Abstract
We assessed the biologic role of signaling through gp130, a signal-transducing receptor (R) component, in human hematopoiesis in vitro. Although peripheral blood-derived CD34+ cells ubiquitously expressed gp130 and interleukin-3 receptor α (IL-3Rα), IL-6Rα was only detected on 80% of these CD34+ cells. We sorted CD34+IL-6R+ or CD34+IL-6R− cells and studied the effect on hematopoietic colony formation of signaling through gp130 activated by IL-6 or a combination of IL-6 and recombinant soluble human IL-6R (sIL-6R) in the presence or absence of stem cell factor (SCF ) and/or IL-3. Signals activated by SCF, IL-6, or IL-6/sIL-6R complex alone did not induce significant colony formation. However, a combination of IL-3, SCF, and IL-6/sIL-6R complex dramatically induced many neutrophil (colony-forming unit-granulocyte [CFU-G]), erythroid burst (burst-forming unit-erythrocyte [BFU-E]), erythrocyte-containing mixed (CFU-Mix), and megakaryocyte (CFU-Meg) colony formations when CD34+IL-6R− cells were used as the target. CFU-G colony formation induced by the three signals was more evident when CD34+IL-6R+ cells were used as the target. This distinct synergistic effect of the three different signals was confirmed by single-cell clone-sorting experiments. Moreover, colony formation (including CFU-G, BFU-E, CFU-Mix, and CFU-Meg) was observed even in the presence of neutralizing antibodies for granulocyte colony-stimulating factor, erythropoietin, and thrombopoietin (c-Mpl), whereas neutralizing antibodies for gp130, IL-6R, IL-3, and SCF partially or completely blocked the synergistic effect. The maturation of neutrophilic, erythroid, and megakaryocytic cells supported by the three signals in serum-free cultures was confirmed by immunostaining using anti-CD66b, antiglycophorin A, antihemoglobin α, and anti-CD41 monoclonal antibodies, respectively. In contrast, any two of the three signals were insufficient for effective blood cell production in the absence of maturation factors. These results suggest that simultaneous activation of the three signals through gp130, c-kit, and IL-3R can induce in vitro proliferation and differentiation of trilineage hematopoietic progenitors in the absence of terminally acting lineage-specific factors.
RECENTLY, OGAWA1 proposed that the growth factors may be divided into three groups, including late-acting lineage-specific factors, intermediate-acting stage-specific factors, and early acting factors affecting the kinetics of cell-cycle dormant primitive stem cells (HSC). His colleagues used mapping studies of blast cell colony formation to show that interleukin-6 (IL-6), granulocyte colony-stimulating factor (G-CSF ), IL-11, stem cell factor (SCF ), leukemia inhibitory factor (LIF ), and IL-12 acted synergistically with IL-3 in support of colony formation from murine HSC.2-5 Interestingly, these early acting cytokines may be grouped together based on their functional similarities.1 In addition, the combination of SCF, a ligand for type III receptor (R) tyrosine kinase (TK), and any of these early acting factors exerts a synergistic action on in vitro hematopoietic colony formation by human and murine cells.1,6-8 Moreover, IL-3, granulocyte-macrophage colony-stimulating factor (GM-CSF ) and IL-4, which are intermediate-acting lineage nonspecific factors,1,9 showed a distinct synergistic effect on hematopoietic colony formation when combined with SCF or IL-11.10-12 Based on these experimental data, he proposed that cytokines belonging to different groups, including early acting factors, stage-specific factors, and ligands for type III RTKs, may synergistically interact with each other to stimulate the proliferation and differentiation of primitive hematopoietic stem/progenitor cells.1
We previously reported that the combinations of SCF or a ligand for flt3 RTK (FL) with IL-3 or GM-CSF showed a distinct synergistic effect on multipotential progenitor cells.11,13 In addition, we found that IL-4, which is also a stage-specific factor,1 shows a distinct synergistic action with G-CSF in support of neutrophil colony formation14 and with SCF in support of erythroid and multipotential colony formation.15 In this context, we have studied interactions between the above-mentioned cytokines in human hematopoiesis in vitro. It is well documented that signals activated by early acting factors (including IL-6, IL-11, and LIF ) are transmitted through a signal-transducing receptor component, gp130,16-18 and that gp130 mRNA is ubiquitously expressed in a wide variety of cells, including hematopoietic stem/progenitor cells.17 Therefore, we studied the role of gp130 signaling in the proliferation and differentiation of human hematopoietic progenitor cells using peripheral blood (PB)-derived highly purified CD34+ cells. Recently, it was reported that signals through gp130 and c-kit promote erythropoietin (Epo)-independent erythrocyte production from umbilical cord blood (CB)- or bone marrow (BM)-derived CD34+ cells.19 However, the combination of these two signals was insufficient to promote erythrocyte production in our culture system. Our results clearly showed that simultaneous activation of three signals through gp130, c-kit, and IL-3R dramatically induced in vitro proliferation and differentiation of trilineage hematopoietic progenitor cells independently of terminally acting lineage-specific factors, such as G-CSF, Epo, and thrombopoietin (TPO).
MATERIALS AND METHODS
Recombinant factors and neutralizing antibodies. Purified bacterially derived recombinant human IL-3, GM-CSF, G-CSF, and SCF, as well as purified Chinese hamster ovary (CHO) cell-derived recombinant human Epo, were generously supplied by Kirin Brewery Co Ltd (Tokyo, Japan). Purified recombinant human TPO was prepared by the TPO Production Group (Kirin Brewery Co Ltd, Maebashi, Japan) and was provided by Dr Hiroshi Miyazaki (Kirin Brewery). Purified bacterially derived recombinant human IL-6 was kindly provided by Ajinomoto Co Inc (Yokohama, Japan) and had a specific activity of 6 × 106 U/mg. Recombinant soluble human IL-6 receptor (sIL-6R) was prepared as reported previously.20
Three antihuman gp130 monoclonal antibodies (MoAbs), including GPX7, GPX22, and GPZ35, were prepared as described previously.21 They recognized different epitopes on gp130 and were shown to inhibit the IL-6–induced association of gp130 and IL-6 receptors. Antihuman IL-6R MoAb (PM1) was prepared as described22 and specifically inhibited binding between the IL-6R and IL-6. In preliminary titration experiments, a combination of three anti-gp130 MoAbs at 1 μg/mL and the anti–IL-6R MoAb at 5 μg/mL completely abrogated the colony formation induced by these signals in clonal cell culture (data not shown).
Rabbit polyclonal antihuman IL-3 and antihuman G-CSF antisera were kindly provided by Kirin Brewery. A 1:100 dilution of each antiserum neutralized 10 ng/mL and 20 ng/mL of the relevant factor in preliminary titration experiments (data not shown). Rabbit polyclonal antihuman TPO (anti-TPO), antihuman c-Mpl (anti–c-Mpl), and antihuman Epo (anti-Epo) Abs were also provided by Dr Takashi Kato (Kirin Brewery). The addition of greater than 10 μg/mL of anti-TPO Ab and greater than 2 μg/mL of anti–c-Mpl Ab completely abrogated the effect of 50 ng/mL TPO on megakaryocyte colony formation in preliminary titration experiments.23 The addition of greater than 1 μg/mL of anti-Epo Ab completely blocked the effect of 2 U/mL Epo on erythroid burst formation (data not shown). A murine MoAb for human SCF was purchased from Genzyme Corp (Boston, MA) and 20 μg/mL of this Ab completely neutralized 20 ng/mL of SCF in our culture system (data not shown).
Cell preparation and staining with MoAbs. After informed consent was obtained, PB mononuclear (MN) cells were collected from 11 patients with testicular tumors by leukapheresis using a Fenwall CS-3000 (Fenwall Laboratories, Inc, Deerfield, IL), as reported elsewhere.11,13,15 These cells contained an average of 5.9% ± 3.4% (n = 11) CD34+ cells by flow cytometric analysis. The samples were washed twice with α-medium containing 5% fetal calf serum (FCS; Flow Laboratories, Inc, McLean, VA) and nonadherent (NA) cells were recovered after overnight culture on plastic dishes. The MNNA cell fraction was further enriched for null cells using nylon wool columns (Wako Pure Chemicals, Osaka, Japan) and rosette formation with neuraminidase-treated sheep erythrocytes, as reported elsewhere.9 The resultant PB-derived null cells contained an average of 47.7% ± 23.5% (n = 11) CD34+ cells. BM MNNA cells were used in part to study the effect of gp130 signaling on colony-forming unit-erythroid (CFU-E).
These null cells were washed twice with Ca2+- and Mg2+-free phosphate-buffered saline (PBS−) containing 2% FCS (staining medium) and then were passed through a stainless steel mesh. Cells were pelleted before staining with the following MoAbs: fluorescein isothiocyanate (FITC)-conjugated HPCA-2 (mouse IgG1 CD34 MoAb; Becton Dickinson Immunocytometry Systems, San Jose, CA); purified antihuman gp130 MoAb (AM64; mouse IgG1)21; purified antihuman IL-6R MoAb (MT18; mouse IgG1)22; purified anti–IL-3R MoAb (mouse IgG1; Pharmingen, San Diego, CA); purified antihuman common β chain MoAb (mouse IgG1; Pharmingen); purified antihuman glycophorin A (GPA; mouse IgG1; Immunotech S.A., Marseille Cedex, France); purified antihuman c-kit MoAb (mouse IgM; Immunotech); and phycoerythrin (PE)-conjugated goat antimouse IgM (Immunotech). Purified antihuman gp130, IL-6R, IL-3R, GPA, and common β chain MoAbs were biotinylated as described elsewhere.24 For staining of CD34 and c-kit antigens, incubation was performed with 20 μL of purified antihuman c-kit MoAb per 5 × 105 cells for 30 minutes at room temperature. After washing twice with the staining medium, the cells were incubated with 20 μL of PE-conjugated goat antimouse IgM per 5 × 105 cells for 30 minutes at room temperature. The cells were then washed twice with the staining medium and incubated with 10 μL of normal mouse serum on ice to block free binding sites of the PE-conjugated secondary Ab. After 15 minutes, staining was performed with 20 μL of HPCA-2 (FITC) per 106 cells on ice for 30 minutes. For detection of gp130, IL-6R, IL-3R, GPA, and common β chain, incubation was performed with 5 μg of the above-mentioned biotinylated MoAbs per 106 cells for 30 minutes on ice. After washing, cells were incubated with streptavidin-PE (Becton Dickinson) for 30 minutes on ice. Subsequently, all cells were washed twice with the staining medium and were kept on ice for cell sorting. Negative controls included unstained cells and cells only stained with the secondary MoAb or with an FITC-conjugated isotype control IgG or only with the streptavidin-PE.
Flow cytometry and cell sorting. All fluorescence-activated cell sorting (FACS) analyses were performed on a FACStar Plus or FACS Vantage (Becton Dickinson) equipped with an argon laser tuned at 488 nm, as reported elsewhere.11 Sorting gates were established for intermediate-forward scatter (FSC) and low side scatter (SSC). A dual-parameter dot diagram displaying FITC (CD34) and PE (gp130, IL-6R, IL-3R, c-kit, and common β chain) fluorescence was then generated from the gated events. For part of the experiments, cultured cells were serially analyzed for the expression of GPA and gp130. Data acquisition was performed using FACStar Plus Research Software or CELLQuest (Becton Dickinson) and at least 20,000 events were analyzed for each sample. Sorting windows were established for CD34+ cells expressing different levels of IL-6R (data not shown). We sorted CD34+IL-6R+ and CD34+IL-6R− cells. The phenotypic purity of the sorted cells consistently exceeded 90% when checked using postsorting flow cytometric analysis. After sorting, the recovered cells were washed twice with α-medium and cultured as described later.
Clonal cell culture. Cultures were performed in 35-mm Lux suspension culture dishes (no. 171099; Nunc Inc, Naperville, IL), as reported elsewhere.11,13,14 One milliliter of culture contained 200 sorted cells, a 1.2% concentration of 1,500 centipoise methylcellulose (Shinetsu Chemical, Tokyo, Japan), 30% FCS, 1% deionized fraction V bovine serum albumin (BSA; Sigma Chemical Co, St Louis, MO), 5 × 10−5 mol/L 2-mercaptoethanol (ME; Sigma), and one or more recombinant human CSFs. For megakaryocyte colony formation, 10% human platelet-poor plasma (PPP), which was prepared as reported elsewhere23 and prescreened for its ability to support megakaryocyte colony formation, was used instead of FCS. The final concentrations of each CSF used were as follows: IL-3, 10 ng/mL; GM-CSF, 10 ng/mL; G-CSF, 20 ng/mL; Epo, 2 U/mL; SCF, 20 ng/mL; and TPO, 100 ng/mL. These concentrations supported maximal total colony formation in preliminary titration experiments (data not shown).
Serum-free culture was performed as reported elsewhere.9,11,15 Briefly, 200 sorted cells were plated in 35-mm Lux suspension culture dishes (Nunc) containing ASF 104 medium,25 1.2% methylcellulose, 1% globulin-free BSA (Sigma) that had been crystallized and deionized, 5 × 10−5 mol/L 2-ME, 300 μg/mL fully iron-saturated human transferrin (TF; 95% pure; Sigma), 10 μg/mL lecithin (Sigma), 6 μg/mL cholesterol (Sigma), and various growth factors.
Dishes were incubated at 37°C in a fully humidified atmosphere flushed with a combination of 5% CO2 , 5% O2 , and 90% N2 . On days 12 through 14 of incubation, all colonies were counted under an inverted microscope. The typical morphologic features reported elsewhere9,11,13-15 23 were used to identify megakaryocyte colonies (CFU-Meg), granulocyte colonies (CFU-G), macrophage colonies (CFU-M), granulocyte-macrophage colonies (CFU-GM), erythroid burst-forming units (BFU-E), eosinophil colonies (CFU-Eo), and erythrocyte-containing mixed (E-Mix) colonies (CFU-Mix). CFU-E–derived small erythroid colony was assayed on day 7 of incubation. In some experiments, we used megakaryocyte-containing mixed (M-Mix) colonies.
The average plating efficiencies of PB-derived CD34+IL-6R+ and CD34+IL-6R− cells were 40% to 60% in the presence of a cocktail of CSFs, including SCF, IL-3, GM-CSF, G-CSF, and Epo (5 CSFs). However, the CD34+IL-6R− cell population contained a large number of BFU-E, CFU-Mix, and CFU-Meg, whereas the CD34+IL-6R+ cell population contained largely CFU-G (Kimura et al, unpublished data). Our data are consistent with that of a recent report.26
Clone-sorting and single-cell culture. Clone-sorting was performed on a FACStar Plus using an Automated Cell Deposition Unit (ACDU), as reported previously.15,23,27 Serum-containing liquid suspension cultures were performed in 96-well plates. Cultures consisted of single cells in wells containing α-medium, 1% BSA, 5 × 10−5 mol/L 2-ME, 30% FCS, and the designated factors. Incubation was performed for 14 days at 37°C in a fully humidified atmosphere flushed with a combination of 5% CO2 , 5% O2 , and 90% N2 . On days 5, 10, and 14, each well was scanned under an inverted microscope. When a positive well was identified, the number of cells per clone was directly counted in situ. Large clones containing greater than 500 cells were picked up on day 14 and the number of cells was counted using a counting chamber. Serum-containing methylcellulose cultures consisting of single cells were also performed as reported.15,23 27
Serum-free liquid suspension culture. PB-derived CD34+ cells were cultured at 2 × 103/mL in 1 mL of ASF 104 medium25 supplemented with 1% crystallized and deionized globulin-free BSA (Sigma), 5 × 10−5 mol/L 2-ME, 300 μg/mL fully iron-saturated human TF (Sigma), 10 μg/mL lecithin (Sigma), 6 μg/mL cholesterol (Sigma), and the optimum concentrations of the designated growth factors. Dishes were incubated at 37°C in a fully humidified atmosphere flushed with a combination of 5% CO2 , 5% O2 , and 90% N2 for 14 days. Viable cells in each dish were counted on days 5, 7, 10, and 14 of incubation. Cytocentrifuged preparations were made for morphologic examination and confirmation of specific lineages, as described below.
Cytochemistry and immunostaining. Cells were harvested from representative colonies or suspension cultures. Cytospin preparations were first stained with May-Gruenwald-Giemsa (M-G) staining for morphologic analysis. Confirmation of the neutrophilic, erythroid, and megakaryocytic nature of the cells was obtained by immunostaining with the alkaline phosphatase antialkaline phosphatase (APAAP) method using the following MoAbs: anti-CD66b (mouse IgG1; Immunotech), anti-GPA (mouse IgG1, Immunotech), anti-hemoglobin (Hb) α (mouse IgG1; Cosmo Bio, Tokyo, Japan), and anti-CD41 (mouse IgG1; Immunotech). Staining was performed using a DAKO APAAP KIT (Dakopatts, Osaka, Japan) according to the instructions of the manufacturer. The final preparations were counterstained with Mayer's hematoxylin (Wako Pure Chemicals).
Determining the size of pure megakaryocyte colonies and the diameter of megakaryocytes. Colony size was estimated through counting the number of cells in pure megakaryocyte colonies by direct microscopic visualization. Pure megakaryocyte colonies were then picked up with a fine Pasteur pipette and spread on glass slides using cytocentrifugation. After immunostaining with anti-CD41 MoAb (APAAP method), the diameters of individual positive cells were measured using a microscope equipped with an ocular micrometer, and the mean of two perpendicular diameters of each cell was calculated.
Assessment of megakaryocyte ploidy. Cytospin preparations of pooled pure megakaryocyte colonies were made as described elsewhere.23 Pooled cells were confirmed to be positive for CD41 before use. Slides were fixed in cold 70% ethanol at 4°C. After treatment with RNase A (0.1% in PBS−; Sigma), the slides were stained with 0.0025% propidium iodide (PI; Sigma) for 15 minutes at 4°C and mounted with 0.0025% PI solution. The relative nuclear DNA content of cultured megakaryocytes was then individually determined using epifluorecent microfluorometry.
Statistical analysis. The significance of differences in mean values was determined using the two-tailed Student's t-test, the Mann-Whitney rank-sum test, or the χ2 test.
RESULTS
Characterization of PB-derived CD34+ cells. To characterize PB-derived CD34+ cells, we investigated the expression of various cell surface antigens/receptors by these cells. As reported elsewhere,27 the majority of PB-derived CD34+ cells expressed HLA-DR and CD38 antigens. In contrast, approximately 60% and 20% of the CD34+ cells expressed CD33 and c-kit antigens, respectively. Interestingly, PB-derived CD34+ cells ubiquitously expressed gp130 and IL-3R. In addition, most of the cells showed weak common β chain expression. On the other hand, 80% of the CD34+ cells expressed IL-6R (data not shown). We used CD34+IL-6R+ and CD34+IL-6R− cells in the following experiments.
Effect of signals activated by IL-6/sIL-6R complex on colony formation by PB-derived CD34+ IL-6R− or CD34+IL-6R+ cells in the presence or absence of SCF and/or IL-3. First, we investigated effect of signals mediated through gp130 and activated by IL-6/sIL-6R complex on colony formation using 200 PB-derived CD34+IL-6R− cells as the target in serum-containing as well as serum-free culture. The pattern of colony formation observed in serum-free culture in the presence of each signal was comparable with that in serum-containing culture. Therefore, representative data from six independent experiments in serum-free culture are presented in Table 1. The signals activated by IL-6/sIL-6R and SCF alone did not induce significant colony formation. However, IL-3 alone supported significant eosinophil colony formation, as reported elsewhere.9 When IL-3 and IL-6/sIL-6R were combined, only a few erythroid burst formations were observed in addition to eosinophil colonies. Combinations of SCF and IL-3, and of SCF and IL-6/sIL-6R induced a small number of neutrophil colonies. Interestingly, a combination of SCF, IL-3, and IL-6/sIL-6R dramatically induced variety of colonies derived from CFU-G, CFU-GM, BFU-E, and CFU-Mix. As described above, CFU-G was enriched in the CD34+IL-6R+ cell fraction. Therefore, the synergistic action of the combination of SCF, IL-3, and IL-6 on neutrophil colony formation was more evident when this population of progenitors was used as the target (see Table 3A). In the separate experiment, the effect of gp130 signaling on CFU-E was investigated. The combination of IL-6/sIL-6R supported a small but significant number of CFU-E. The combinations of IL-3 + IL-6/sIL-6R, SCF + IL-6/sIL-6R, and three signals significantly enhanced CFU-E colony formation, implying the presence of gp130, c-kit, and IL-3R on CFU-E progenitors (data not shown).
Colony Formation by PB-Derived CD34+IL-6R− Cells in Serum-Free Culture
| Factors . | Colony Types . | % of . | ||||||
|---|---|---|---|---|---|---|---|---|
| . | G . | M . | GM . | B . | Eo . | E-Mix . | Total . | Control . |
| None | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.0 |
| SCF | 1 ± 1 | 0 | 0 | 0 | 0 | 0 | 1 ± 1 | 1.0 |
| IL-3 | 0 | 0 | 2 ± 1 | 0 | 8 ± 1 | 0 | 9 ± 0 | 8.7 |
| IL-6 + sIL-6R | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.0 |
| SCF + IL-3 | 4 ± 2 (NS) | 0 | 5 ± 2 | 0 | 5 ± 1 | 0 | 14 ± 3 | 13.6 |
| SCF + IL-6 + sIL-6R | 2 ± 1 (NS) | 0 | 2 ± 0 | 1 ± 0 (NS) | 0 | 2 ± 1 (NS) | 6 ± 0 | 5.8 |
| IL-3 + IL-6 + sIL-6R | 0 | 0 | 4 ± 1 | 2 ± 0 (NS) | 6 ± 1 | 1 ± 0 (NS) | 12 ± 0 | 11.7 |
| SCF + IL-3 + IL-6 + sIL-6R | 8 ± 1* | 0 | 5 ± 1 | 52 ± 3† | 5 ± 2 | 19 ± 1† | 88 ± 3 | 85.4 |
| CSFs | 11 ± 1 | 1 ± 1 | 18 ± 7 | 55 ± 6 | 2 ± 1 | 18 ± 2 | 130 ± 9 | 100.0 |
| Factors . | Colony Types . | % of . | ||||||
|---|---|---|---|---|---|---|---|---|
| . | G . | M . | GM . | B . | Eo . | E-Mix . | Total . | Control . |
| None | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.0 |
| SCF | 1 ± 1 | 0 | 0 | 0 | 0 | 0 | 1 ± 1 | 1.0 |
| IL-3 | 0 | 0 | 2 ± 1 | 0 | 8 ± 1 | 0 | 9 ± 0 | 8.7 |
| IL-6 + sIL-6R | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.0 |
| SCF + IL-3 | 4 ± 2 (NS) | 0 | 5 ± 2 | 0 | 5 ± 1 | 0 | 14 ± 3 | 13.6 |
| SCF + IL-6 + sIL-6R | 2 ± 1 (NS) | 0 | 2 ± 0 | 1 ± 0 (NS) | 0 | 2 ± 1 (NS) | 6 ± 0 | 5.8 |
| IL-3 + IL-6 + sIL-6R | 0 | 0 | 4 ± 1 | 2 ± 0 (NS) | 6 ± 1 | 1 ± 0 (NS) | 12 ± 0 | 11.7 |
| SCF + IL-3 + IL-6 + sIL-6R | 8 ± 1* | 0 | 5 ± 1 | 52 ± 3† | 5 ± 2 | 19 ± 1† | 88 ± 3 | 85.4 |
| CSFs | 11 ± 1 | 1 ± 1 | 18 ± 7 | 55 ± 6 | 2 ± 1 | 18 ± 2 | 130 ± 9 | 100.0 |
Data represent the mean ± SD of duplicate cultures containing 200 CD34+IL-6R− cells/dish. CSFs contained SCF, IL-3, GM-CSF, G-CSF, and Epo.
Abbreviations: G, granulocyte; M, macrophage; GM, granulocyte-macrophage; B, erythroid burst; Eo, eosinophil; E-Mix, erythrocyte-containing mixed colony; NS, not significant.
P < .02.
P < .01.
Effects of Neutralizing Antibodies or Antisera on Colony Formation by PB-Derived CD34+IL-6R+ CD34+IL-6R− Cells
| Factors . | Antibodies or Antisera . | Colony Types . | ||||||
|---|---|---|---|---|---|---|---|---|
| . | . | G . | M . | GM . | B . | Eo . | E-Mix . | Total . |
| (A) CD34+IL-6R+ cells | ||||||||
| SCF + IL-3 | — | 7 ± 1 | 8 ± 1 | 3 ± 2 | 0 | 13 ± 2 | 0 | 32 ± 1 |
| SCF + IL-6 | — | 7 ± 1 | 3 ± 2 | 0 | 0 | 0 | 0 | 9 ± 1 |
| IL-3 + IL-6 | — | 0 | 11 ± 3 | 3 ± 2 | 0 | 18 ± 1 | 0 | 31 ± 2 |
| SCF + IL-3 + IL-6 | — | 18 ± 2 | 8 ± 2 | 5 ± 3 | 7 ± 2 | 11 ± 1 | 2 ± 1 | 51 ± 3 |
| Control sera3-150 | 19 ± 2 (NS) | 7 ± 4 | 6 ± 1 | 7 ± 2 | 11 ± 2 | 1 ± 0 | 51 ± 1 | |
| Anti–G-CSF serum3-151 | 21 ± 2 (NS) | 6 ± 2 | 4 ± 1 | 7 ± 2 | 12 ± 1 | 1 ± 1 | 51 ± 2 | |
| Anti–SCF Ab3-152 | 03-167 | 11 ± 2 | 3 ± 1 | 0 | 18 ± 2 | 0 | 32 ± 3 | |
| Anti–IL-3 serum3-151 | 5 ± 13-160 | 4 ± 1 | 0 | 0 | 0 | 0 | 9 ± 0 | |
| Anti–IL-6R Abρ | 6 ± 23-160 | 10 ± 1 | 3 ± 1 | 0 | 15 ± 2 | 0 | 34 ± 4 | |
| Anti–gp130 Abs3-155 | 8 ± 13-160 | 8 ± 4 | 4 ± 1 | 0 | 15 ± 3 | 0 | 34 ± 5 | |
| Combination of Abs3-154 | 03-167 | 0 | 0 | 0 | 0 | 0 | 0 | |
| CSFs | — | 40 ± 2 | 12 ± 1 | 3 ± 1 | 41 ± 3 | 6 ± 2 | 3 ± 1 | 105 ± 3 |
| (B)CD34+IL-6R− cells | ||||||||
| SCF + IL-3 | — | 4 ± 1 | 5 ± 1 | 3 ± 2 | 0 | 12 ± 2 | 0 | 24 ± 1 |
| SCF + IL-6 + sIL-6R | — | 2 ± 1 | 4 ± 2 | 0 | 3 ± 1 | 0 | 2 ± 1 | 18 ± 2 |
| IL-3 + IL-6 + sIL-6R | — | 0 | 5 ± 2 | 3 ± 1 | 14 ± 1 | 3 ± 1 | 7 ± 1 | 32 ± 2 |
| SCF + IL-3 + IL-6 + sIL-6R | — | 5 ± 1 | 4 ± 1 | 3 ± 2 | 51 ± 3 | 4 ± 1 | 23 ± 1 | 90 ± 4 |
| Control sera | 5 ± 1 | 3 ± 2 | 2 ± 1 | 52 ± 2 (NS) | 4 ± 2 | 24 ± 1 | 87 ± 3 | |
| Anti-Epo Abρ | 4 ± 2 | 3 ± 2 | 4 ± 1 | 50 ± 2 (NS) | 3 ± 2 | 25 ± 1 | 89 ± 4 | |
| Anti-SCF Ab | 0 | 5 ± 1 | 3 ± 1 | 16 ± 13-160 | 4 ± 2 | 7 ± 2 | 33 ± 1 | |
| Anti–IL-3 serum | 2 ± 1 | 3 ± 1 | 0 | 1 ± 13-167 | 0 | 1 ± 1 | 7 ± 3 | |
| Anti–IL-6R Ab | 4 ± 2 | 6 ± 2 | 3 ± 1 | 03-167 | 12 ± 2 | 0 | 24 ± 4 | |
| Anti-gp130 Ab | 4 ± 2 | 5 ± 1 | 5 ± 2 | 03-167 | 13 ± 1 | 0 | 26 ± 3 | |
| Combination of Abs | 0 | 0 | 0 | 03-167 | 0 | 0 | 0 | |
| CSFs | — | 14 ± 2 | 6 ± 2 | 5 ± 2 | 60 ± 4 | 4 ± 2 | 18 ± 2 | 106 ± 1 |
| Factors . | Antibodies or Antisera . | Colony Types . | ||||||
|---|---|---|---|---|---|---|---|---|
| . | . | G . | M . | GM . | B . | Eo . | E-Mix . | Total . |
| (A) CD34+IL-6R+ cells | ||||||||
| SCF + IL-3 | — | 7 ± 1 | 8 ± 1 | 3 ± 2 | 0 | 13 ± 2 | 0 | 32 ± 1 |
| SCF + IL-6 | — | 7 ± 1 | 3 ± 2 | 0 | 0 | 0 | 0 | 9 ± 1 |
| IL-3 + IL-6 | — | 0 | 11 ± 3 | 3 ± 2 | 0 | 18 ± 1 | 0 | 31 ± 2 |
| SCF + IL-3 + IL-6 | — | 18 ± 2 | 8 ± 2 | 5 ± 3 | 7 ± 2 | 11 ± 1 | 2 ± 1 | 51 ± 3 |
| Control sera3-150 | 19 ± 2 (NS) | 7 ± 4 | 6 ± 1 | 7 ± 2 | 11 ± 2 | 1 ± 0 | 51 ± 1 | |
| Anti–G-CSF serum3-151 | 21 ± 2 (NS) | 6 ± 2 | 4 ± 1 | 7 ± 2 | 12 ± 1 | 1 ± 1 | 51 ± 2 | |
| Anti–SCF Ab3-152 | 03-167 | 11 ± 2 | 3 ± 1 | 0 | 18 ± 2 | 0 | 32 ± 3 | |
| Anti–IL-3 serum3-151 | 5 ± 13-160 | 4 ± 1 | 0 | 0 | 0 | 0 | 9 ± 0 | |
| Anti–IL-6R Abρ | 6 ± 23-160 | 10 ± 1 | 3 ± 1 | 0 | 15 ± 2 | 0 | 34 ± 4 | |
| Anti–gp130 Abs3-155 | 8 ± 13-160 | 8 ± 4 | 4 ± 1 | 0 | 15 ± 3 | 0 | 34 ± 5 | |
| Combination of Abs3-154 | 03-167 | 0 | 0 | 0 | 0 | 0 | 0 | |
| CSFs | — | 40 ± 2 | 12 ± 1 | 3 ± 1 | 41 ± 3 | 6 ± 2 | 3 ± 1 | 105 ± 3 |
| (B)CD34+IL-6R− cells | ||||||||
| SCF + IL-3 | — | 4 ± 1 | 5 ± 1 | 3 ± 2 | 0 | 12 ± 2 | 0 | 24 ± 1 |
| SCF + IL-6 + sIL-6R | — | 2 ± 1 | 4 ± 2 | 0 | 3 ± 1 | 0 | 2 ± 1 | 18 ± 2 |
| IL-3 + IL-6 + sIL-6R | — | 0 | 5 ± 2 | 3 ± 1 | 14 ± 1 | 3 ± 1 | 7 ± 1 | 32 ± 2 |
| SCF + IL-3 + IL-6 + sIL-6R | — | 5 ± 1 | 4 ± 1 | 3 ± 2 | 51 ± 3 | 4 ± 1 | 23 ± 1 | 90 ± 4 |
| Control sera | 5 ± 1 | 3 ± 2 | 2 ± 1 | 52 ± 2 (NS) | 4 ± 2 | 24 ± 1 | 87 ± 3 | |
| Anti-Epo Abρ | 4 ± 2 | 3 ± 2 | 4 ± 1 | 50 ± 2 (NS) | 3 ± 2 | 25 ± 1 | 89 ± 4 | |
| Anti-SCF Ab | 0 | 5 ± 1 | 3 ± 1 | 16 ± 13-160 | 4 ± 2 | 7 ± 2 | 33 ± 1 | |
| Anti–IL-3 serum | 2 ± 1 | 3 ± 1 | 0 | 1 ± 13-167 | 0 | 1 ± 1 | 7 ± 3 | |
| Anti–IL-6R Ab | 4 ± 2 | 6 ± 2 | 3 ± 1 | 03-167 | 12 ± 2 | 0 | 24 ± 4 | |
| Anti-gp130 Ab | 4 ± 2 | 5 ± 1 | 5 ± 2 | 03-167 | 13 ± 1 | 0 | 26 ± 3 | |
| Combination of Abs | 0 | 0 | 0 | 03-167 | 0 | 0 | 0 | |
| CSFs | — | 14 ± 2 | 6 ± 2 | 5 ± 2 | 60 ± 4 | 4 ± 2 | 18 ± 2 | 106 ± 1 |
Data represent the mean ± SD of triplicate cultures containing 200 cells/dish with the specified factors and 30% FCS. Abbreviations are defined in the legend for Table 1.
Normal mouse serum + normal rabbit serum.
(1:100) dilution.
20 μg/mL.
ρ 5 μg/mL.
Combination of three anti-gp130 Abs (GPX-7, GPX-22, and GPZ-35) at 1 μg/mL each.
Anti-SCF Ab + anti–IL-3 serum + anti–IL-6R Ab + anti-gp130 Abs.
P < .001.
P < .01.
The neutrophilic and erythroid nature of the cells was confirmed by immunostaining for CD66b, GPA, and Hbα. The number of colonies supported by the three signals was approximately 85% of that supported by a combination of SCF, IL-3, GM-CSF, G-CSF, and Epo (5 CSFs). In a series of experiments, 100 U/mL of IL-6, 1 μg/mL of sIL-6R, and 20 ng/mL of SCF were added to the cultures based on the data obtained by preliminary titration experiments. These concentrations supported maximal colony formation (data not shown).
Our data indicate that the combination of SCF + IL-3 + IL-6/sIL-6R can induce not only erythroid burst and erythrocyte-containing mixed colonies, but also neutrophil colony in the absence of Epo or G-CSF. Moreover, single-cell clone-sorting experiments performed in methylcellulose culture provided strong evidence that the dramatic synergistic action of the three signals on hematopoietic colony formation is independent of the presence of accessory cells (data not shown).
Effect of signals activated by IL-6/sIL-6R complex on megakaryocyte colony formation by PB-derived CD34+ IL-6R− cells in the presence or absence of SCF and/or IL-3. Next, we investigated the effects of combinations of two or three signals activated by SCF, IL-3, and IL-6/sIL-6R complex on megakaryocyte colony formation in plasma-containing as well as serum-free culture. Representative data of three independent experiments performed in serum-free culture are shown in Table 2. The combination of any two of the three signals activated by SCF, IL-3, and IL-6/sIL-6R supported a comparable number of pure and mixed megakaryocyte colonies. The most striking colony formation was obtained in the presence of SCF + IL-3 + IL-6/sIL-6R, with almost all of them being megakaryocyte-containing mixed colonies. The megakaryocytic nature of the cells was confirmed by M-G staining and immunostaining with anti-CD41 MoAb. In contrast, TPO alone only supported pure megakaryocyte colonies. These results indicate that the signals activated by SCF, IL-3, and IL-6/sIL-6R complex supported megakaryocyte colony formation independently of the presence of TPO.
Pure and Mixed Megakaryocyte Colony Formation by PB-Derived CD34+IL-6R− Cells in Serum-Free Culture
| Factors . | Colony Types . | |||
|---|---|---|---|---|
| . | Meg . | M-Mix . | Others . | Total . |
| TPO | 140 | 0 | 0 | 140 |
| SCF | 0 | 0 | 5 | 5 |
| IL-3 | 35 | 24 | 131 | 190 |
| SCF + IL-3 | 38 | 32 | 227 | 297 |
| SCF + IL-6 + sIL-6R | 47 | 24 | 76 | 147 |
| IL-3 + IL-6 + sIL-6R | 20 | 57 | 332 | 409 |
| SCF + IL-3 + IL-6 + sIL-6R | 7 | 135 | 607 | 749 |
| Factors . | Colony Types . | |||
|---|---|---|---|---|
| . | Meg . | M-Mix . | Others . | Total . |
| TPO | 140 | 0 | 0 | 140 |
| SCF | 0 | 0 | 5 | 5 |
| IL-3 | 35 | 24 | 131 | 190 |
| SCF + IL-3 | 38 | 32 | 227 | 297 |
| SCF + IL-6 + sIL-6R | 47 | 24 | 76 | 147 |
| IL-3 + IL-6 + sIL-6R | 20 | 57 | 332 | 409 |
| SCF + IL-3 + IL-6 + sIL-6R | 7 | 135 | 607 | 749 |
Data represent the total number of colonies derived from 10 dishes each containing 200 CD34+IL-6R− cells.
Abbreviations: Meg, pure megakaryocyte colony; M-Mix, megakaryocyte-containing mixed colony.
Effect of neutralizing antibodies or antisera on colony formation by PB-derived CD34+IL-6R+ or CD34+IL-6R− cells. To confirm that the signals activated by SCF, IL-3, and IL-6/sIL-6R complex actually supported CFU-G, BFU-E, and CFU-Meg independently of G-CSF, Epo, and TPO, we investigated the effect on colony formation of neutralizing Abs (antiserum) for G-CSF, Epo, and TPO (c-Mpl). The synergistic action of the three signals on CFU-G and BFU-E colonies in the presence of neutralizing Abs or antisera for SCF, IL-3, IL-6R, and gp130 was also studied. CFU-G was highly enriched in the CD34+IL-6R+ cell population, so the effect of neutralizing Abs on neutrophil colony formation was studied using this population of progenitors, whereas the CD34+IL-6R− cell population was used to study effects of neutralizing Abs on BFU-E and CFU-Meg (M-Mix).
As shown in Table 3A, anti–IL-3 serum, anti–IL-6R Ab, and anti-gp130 Abs significantly reduced the number of CFU-G supported by the three signals. In contrast, anti-SCF Ab and a combination of all of the Abs completely abrogated neutrophil colony formation, suggesting that signaling through c-kit plays a pivotal role in neutrophil production. However, the addition of anti–G-CSF serum did not affect colony formation, indicating that the three signals could support CFU-G independently of the presence of G-CSF. The effect of these neutralizing Abs on erythroid burst formation was then investigated using CD34+IL-6R− cells (Table 3B). The addition of anti–IL-3 serum, anti–IL-6R Ab, anti-gp130 Abs, and a combination of all of the Abs completely abrogated erythroid burst formation. The number of BFU-E supported by the three signals did not change even in the presence of an anti-Epo Ab, indicating that the observed erythroid burst formation was independent of Epo.
Finally, we studied the effect of these neutralizing Abs on megakaryocyte colony formation. Anti-SCF Ab, anti–IL-6R Ab, and anti-gp130 Abs significantly reduced the number of M-Mix colonies supported by the three signals. In contrast, anti–IL-3 serum and a combination of all of the Abs completely abrogated colony formation. However, the addition of anti-TPO Ab or anti–c-Mpl Ab did not affect megakaryocyte colony formation, indicating that the three signals could support M-Mix colonies independently of the presence of TPO (data not shown).
Alternatively, the addition of anti-gp130 MoAbs to the cultures containing SCF + IL-3 + G-CSF or EPO, or TPO alone, did not affect colony formation of these terminally acting factors (data not shown).
These results clearly showed that the observed synergistic action was initiated through interaction of the IL-6/sIL-6R complex with membrane-anchored gp130 and further confirmed that the three signals activated by SCF, IL-3, and IL-6/sIL-6R complex synergistically support committed progenitors, including CFU-G, BFU-E, CFU-Mix, and CFU-Meg (M-Mix) independently of the relevant lineage-specific factors such as G-CSF, Epo, and TPO.
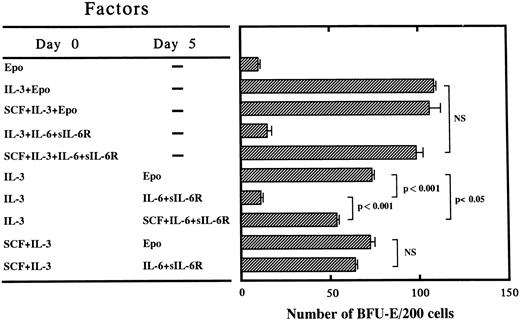
Effect of delayed addition of Epo or IL-6/sIL-6R or SCF + IL-6/sIL-6R on erythroid burst formation by PB-derived CD34+IL-6R− cells supported by IL-3 or SCF + IL-3 in serum-containing culture. The hematopoietic action of signaling through gp130 was further investigated by the delayed addition of Epo or IL-6/sIL-6R or SCF + IL-6/sIL-6R on day 5 of cultures containing IL-3 or SCF + IL-3 from day 0. Representative data for three independent experiments are shown in Fig 1. The number of BFU-E supported by SCF, IL-3, and IL-6/sIL-6R from day 0 was almost comparable with that supported by IL-3 (the most potent burst-promoting activity)11 plus Epo. When Epo was added on day 5 to cultures containing IL-3 from day 0, approximately 70% of the BFU-E survived relative to those supported by day 0 IL-3 + EPO. In contrast, most of the BFU-E failed to recover when IL-6/sIL-6R was added on day 5 instead of Epo. When SCF + IL-6/sIL-6R was added on day 5 to cultures containing IL-3 from day 0, a significant number of BFU-E recovered, although the number of BFU-E was still significantly smaller than that supported by day 0 IL-3 followed by day 5 Epo. Interestingly, when IL-6/sIL-6R was added on day 5 to cultures containing SCF + IL-3 from day 0, a significant number of BFU-E recovered, indicating that signaling through gp130 activated by IL-6/sIL-6R induced maturation of BFU-E in the presence of SCF plus IL-3, but not in the presence of IL-3 alone. In other words, these results suggest that both IL-3 and SCF are key cytokines for the survival and/or proliferation of BFU-E and that signaling through gp130 is mainly important for terminal maturation.
Effects of delayed addition of Epo, IL-6/sIL-6R, or SCF + IL-6/sIL-6R on erythroid burst formation by 200 PB-derived CD34+IL-6R− cells supported by IL-3 or SCF + IL-3 in serum-containing cultures. (▨) The mean ± SD of the number of BFU-E is shown. Statistical analysis was performed using the two-tailed Student's t-test. NS, not significant.
Effects of delayed addition of Epo, IL-6/sIL-6R, or SCF + IL-6/sIL-6R on erythroid burst formation by 200 PB-derived CD34+IL-6R− cells supported by IL-3 or SCF + IL-3 in serum-containing cultures. (▨) The mean ± SD of the number of BFU-E is shown. Statistical analysis was performed using the two-tailed Student's t-test. NS, not significant.
Pattern of proliferation of single CD34+IL-6R− cells in liquid suspension culture. Next, the hematopoietic action of the signals activated by SCF, IL-3, and IL-6/sIL-6R complex was further investigated using single-cell suspension cultures. Single PB-derived CD34+IL-6R− cells were deposited in wells and the pattern of proliferation was serially observed on days 5, 10, and 14 using an inverted microscope. Wells containing greater than 8 cells were scored as positive clones and are shown in Fig 2. SCF and IL-6/sIL-6R complex alone did not induce significant proliferation of single cells. IL-3 alone did induce proliferation of single CD34+IL-6R− cells, but half of the clones disappeared on day 10. In the presence of SCF + IL-3 or IL-3 + IL-6/sIL-6R, the proliferation of single CD34+IL-6R− cells was enhanced compared with that in the presence of IL-3 alone. Subsequently, the number of clones proliferating markedly decreased on day 14 in cultures containing SCF + IL-3, suggesting that the clones died during culture. In cultures containing IL-3 + IL-6/sIL-6R, 80% of the clones still survived on day 14, suggesting that the cells had differentiated along with the maturation process. In contrast, the number of clones was smaller in the cultures containing SCF + IL-6/sIL-6R, but the positive wells were maintained on day 14 of culture. The most striking proliferation of single CD34+IL-6R− cells was observed in cultures containing SCF + IL-3 + IL-6/sIL-6R. Half of the clones contained greater than 500 cells on day 14 of culture, and the number of positive wells did not change during the observation period. Huge clones of greater than 1,000 cells were only observed in the presence of the three signals. These results again showed a distinct synergistic action of the three signals activated by SCF, IL-3, and IL-6/sIL-6R complex on the proliferation of hematopoietic progenitors at the single progenitor cell level.
Proliferation of single PB-derived CD34+IL-6R− cells deposited in the wells of a 96-well flat-bottomed microtiter plate in the presence of the designated factors was serially analyzed on day 5 (left bar), day 10 (center bar), and day 14 (right bar) of culture. Each well was scanned under an inverted microscope. The number of cells per clone was directly counted in situ and wells containing greater than 8 cells were scored as positive clones. Large clones containing greater than 500 cells were picked up on day 14 and the number of cells was counted using a counting chamber. Shown are the clones of (□) 8 to 50, () 51 to 500, and (▨) greater than 500 cells.
Proliferation of single PB-derived CD34+IL-6R− cells deposited in the wells of a 96-well flat-bottomed microtiter plate in the presence of the designated factors was serially analyzed on day 5 (left bar), day 10 (center bar), and day 14 (right bar) of culture. Each well was scanned under an inverted microscope. The number of cells per clone was directly counted in situ and wells containing greater than 8 cells were scored as positive clones. Large clones containing greater than 500 cells were picked up on day 14 and the number of cells was counted using a counting chamber. Shown are the clones of (□) 8 to 50, () 51 to 500, and (▨) greater than 500 cells.
Effects of the three signals activated by IL-3, SCF, and IL-6/sIL-6R complex on proliferation and differentiation of megakaryocyte progenitor cells in serum-free culture. We studied the effect of the three signals activated by IL-3, SCF, and IL-6/sIL-6R complex singly or in combination on megakaryocyte colony formation in serum-free cultures. The mean number of megakaryocytes in individual colonies supported by IL-3 was 54.4 ± 12.2 cells. A combination of SCF + IL-3 or SCF + IL-3 + IL-6/sIL-6R significantly increased the size of the colonies to 70.7 ± 16.0 (P < .05) and 91.6 ± 15.6 (P < .01) cells, respectively.
On the other hand, the mean diameter of megakaryocytes supported by IL-3 alone was 24.6 ± 8.0 μm. SCF did not enlarge megakaryocytes, but the signal through gp130 activated by IL-6/sIL-6R complex significantly increased the mean diameter of megakaryocytes in the presence of IL-3 or SCF. In the presence of the three signals, the mean diameter significantly increased to 48.8 ± 20.3 μm (P < .02). However, the mean diameter was still significantly less than that (60.6 ± 19.6 μm) supported by TPO alone (P < .001).
Next, we examined the nuclear DNA content of megakaryocytes in pure megakaryocyte colonies to study the effect of the three signals on megakaryocytic maturation (Table 4). Approximately 70% of the megakaryocytes supported by IL-3 or SCF + IL-3 were 2 N in ploidy and there was no significant difference of the mean DNA content. In contrast, the mean ploidy of megakaryocytes was significantly increased in the presence of IL-6/sIL-6R complex. In the presence of three signals, the mean DNA content was 6.62 ± 7.35 N and higher ploidy classes (16 N and 32 N) showed a significant increase (P < .01). However, the mean DNA content was still significantly less than that supported by TPO alone (P < .01).
Ploidy Distribution of Megakaryocytes Supported by Specified Factors in Serum-Free Culture
| Factors . | No. of Megakaryocytes . | Ploidy Class (%)4-150 . | Mean ± SD . | ||||
|---|---|---|---|---|---|---|---|
| . | Analyzed . | 2N . | 4N . | 8N . | 16N . | 32N . | . |
| IL-3 | 150 | 72.7 | 19.3 | 7.3 | 0.7 | 2.92 ± 1.97 | |
| SCF + IL-3 (NS) | 150 | 66.7 | 23.3 | 10.0 | 3.07 ± 1.84 | ||
| SCF + IL-6 + sIL-6R4-151 | 150 | 54.0 | 26.0 | 20.0 | 3.72 ± 2.30 | ||
| IL-3 + IL-6 + sIL-6R‡ | 200 | 48.5 | 29.0 | 12.5 | 10.0 | 4.63 ± 4.22 | |
| SCF + IL-3 + IL-6 + sIL-6Rρ | 200 | 44.0 | 18.5 | 23.5 | 8.5 | 5.5 | 6.62 ± 7.35 |
| TPOρ | 150 | 19.3 | 22.0 | 28.7 | 24.7 | 5.3 | 9.21 ± 7.45 |
| Factors . | No. of Megakaryocytes . | Ploidy Class (%)4-150 . | Mean ± SD . | ||||
|---|---|---|---|---|---|---|---|
| . | Analyzed . | 2N . | 4N . | 8N . | 16N . | 32N . | . |
| IL-3 | 150 | 72.7 | 19.3 | 7.3 | 0.7 | 2.92 ± 1.97 | |
| SCF + IL-3 (NS) | 150 | 66.7 | 23.3 | 10.0 | 3.07 ± 1.84 | ||
| SCF + IL-6 + sIL-6R4-151 | 150 | 54.0 | 26.0 | 20.0 | 3.72 ± 2.30 | ||
| IL-3 + IL-6 + sIL-6R‡ | 200 | 48.5 | 29.0 | 12.5 | 10.0 | 4.63 ± 4.22 | |
| SCF + IL-3 + IL-6 + sIL-6Rρ | 200 | 44.0 | 18.5 | 23.5 | 8.5 | 5.5 | 6.62 ± 7.35 |
| TPOρ | 150 | 19.3 | 22.0 | 28.7 | 24.7 | 5.3 | 9.21 ± 7.45 |
One hundred fifty or 200 megakaryocytes were analyzed for relative nuclear DNA content. Statistical significance was calculated by the χ2 test.
Abbreviation: NS, not significant.
Percentage of megakaryocytes in each ploidy class. Ploidy distribution was compared between IL-3 and the specified factors. The mean nuclear DNA content supported by the three signals was significantly less than that supported by TPO (P < .01). Granulocytes obtained from pure neutrophilic colonies formed in the cultures were used as the diploid standards.
P < .05.
P < .02.
ρ P < .01.
Based on these data, it appears that SCF promoted the proliferation of CFU-Meg initially supported by IL-3, whereas the predominant effect of the signal through gp130 activated by IL-6/sIL-6R complex was induction of megakaryocyte maturation.
Serial analysis of the expression of GPA on the surface of cultured cells in the presence of the three signals or SCF + IL-3+ Epo in serum-free culture. In this experiment, 2 × 103 CD34+IL-6R− cells were cultured per dish in the presence of SCF + IL-3 + IL-6/sIL-6R or SCF + IL-3 + Epo. The expression of GPA on the surface of cultured cells was serially analyzed on days 7, 10, and 14 of culture by flow cytometry. The expression of GPA gradually increased in both culture conditions. However, the peak fluorescence intensity on day 14 was much higher in the culture supported by SCF + IL-3+ Epo than that supported by the three signals (data not shown). Moreover, cytologic analysis of cytospin preparations of cultured cells stained with May-Gruenwald-Giemsa showed that the percentage of erythroid cells (erythroblasts + erythrocytes) was 84.5% in the presence of SCF + IL-3 + Epo, whereas that was only 33.5% in the presence of the three signals. The absolute number of erythrocytes produced in the culture containing SCF + IL-3 + Epo was approximately 5 times more than that produced in the culture supported by the three signals (3.42 × 104v 1.61 × 105 cells). These results clearly indicated that Epo induces the maturation of erythroid cells more efficaciously than does the signal through gp130.
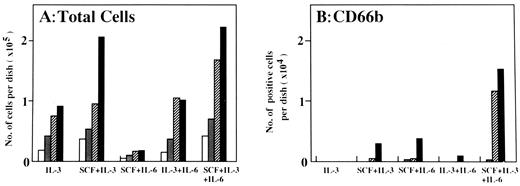
Serial analysis of the maturation of progenitors supported by IL-3, SCF, and IL-6/sIL-6R singly and/or in combination in serum-free liquid suspension culture. Finally, we examined the growth pattern of CD34+IL-6R− and CD34+IL-6R+ cells in serum-free liquid suspension culture, and serially analyzed the number of CD41+, GPA+, Hbα+, and CD66b+ cells using the APAAP method on days 5, 7, 10, and 14 of culture. In these experiments, 2 × 103 CD34+IL-6R− or CD34+IL-6R+ cells were cultured per dish in the presence of the designated factors. Representative data from two experiments are shown in Figs 3 and 4. The pattern of proliferation of CD34+IL-6R− cells (Fig 3) was almost identical with that seen in single-cell culture (Fig 2). IL-3 supported the proliferation of CD34+IL-6R− cells and SCF significantly promoted their growth. In the presence of the three signals, proliferation was most striking and the number of cells reached approximately 9 × 105/dish on day 14 (Fig 3A). In the presence of SCF + IL-3 or IL-3 + IL-6/sIL-6R, approximately 1.5 × 104 CD41+ cells were observed on day 7. However, they markedly decreased in the presence of SCF + IL-3 on day 14. In contrast, CD41+ cells could be maintained in the presence of IL-3 + IL-6/sIL-6R. Moreover, the number of CD41+ cells supported by the three signals was twice that supported by IL-3 + IL-6/sIL-6R (Fig 3B).
Number of viable cells (A) and number of cells positive for immunostaining with CD41 (B), GPA (C), or Hbα (D) on days 5, 7, 10, and 14 of culture in the presence of the designated factors. Cultures were initiated with 2 × 103 PB-derived CD34+IL-6R− cells per dish. Shown are the numbers of cells on days (□) 5, () 7, (▨) 10, and (▪) 14.
Number of viable cells (A) and number of cells positive for immunostaining with CD41 (B), GPA (C), or Hbα (D) on days 5, 7, 10, and 14 of culture in the presence of the designated factors. Cultures were initiated with 2 × 103 PB-derived CD34+IL-6R− cells per dish. Shown are the numbers of cells on days (□) 5, () 7, (▨) 10, and (▪) 14.
Number of viable cells (A) and number of cells positive for immunostaining with CD66b (B) on days 5, 7, 10, and 14 of culture in the presence of the designated factors. Cultures were initiated with 2 × 103 PB-derived CD34+IL-6R+ cells per dish. Shown are the numbers of cells on days (□) 5, () 7, (▨) 10, and (▪) 14.
Number of viable cells (A) and number of cells positive for immunostaining with CD66b (B) on days 5, 7, 10, and 14 of culture in the presence of the designated factors. Cultures were initiated with 2 × 103 PB-derived CD34+IL-6R+ cells per dish. Shown are the numbers of cells on days (□) 5, () 7, (▨) 10, and (▪) 14.
On the other hand, SCF + IL-3 could not induce GPA+ and Hbα+ cells under the same culture conditions (Fig 3C and D). In the presence of SCF + IL-6/sIL-6R or IL-3 + IL-6/sIL-6R, a few GPA+ and Hbα+ cells could be detected. Interestingly, in the presence of the three signals, greater than 2 × 105 cells were positive for GPA and Hbα immunostaining on day 14. Approximately 98.5% of the CD34+IL-6R− cells were blastoid in morphology at the initiation of culture, but 24.0% and 5.5% of the cells were respectively erythroblasts and mature erythrocytes on day 14. This findings was highly consistent with the immunostaining data. These results clearly indicated that signaling through gp130 significantly induced maturation of megakaryocytic as well as erythroid progenitor cells in the presence of IL-3 or SCF + IL-3.
When CD34+IL-6R+ cells were cultured under the same conditions, the number of cells reached a peak level (2 × 105/dish) on day 14 in both the presence of SCF + IL-3 and SCF + IL-3 + IL-6 (Fig 4A). However, only in the presence of the three signals did 7% of the cells become CD66b+ on day 14 (Fig 4B), which was consistent with morphologic examination by M-G staining. These results again showed that signaling through gp130 may play an important role on the maturation of neutrophilic progenitor cells in the presence of SCF and IL-3. Our findings further suggested simultaneous activation of the three signals through gp130, c-kit, and IL-3R induced not only proliferation, but also maturation of neutrophilic, erythroid, and megakaryocytic progenitor cells in the absence of terminally acting factors, including G-CSF, Epo, and TPO.
DISCUSSION
An important finding of this study is that a combination of three signals through gp130, c-kit, and IL-3R exerted a dramatic synergistic action on hematopoietic colony formation. In the presence of the three signals, committed and multipotential progenitors (including CFU-G, BFU-E, CFU-Meg, and CFU-Mix) could proliferate and differentiate to form colonies in serum-free culture in the absence of terminally acting lineage-specific factors, such as G-CSF, Epo, and TPO. Single-cell clone-sorting experiments in liquid suspension culture clearly showed that all three signals were essential for a dramatic synergistic effect on the proliferation and differentiation of hematopoietic progenitor cells. Moreover, data from single-cell suspension cultures suggested that IL-3 support was important for survival and initial proliferation, whereas SCF was important for enhancement of the proliferation of progenitors. The signal through gp130 activated by IL-6/sIL-6R complex may mainly contribute to the maturation of progenitors in the presence of SCF and IL-3, as suggested by the data from delayed addition experiments and serum-free suspension cultures. In addition, approximately 95% of mature hematopoietic cells, which are derived from the day-14 culture supported by the three signals and consist of 32.5% myeloid cells, 21.0% monocytes, 9.0% eosinophils and basophils, 33.5% erythroid cells, and 2% megakaryocytes, expressed gp130 by flow cytometric analysis (Kimura et al, unpublished data ). These findings further support that signals through gp130 function as the maturation stimuli.
Our interpretation of the role of SCF is consistent with an earlier report that it enhances the proliferation but not maturation of murine megakaryocyte progenitors.28 A maturation-promoting effect of IL-6 on megakaryocytes has already been reported.29 Moreover, the IL-6 family of cytokines, including IL-6, IL-11, LIF, and oncostatin M, uses gp130 as a signal transducer and functions as a megakaryocyte-potentiating activity.18 Subsequently, Debili et al30 clearly indicated that a combination of SCF, IL-6, and IL-3 promoted the maturation of CFU-Meg and led to the synthesis of demarcation membranes and platelet shedding, further supporting our results. Moreover, Papayannopoulou et al31 reported that SCF exerted synergy with IL-3 in supporting the survival and/or amplification of early and late erythroid progenitors in the absence of Epo. These erythroid progenitors could differentiate to the stage of globin-producing cells, but were unable to complete the maturation process, including GPA expression. These results suggest that two signals through c-kit and IL-3R are important for BFU-E development, but are insufficient for the terminal maturation of erythroid progenitors, and that Epo is not critical for the generation of globin+ cells in the presence of IL-3 + SCF. Our data are consistent with their conclusions and suggest that signaling through gp130 can partially substitute for Epo and induce further maturation of erythroid progenitors. The effects of SCF and SCF + IL-3 on myeloid colony formation have been reported previously,12 32 but it was still uncertain whether signaling through gp130 promotes the maturation of neutrophilic progenitors. Our immunostaining study using anti-CD66b MoAb, which detects mature neutrophils, clearly showed that the three signals greatly enhanced the maturation of neutrophilic progenitors in serum-free cultures.
The present study provides an evidence that signals through gp130 induced the maturation of trilineage hematopoietic progenitor cells in the presence of SCF and IL-3. However, the mean DNA content of the megakaryocytes supported by the three signals was still significantly less than that supported by TPO alone, suggesting that the signaling through gp130 is not as effective as TPO. Moreover, the expression of GPA on the surface of mature erythroid cells obtained in the culture supported by the three signals was apparently less than that in the culture supported by SCF + IL-3 + Epo. These results indicate that signals through gp130 cannot replace the function of physiologic maturation factors completely.
Very recently, Sui et al33 reported that the IL-6/sIL-6R complex shows distinct synergy with SCF in promoting the proliferation of CB-derived CD34+ cells. It was also reported that signaling through gp130 and c-kit dramatically promoted erythropoiesis from human CB- and BM-derived CD34+ cells.19 However, we could not induce such a potent hematopoietic action by these two signals in our cultures. Our data clearly indicated that signaling through IL-3R has a pivotal role and that a combination of the three signals through gp130, c-kit, and IL-3R exerts a dramatic hematopoietic action in vitro. These discrepancies between studies may reflect differences in experimental methods as well as the target cell population. The most striking difference between PB- and CB-derived CD34+ cells is expression of the IL-6R. We found that 80% of the PB-derived CD34+ cells expressed the IL-6R, whereas only 20% of CB-derived CD34+ cells express this receptor (Sakabe et al, unpublished data).
Broudy et al34 recently reported that TPO-induced colony growth and nuclear maturation of CFU-Meg were not blocked by the addition of neutralizing anti-gp130 MoAb. In contrast, IL-3–induced colony growth and nuclear maturation of CFU-Meg were impaired by the same neutralizing MoAb. These results suggest that signals induced by IL-3 are mediated in part through gp130. It was reported that c-kit antisense oligonucleotides significantly inhibited colony formation by BM-derived CD34+ cells in the presence of IL-3.35 In addition, Ratajczak et al36 reported that c-kit antisense oligonucleotides selectively inhibited erythroid-burst formation induced by IL-3 + Epo. These results suggest that c-kit/SCF system predominantly functions in erythropoiesis in association with IL-3. Our previous report11 was consistent with these results and further suggest that IL-3 may share a common signal transduction pathway with SCF in erythropoiesis. Collectively, IL-3 signaling may function as a key signal for both gp130 and c-kit signal transduction pathways.
Recent studies18,37 have shown that activation of JAK 2 TK is associated with signal transduction of gp130 as well as receptors for terminally acting lineage-specific factors, including Epo, G-CSF, and TPO, suggesting that gp130 and these receptors (Epo-R, G-CSF-R, and c-Mpl) may share a common intracellular signaling pathway. In addition, signals through common β initiated by IL-3 also activated JAK 2 TK,37 implying that JAK 2 may play an important role in the cross-talk between these cytokines (signals).
The physiologic significance of sIL-6R may be suggested by its presence in human sera.38,39 In addition, sIL-6R found in sera is biologically active and can bind to IL-6 and stimulate gp130.17,38 Furthermore, gp130 knock-out mice showed a marked reduction of the number of colony-forming units in spleen (CFU-S), CFU-GM, and BFU-E in fetal livers.40 In this study, some gp130−/− embryos show anemia, further suggesting the physiologic role of the signal through gp130 in embryonic hematopoiesis. In addition, Bernad et al41 suggested that the terminal maturation of myeloid as well as erythroid progenitor cells is impaired in IL-6–deficient mice. Their interpretation of the role of IL-6 is consistent with our results. Very recently, it was reported that IL-6/sIL-6R double transgenic mice displayed a marked hepatosplenomegaly caused by an extreme expansion of extramedullary hematopoietic progenitors.42 In these double transgenic mice, numbers of neutrophilic granulocytes, platelets, and red blood cells markedly increased in the peripheral blood. This study clearly showed that continuous activation of the gp130 signal transducer leads to an effective production of mature blood cells, further demonstrating in vivo role of gp130 signaling.
Alternatively, it was reported that G-CSF and c-Mpl knock-out mice have mature neutrophils and platelets at 20% to 30% and 6% of the levels in wild-type mice.43,44 In addition, a few primitive erythrocytes are produced by Epo or Epo-R knock out mice, indicating that some erythroid progenitors can proliferate and differentiate in the complete absence of either Epo or Epo-R.45 It was also reported that a few definitive erythroid cells could terminally differentiate in Epo-R knock-out mice and that the number of fetal liver-derived CFU-E greatly increased when cultured with SCF and spleen cell-conditioned medium (a source of IL-3 and other cytokines).46 These results suggest that maturation signals through G-CSF-R, Epo-R, and c-Mpl can be replaced by some other signals and that there may be functional redundancy between specific cytokines (signals). In the present study, we showed that simultaneous activation of the three signals induced proliferation and differentiation of trilineage hematopoietic progenitor cells. Signaling through gp130 may mainly induce maturation of these progenitors and this may partly explain why some mature blood cells are found in the lineage-specific factor and/or receptor knock-out mice. However, our data also indicate that the three signals are apparently less effective than physiologic maturation factors qualitatively as well as quantitatively in vitro. Therefore, they cannot possibly provide quantitatively enough numbers of mature blood cells that are required to maintain steady-state hematopoiesis in the absence of physiologic maturation factors.
From the other point of view, it was reported that SCF, IL-6, and sIL-6R are detectable in human sera.38,39 In contrast, IL-3 is only produced by activated T cells and mast cells47 48 and is not detectable in human serum. Taken together, there is a possibility that the three signals cannot function in normal physiologic hematopoiesis in vivo.
In conclusion, the present study provides support for a new concept that terminal maturation of hematopoietic progenitors may be achieved by simultaneous activation of signals through gp130, c-kit, and IL-3R in vitro. It is still uncertain whether there is a cross-talk between the three signals. However, Wu et al49 recently reported that SCF induced tyrosine phosphorylation of Epo-R and suggested that the signaling through c-kit may activate the Epo/Epo-R signal transduction pathway. More recently, they clearly showed that a functional interaction between c-kit and the Epo-R is essential for the function of CFU-E progenitors.50 It was also reported that various chimeric receptors composed of extracellular domains of Epo-R and cytoplasmic domains of IL-2 or IL-3 receptors can induce erythroid-specific gene expression in nonerythroid IL-3–dependent pro-B cells (Ba/F3 cells), suggesting that the cytoplasmic domains of the IL-3R can induce the expression of erythroid-specific gene, including globin, GATA-1, and SCL.51 Collectively, activation of three signals may lead to cross-activation of additional signals that induce the terminal maturation of hematopoietic progenitor cells. Recent molecular biologic analysis of signal transduction of various cytokines18 (including IL-6, SCF, and IL-3) may provide a better understanding of the interactions among signals through gp130 and specific cytokine receptors and/or receptor type TKs.
ACKNOWLEDGMENT
The authors are grateful to Kirin Brewery Co, Ltd for providing the various growth factors used in this study. The authors also thank Dr Emi Hirai (First Department of Pathology, Kyoto Prefectural University of Medicine, Kyoto, Japan) for her advice on epifluorecent microfluorometry and Dr Zhaozhu Zeng, Miho Kinugawa, and Mayuko Adachi for their excellent technical assistance.
Supported in part by Grants-in-Aid for Scientific Research on Priority Areas (Grants No. 08261213 and 09250212) and for Scientific Research B (Grant No. 09470232) from the Ministry of Education, Science and Culture of Japan.
Address reprint requests to Yoshiaki Sonoda, MD, The Department of Hygiene, Kyoto Prefectural University of Medicine, Kawaramachi-Hirokoji, Kamigyoku, Kyoto 602, Japan.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal