in the tyrosine kinase activity of p60c-src. It may be that only a small percentage of the total p60c-src is activated, which then becomes associated with p72syk.
Shc, a member of the adaptor protein family, does not possess tyrosine kinase activity per se, but does act as an adaptor to facilitate the binding of different signaling molecules.31-33 The presence of the SH2 domain that binds to phosphorylated tyrosine residues appears to be essential for this function, and Shc itself can be tyrosine-phosphorylated to facilitate its binding to the SH2 domain of other molecules.33,43 In platelets, Shc phosphorylation and its association with Grb2 occurs upon thrombopoietin or thrombin stimulation.44 Shc tyrosine phosphorylation and its association with Grb2 is known to activate MAP kinase that lies downstream. We found that Shc is tyrosine-phosphorylated by vWF-botrocetin activation, but we were not able to identify its association with other molecules including Grb2. Furthermore, vWF-botrocetin did not induce MAP kinase activation, whereas thrombin did activate MAP kinase, as assessed by renatured kinase assays.27 It is likely that the Shc-related signaling pathway in vWF-botrocetin activation is distinct from that of thrombin.
Upon platelet activation, a number of proteins, including p60c-src, translocate to the Triton-X–insoluble cytoskeletal fraction.45,46 Although the precise role of cytoskeletal association of tyrosine kinases remains largely unknown, it may contribute to stabilizing platelet aggregates or to inducing clot retraction.47 We have recently found that p54/58lyn and p60c-src, but not p72syk, translocate to the cytoskeletal fraction upon platelet activation induced by collagen or thrombin receptor activation peptides,37 whereas thrombin induced p72syk translocation to cytoskeletons. In the present study, we have shown that vWF-botrocetin stimulation also induced the cytoskeletal association of p60c-src and p54/58lyn, but not that of p72syk. These findings suggest that there is a qualitative difference in the translocation of tyrosine kinases to the cytoskeleton, between vWF-GPIb–induced and thrombin-induced platelet activation. Jararaca GPIb-BP delayed but did not eliminate the translocation of p60c-src and p54/58lyn to cytoskeletal fractions (Fig 8A and B). We assume that the blockade of botrocetin-induced vWF binding to GPIb by jararaca GPIb-BP may not be complete and that some signaling induced by vWF-GPIb, although attenuated, remained in effect, because the binding of jararaca GPIb-BP and vWF is competitive.48
The findings thus far have clearly shown the involvement of tyrosine kinases in the signal transduction pathway in botrocetin-vWF activation, whereas their significance, mutual interactions, and place in the sequential propagation of activation signals remain largely unknown. What signal lies most proximal to the GPIb-vWF interaction? In platelets as well as in other cells, a number of membrane signaling molecules appear to associate with tyrosine kinases upon activation. In B cells, the B-cell antigen receptor colocalizes with p54/58lyn. In Fc receptor-mediated activation, the association between the Fc receptor and tyrosine kinases appears to be the initial step of activation.49 In the same line of reasoning, GPIb may associate with a kinase activity upon botrocetin-vWF activation. Thus, we sought to address this issue by evaluating the kinase activity coprecipitated with GPIb.
We first used several anti-GPIb MoAbs that inhibit ristocetin/botrocetin-induced platelet agglutination/aggregation for immunoprecipitation GPIb, only to find that no kinase activity associated with GPIb upon botrocetin-vWF activation. However, when we immunoprecipitated GPIb with two nonfunctional anti-GPIb MoAbs, the GPIb-linked kinase activity was increased upon platelet activation. Phosphoamino acid analysis showed that the kinase activity was due to a tyrosine kinase associated with GPIb. Under our immunoprecipitation conditions, at least GPIb and GPIX retain a complex form. Accordingly, we cannot draw a conclusion on which component of the GPIb-IX and V complex is associated with the tyrosine kiase activity observed in this study. The tyrosine kinase appeared not to be autophosphorylated and was not identical with p54/58lyn, p60c-src, p59fyn, p72syk, or p125FAK as assessed by Western blotting. However, the failure to detect phosphotyrosine residues by Western blotting may be due to a low sensitivity of this method for evaluating tyrosine kinase coassociation in the GPIb immunoprecipitate. Hence, it may be inappropriate to draw conclusions regarding whether the tyrosine kinase associated with GPIb is autophosphorylated or whether it corresponds to known platelet tyrosine kinases. The increase in the tyrosine kinase activity associated with GPIb occurred before translocation of tyrosine kinases to the cytoskeleton or Shc phosphorylation. These results imply that the unidentified tyrosine kinase plays an essential role in the proximal step of the signal transduction pathway in vWF-GPIb–induced platelet interactions.
In conclusion, we show that the vWF-GPIb interaction induces several tyrosine kinase-related intracellular changes, including p72syk activation, its association with p60c-src, Shc tyrosine phosphorylation, and cytoskeletal association of p60c-src and p54/58lyn. An unidentified tyrosine kinase activity associated with GPIb appears to be one of the earliest intracellular signals. However, more work is necessary to determine whether it binds directly or indirectly to GPIb.
ACKNOWLEDGMENT
The authors are grateful to Dr N. Yamamoto and Dr S. Nomura for providing us with valuable anti-GPIb MoAbs. We are also indebted to Dr G. Kato (Yamanashi Medical University, Yamanashi, Japan) for his valuable advice.
Address reprint requests to Yukio Ozaki, MD, Department of Clinical and Laboratory Medicine, Yamanashi Medical University, Shimokato 1110, Tamaho, Nakakoma, Yamanashi 409-38, Japan.


![Fig. 6. Measurement of p42mapk and p44mapk induced by botrocetin-vWF. Platelets (109/mL) were stimulated with 1 U/mL of thrombin or 3 μg/mL of botrocetin and 10 μg/mL of vWF for the indicated time intervals, and reactions were terminated with an equal volume of SDS sample buffer. Samples were boiled for 5 minutes and diluted 40-fold with Tris-buffered saline. p42mapk was immunoprecipitated using anti-p42mapk MoAb and the immunoprecipitates were subjected to electrophoresis on 10% SDS-PAGE gels containing 0.5 mg/mL of MBP. The MAP kinase in gel was then renatured, and the gels were incubated with the kinase assay buffer containing [γ-32P]ATP. The autoradiograph shows the band of renatured p42mapk activity. The data are representative of three experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/12/10.1182_blood.v90.12.4789/3/m_bl_0023f6.jpeg?Expires=1769132188&Signature=PjPFWLgoloLWVfJp3qIFWMeSlsKC4k68FKq5LAtuRMSDa37Qu8z785PM~VWF1K-7lGJpAZ~db~Vs2C7F48rV3Q~JZTKdeWxyg2JFDWEtZwhpZfA3AIGgLM9OE1BgI3tEBuCJJ26nRzNKmGsx4lJdX2a6vsEiIrHt5P8EvGuUTBQwQInlzyaYh5xhnEgFdsZ5GgX2LGXX3j9VZL3EQivk7FvER9Dm2I7ML2bBwkPLDKIcHjR542g-tYY8imYp~C3WCvW4doYJJWWjzqDHKSQYhc2Dro4VjxR9dKMWFb9roYTx3AqZlFyKFuAnpzeknCGOM7Th1W8XviHMAPF7P25vMQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal