Abstract
The granulocyte-macrophage colony-stimulating factor (GM-CSF) receptor (GMR) is a heterodimeric receptor expressed by myeloid lineage cells. Binding of GM-CSF activates at least one receptor-associated tyrosine kinase, JAK2, and rapidly induces tyrosine phosphorylation of the GMR βc-chain (GMRβ), but not the GMR α-chain (GMRα). To examine the role of GMRβ tyrosine phosphorylaiton, each of the 8 tyrosine residues in the cytoplasmic domain of the human GMRβ was mutated to phenylalanine (GMRβ-F8), and this mutant receptor was expressed with wild-type GMRα in the interleukin-3–dependent murine hematopoietic cell line, Ba/F3. GM-CSF induced tyrosine phosphorylation of multiple cellular proteins in cells expressing GMRβ-F8 , including JAK2 and STAT5. However, GM-CSF–induced tyrosine phosphorylation of both SHP2 and SHC was reduced or absent compared with wild-type. Next, a series of 8 receptors were generated, each containing only a single, restored, tyrosine residue. Tyrosine 577 was found to be sufficient to regenerate GM-CSF–dependent phosphorylation of SHC, and any of Y577, Y612, or Y695 was sufficient to regenerate GM-CSF–inducible phosphorylation of SHP2. Despite the signaling defect to SHC and SHP2, Ba/F3 cells expressing GMRβ-F8 were still able to proliferate in response to 10 ng/mL of human GM-CSF, although mitogenesis was impaired compared with wild-type GMRβ, and this effect was even more prominent at lower concentrations of GM-CSF (1 ng/mL). Overall, these results indicate that GMRβ tyrosine residues are not necessary for activation of the JAK/STAT pathway or for proliferation, viability, or adhesion signaling in Ba/F3 cells, although tyrosine residues significantly affect the magnitude of the response. However, specific tyrosine residues are needed for activation of SHC and SHP2.
THE GRANULOCYTE-MACROPHAGE colony-stimulating factor (GM-CSF) receptor (GMR) is a heterodimeric receptor consisting of a unique α-chain (GMRα) and a common β-chain (GMRβ) shared with the interleukin-3 (IL-3) and IL-5 receptors (reviewed in Bagley et al1 and Sakamoto et al2 ). Both GMR chains are members of the cytokine receptor superfamily. GMRα alone binds GM-CSF with low affinity and cannot transmit mitogenic or viability signals. GMRβ cannot bind ligand by itself, but is required for both high-affinity binding and normal signal transduction.1 Signal transduction pathways activated by the GMR are not well understood. Several cytoplasmic proteins, including GMRβ itself, are rapidly tyrosine phosphorylated upon GM-CSF stimulation of cells containing the GMR, and this is associated with activation of the JAK2 tyrosine kinase.3 Multiple studies have shown that JAK2 associates with specific regions of the GMRβ, termed box 1 domains, and that mutations of the common β-chain that disrupt binding or activation of JAK2 result in nonfunctional receptors.3 4
It is of great interest to link specific signaling pathways to specific biologic events, and several efforts have been made to define functional domains with the GMRα and GMRβ. For example, deletion mutants of GMRβ have defined a membrane proximal domain (amino acids 456-517) to be essential for proliferation, activation of JAK2 and pim-1, and induction of c-myc.3,5-7 There are data to suggest that JAK2 is constitutively associated with the GMRβ, but its kinase activity is activated only after GM-CSF binding.8 A second domain (amino acids 627-763) was found to be necessary for activation of SHC, p21ras, Raf-1, and MAP kinase, as well as for induction of c-fos and c-jun.6,7,9 Importantly, deletion of this domain was also shown to reduce the tyrosine phosphorylation of GMRβ. Truncation of GMRβ at amino acid 626 did not affect the ability of the receptor to support short term proliferation in fetal calf serum (FCS)-containing medium, although the truncated receptor was defective in medium lacking FCS.10 Recent studies have also indicated that several domains within the GMRβ contribute to GM-CSF–induced differentiation of WEHI-3B D+ cells and M1 cells and specifically that regions distal to amino acid 558 are critical.11
In this study, we evaluated the functions of the 8 tyrosine residues of the GMRβ by using site-directed mutagenesis to either change all 8 tyrosines to phenylalanine residues (GMRβ-F8 ) or mutate 7 of the 8 tyrosines, leaving a single tyrosine residue intact. The results indicate that specific tyrosine residues contribute to the pathways involving SHC and SHP2. However, even a β chain with no tyrosine residues is still capable of supporting proliferation, viability, and adhesion to fibronectin-coated surfaces.
MATERIALS AND METHODS
Cells and cell culture.Ba/F3, an IL-3–dependent murine pro-B–cell line, was transfected with a plasmid containing human GMRα and a hygromycin resistance selection marker (Ba/F3-GMRα; a gift of Atsushi Miyajima, Institute of Molecular and Cellular Biosciences, University of Tokyo, Tokyo, Japan).6 Ba/F3-GMRα cells were cultured in RPMI 1640, 10% FCS, and 15% medium conditioned by the WEHI-3B cell line as a source of murine IL-3 and 0.5 mg/mL hygromycin B (Boehringer Mannheim, Mannheim, Germany). Ba/F3-GMRα cells were then transfected by electroporation using a Bio-Rad Gene Pulser (Richmond, CA) with GMRβ tyrosine mutants. G418-resistant clones were selected and analyzed for expression of GMRβ using a monoclonal antibody (MoAb) generated against the extracellular domain of GMRβ (a gift of Angel Lopez, The Hansen Center, Adelaide, Australia). Ba/F3-GMRα cells stably expressing human wild-type GMRβ are referred to as Ba/F3-GMRαβ-WT. Cell lines expressing mutant receptors are indicated by the β chain mutation (Ba/F3-GMRαβ-F8 , all 8 cytoplasmic tyrosine residues mutated to phenylalanine; Ba/F3-GMRβ-Y3, the third tyrosine distal to the transmembrane domain, Y577, is intact, with the other 7 tyrosine residues mutated to phenylalanine; Ba/F3-GMRαβ-Y4, a single tyrosine residue at the fourth tyrosine distal to the transmembrane domain, Y612, is intact, with the other 7 tyrosine residues mutated to phenylalanine; etc). The nomenclature used is that of Sakamaki et al.6 Two or more independent Ba/F3 subclones for each mutant were characterized.
For viability assays, cells were washed four times in phosphate-buffered saline and then resuspended in a 1:1 mixture of RPMI-1640 and AIM-V (GIBCO BRL, Grand Island, NY; a serum-free, chemically defined medium that contains human serum albumin, human transferrin, and recombinant human insulin) with the indicated concentrations of GM-CSF (purified recombinant human factor; Genetics Institute, Cambridge, MA) with or without FCS. Viability was determined by trypan blue exclusion.
Antibodies.Anti-JAK2 antibody was purchased from UBI (Lake Placid, NY). Anti-SHC, GRB2, and anti-SHP2 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antiphosphotyrosine MoAb 4G10 was a gift of Brian Druker (University of Oregon Health Sciences Center, Portland, OR). A rabbit antibody recognizing tyrosine phosphorylated STAT 5 was obtained from David Frank (Dana-Farber Cancer Institute, Boston, MA).12 Polyclonal anti-GMRβ antibody was raised against the extracellular domain of GMRβ expressed as a GST fusion protein and was used for immunoprecipitations.
Immunoblotting and immunoprecipitation.Cells were starved for 14 hours in RPMI-1640 (Sigma, St Louis, MO) and 0.5% bovine serum albumin and then incubated with GM-CSF as indicated. Cells were washed twice with ice-cold phophate-buffered saline and lysed with RIPA buffer (50 mmol/L Tris, pH 8.0, 150 mmol/L NaCl, 1% NP-40, 0.5% deoxycholate, 0.1% sodium dodecyl sulfate [SDS], 1 mmol/L Na3VO4 , 1 mmol/L phenylmethylsulfonyl fluoride, 10 μg/mL aprotinin, and 10 μg/mL leupeptin). Lysates were incubated on ice for 30 minutes and then clarified by centrifugation at 12,000g for 15 minutes. Supernatants were incubated with antisera as indicated for 2 hours on ice, immune complexes were collected on protein A- (rabbit antisera) or protein G- (mouse MoAbs) Sepharose (Pharmacia Biotech, Inc, Uppsala, Sweden) for 1 hour at 4°C, and then pellets were washed four times with lysis buffer and boiled for 5 minutes in sample buffer (62.5 mmol/L Tris-HCl, pH 6.8, 2% SDS, 5% glycerol, 2% β-mercaptoethanol, 0.001% bromophenol blue). Pellets and lysates were subjected to SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotted using a chemiluminescence detection system as previously described.13
Site-directed mutagenesis.The complete GMRβ coding sequence was cloned into pALTER (Promega Biotech, Madison, WI) as an Xho I-BamHI fragment. Mutagenesis was performed according to the manufacturer's specifications. The identity of the mutation as well as the fidelity of the synthesis reaction were confirmed by sequencing of each mutant. The Xho I-BamHI fragment of the mutated pALTER-GMRβ was subcloned into pLXSN, which was then used for transfections into Ba/F3-GMRα cells, as described.10 13
RESULTS
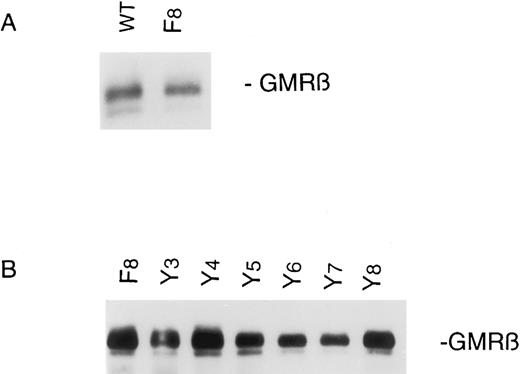
Expression of human GMRβ in murine Ba/F3α cells.GMRβ tyrosine mutants were stably transfected into a clone of Ba/F3 cell lines already expressing the human GMRα as described in the Materials and Methods. Two to four independently derived transfected cell lines were established for all cell lines described in this report, and the data presented are representative of analysis of at least two independent cell lines for each GMRβ mutant. Expression of GMRβ protein was analyzed by immunoblot with an anti-GMRβ MoAb, demonstrating approximately equivalent levels in the mutant cell lines, although generally lower than in the cells transfected with the wild-type GMRβ receptor (Fig 1). The surface expression of the GM-CSF receptor was analyzed by immunofluorescence cytometry, using an MoAb to GMRβ, which showed low, but detectable expression of GMRβ in all cell lines (data not shown). Although the murine pro-B–cell line, Ba/F3, expresses murine GMRβ, the antihuman GMRβ MoAb used here does not recognize the murine GMRβ, nor does human GM-CSF activate the murine receptor at the concentrations used in this study.
GMRβ expression in Ba/F3-GMRαβ cells. Stable transfectants were constructed as described in the Materials and Methods. Cells (107) were lysed and cellular proteins were immunoprecipitated with antihuman GMRβ MoAb. Immunoprecipitates were subjected to SDS-PAGE and immunoblotted with the same anti-GMRβ antibody.
GMRβ expression in Ba/F3-GMRαβ cells. Stable transfectants were constructed as described in the Materials and Methods. Cells (107) were lysed and cellular proteins were immunoprecipitated with antihuman GMRβ MoAb. Immunoprecipitates were subjected to SDS-PAGE and immunoblotted with the same anti-GMRβ antibody.
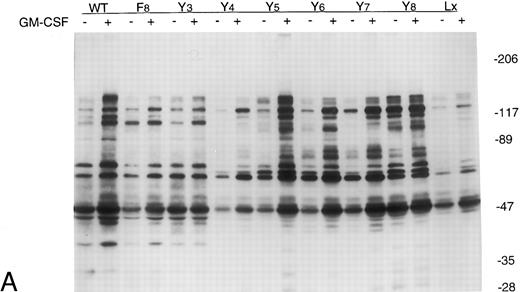
GM-CSF induces tyrosine phosphorylation of cellular proteins in Ba/F3 cells expressing GMRβ-F8 . In an attempt to identify defects in GMRβ signaling in the mutant cell lines, overall GM-CSF–induced tyrosine phosphorylation of cellular proteins was examined. Cells were stimulated with 10 ng/mL GM-CSF for 15 minutes at 37°C, and cellular lysates were immunoprecipitated with antibody against phosphotyrosine and analyzed through immunoblotting with the phosphotyrosine antibody. Figure 2A shows an immunoblot analysis of all the βc mutants. Increased tyrosine phosphorylation of several proteins was observed in response to GM-CSF in Ba/F3-GMRαβ WT cells, as anticipated. Increased tyrosine phosphorylation was also observed in Ba/F3-GMRαβ F8 cells, although some bands appeared less intense (Fig 2A). Other mutants in which one tyrosine was replaced (GMRβ-Y3,4,5,6,7, and 8 ; to be discussed in more detail below) were also studied. The Y8 mutant repeatedly had higher levels of tyrosine phosphorylation after growth factor deprivation than the other mutants (Fig 2A). However, overall, these results suggest that GMRβ tyrosine residues are not required for activation of many tyrosine phosphorylation events in Ba/F3 cells. GM-CSF–induced tyrosine phosphorylation of the β chain itself was examined by performing immunoprecipitation with anti-β chain and immunoblotting with antiphosphotyrosine MoAb (Fig 2B). β Chain tyrosine phosphorylation was detected in cells expressing wild-type β chain stimulated for 15 minutes with GM-CSF, but not in Ba/F3-GMRαβ-F8 cells or in cells expressing β chain mutants with single tyrosine residues. A phosphotyrosine protein migrating slightly faster than the β chain was precipitated with anti-β antibody in some experiments. This may represent JAK2.
Tyrosine phosphorylation of cellular proteins in Ba/F3-GMRα cell lines expressing mutant GMRβ. Cells (107) were incubated in the absence or presence of GM-CSF (10 ng/mL) for 15 minutes. Cell lysates were immunoprecipitated with antiphosphotyrosine antibody (A) or anti-β chain antibody (B), electrophoresed through 6% to 12% gradient SDS-PAGE, and then analyzed by immunoblotting with antiphosphotyrosine antibody 4G10.
Tyrosine phosphorylation of cellular proteins in Ba/F3-GMRα cell lines expressing mutant GMRβ. Cells (107) were incubated in the absence or presence of GM-CSF (10 ng/mL) for 15 minutes. Cell lysates were immunoprecipitated with antiphosphotyrosine antibody (A) or anti-β chain antibody (B), electrophoresed through 6% to 12% gradient SDS-PAGE, and then analyzed by immunoblotting with antiphosphotyrosine antibody 4G10.
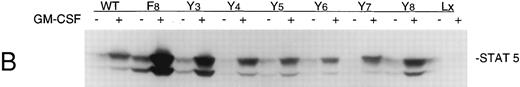
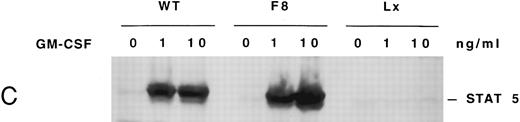
GMRβ-F8 induces tyrosine phosphorylation of JAK2 and STATs, but is impaired in phosphorylating SHC and SHP2.In all GMRβ mutants, stimulation with GM-CSF induced an increase in the level of both JAK2 and STAT 5 tyrosine phosphorylation compared with the unstimulated controls (Fig 3A). To determine if STAT phosphorylation was also detected at lower concentrations of GM-CSF, both 1 and 10 ng/mL concentrations were tested (Fig 3B). No difference between Ba/F3-GMRαβ wild-type and Ba/F3-GMRαβ F8 cells were detected in these experiments.
(A) Phosphorylation of JAK2 in mutant cell lines. Cells (107) were incubated in the absence or presence of 10 ng/mL GM-CSF for 15 minutes. Total cellular proteins were immunoprecipitated with anti-JAK2 antibody and subjected to 7.5% SDS-PAGE and immunoblot analysis with antiphosphotyrosine antibody. (B) Phosphorylation of STAT5 in mutant cell lines. Cells (5 × 106) were incubated in the absence or presence of 10 ng/mL GM-CSF for 15 minutes and cellular proteins were subjected to 7.5% SDS-PAGE and immunoblot analysis with anti-phospho-STAT antibody. (C) Phosphorylation of STAT5 in Ba/F3 −GMRαβ F8 cells in response to two concentrations of GM-CSF.
(A) Phosphorylation of JAK2 in mutant cell lines. Cells (107) were incubated in the absence or presence of 10 ng/mL GM-CSF for 15 minutes. Total cellular proteins were immunoprecipitated with anti-JAK2 antibody and subjected to 7.5% SDS-PAGE and immunoblot analysis with antiphosphotyrosine antibody. (B) Phosphorylation of STAT5 in mutant cell lines. Cells (5 × 106) were incubated in the absence or presence of 10 ng/mL GM-CSF for 15 minutes and cellular proteins were subjected to 7.5% SDS-PAGE and immunoblot analysis with anti-phospho-STAT antibody. (C) Phosphorylation of STAT5 in Ba/F3 −GMRαβ F8 cells in response to two concentrations of GM-CSF.
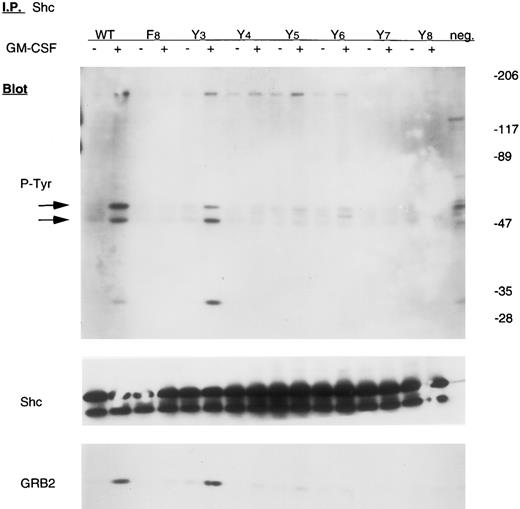
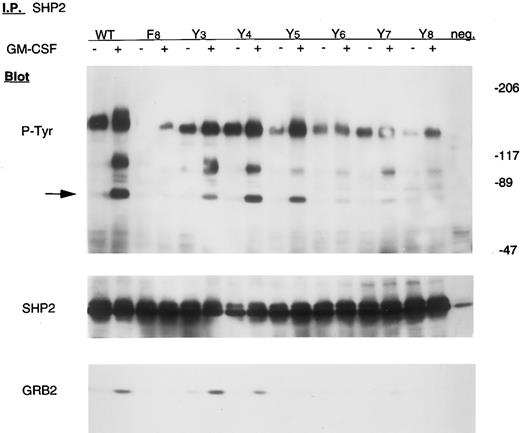
However, two proteins implicated in signaling in the p21ras pathway, SHC and SHP2, were not tyrosine phosphorylated in Ba/F3-GMRαβ F8 cells after GM-CSF stimulation, but were phosphorylated in wild-type control cells (Figs 4 and 5). The adapter protein GRB2 is known to associate with both SHC and SHP2 after activation of many growth factor receptors, including GMR, but was not detected in either SHC or SHP2 immunoprecipitates from Ba/F3-GMRαβ-F8 cells (Figs 4 and 5), consistent with the known requirement for tyrosine phosphorylation of SHC and SHP2 to bind the SH2 domain of GRB2.14 15
Tyrosine phosphorylation of Shc in mutant cell lines. Cells were incubated in the absence or presence of 10 ng/mL GM-CSF for 15 minutes. Lysates from 107 cells were immunoprecipitated with anti-SHC antibody and subjected to 6% to 12% gradient SDS-PAGE. Immunoblot analyses were performed with either antiphosphotyrosine, anti-SHC, or anti-GRB2 antibody using the same membrane, as indicated.
Tyrosine phosphorylation of Shc in mutant cell lines. Cells were incubated in the absence or presence of 10 ng/mL GM-CSF for 15 minutes. Lysates from 107 cells were immunoprecipitated with anti-SHC antibody and subjected to 6% to 12% gradient SDS-PAGE. Immunoblot analyses were performed with either antiphosphotyrosine, anti-SHC, or anti-GRB2 antibody using the same membrane, as indicated.
Tyrosine phosphorylation of SHP2 in mutant cell lines. Cells were incubated in the absence or presence of 10 ng/mL GM-CSF for 15 minutes. Lysates from 107 cells were immunoprecipitated with anti-SHP2 antibody and subjected to 6% to 12% gradient SDS-PAGE and immunoblot analysis with antiphosphotyrosine antibody. The same blot was stripped and reprobed with anti-SHP2 and anti-GRB2 antibody, as indicated.
Tyrosine phosphorylation of SHP2 in mutant cell lines. Cells were incubated in the absence or presence of 10 ng/mL GM-CSF for 15 minutes. Lysates from 107 cells were immunoprecipitated with anti-SHP2 antibody and subjected to 6% to 12% gradient SDS-PAGE and immunoblot analysis with antiphosphotyrosine antibody. The same blot was stripped and reprobed with anti-SHP2 and anti-GRB2 antibody, as indicated.
Y577 is sufficient for tyrosine phosphorylation of SHC.The SHC gene encodes at least two SH2-containing signaling proteins with molecular weight of 46 to 52 kD that are known to be transiently tyrosine phosphorylated in response to GM-CSF.14,16 As shown in Fig 4, GM-CSF failed to stimulate tyrosine phosphorylation of SHC in Ba/F3-GMRαβ-F8 cells. We therefore examined SHC phosphorylation in a series of Ba/F3 cells expressing GMRβ mutants in which a single tyrosine had been replaced (GMRβ-Y3,4,5,6,7, or 8 ). Approximately equivalent expression of each mutant βc chain was confirmed by immunoblot (Fig 1). Only the Y3 mutant (tyrosine residue 577) reconstituted GM-CSF–induced tyrosine phosphorylation of SHC and coprecipitation of GRB2 with SHC (Fig 4). These results are consistent with our previous studies showing that mutation of this tyrosine residue to phenylalanine reduced SHC phosphorylation.13
Y577, 612, or 695 is sufficient for tyrosine phosphorylation of SHP2.SHP2, an SH2-containing protein tyrosine phosphatase, is believed to link some activated growth factor receptors to the p21ras pathway.17 As shown in Fig 5, tyrosine phosphorylation of SHP2 is reduced in Ba/F3-GMRαβ-F8 cells. Interestingly, we found that replacing either F577, F612, or F695 with tyrosine (to generate GMRβ-Y3,4, and 5 , respectively) dramatically enhances SHP2 tyrosine phosphorylation. GM-CSF–induced coprecipitation of GRB2 with SHP2 was readily detected in Ba/F3-GMRαβ-Y3 and -Y4 cells, as well as in Ba/F3-GMRαβ-wild-type, but not clearly in Ba/F3-GMRαβ-Y5 (Fig 5). After GM-CSF stimulation, we have previously shown that SHP2 associates transiently with at least two other unknown cellular proteins that are tyrosine phosphorylated, migrating at 95 kD and 140 kD.30 Binding of these proteins to SHP2 was not induced by GM-CSF in Ba/F3-GMRαβ-F8 cells, but these proteins were readily detected after replacing either F577 or F612 with tyrosine (Y3 and Y4 mutants, respectively; Fig 5). Interestingly, the F-Y695 (Y5 ) mutant does not coprecipitate with pp95, but still does precipitate with pp140, despite significant tyrosine phosphorylation of SHP2 by GM-CSF in these cells.
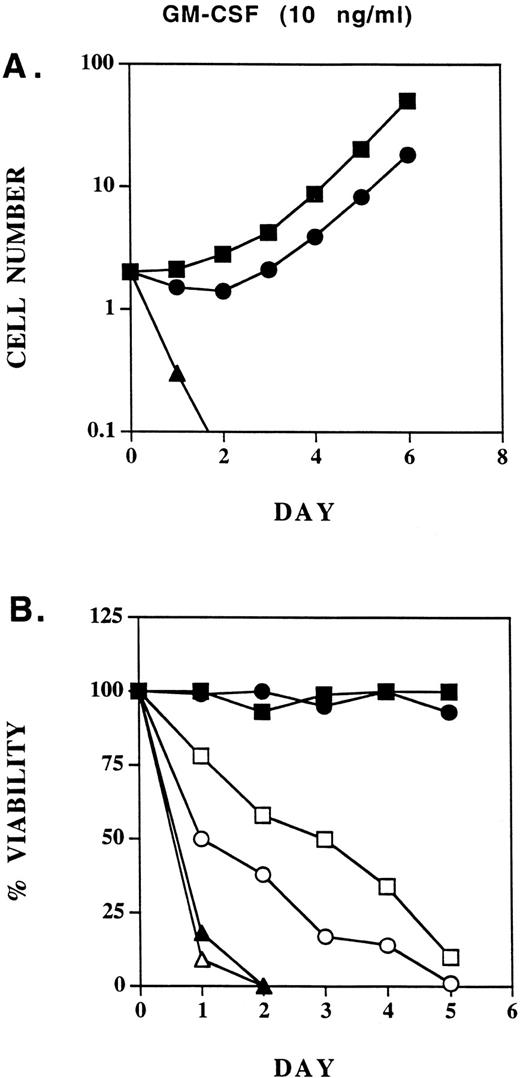
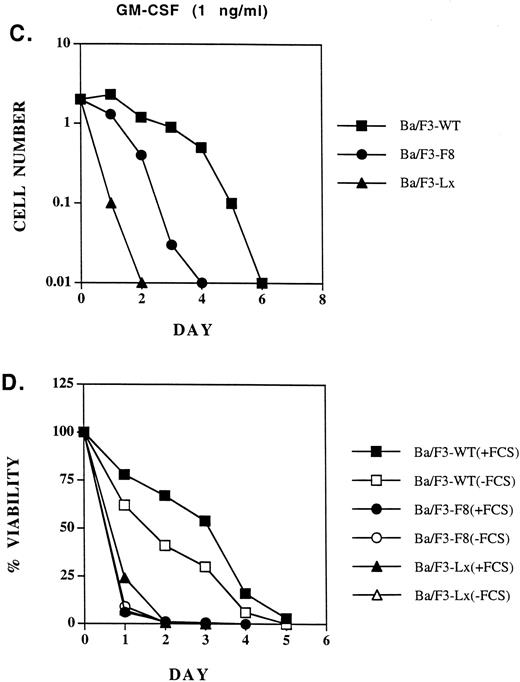
Biologic activities of GMRβ-F8 in Ba/F3 cells in response to different concentrations of GM-CSF.GM-CSF induces cell proliferation, viability, and adhesion to fibronection of a variety of hematopoietic cell lines in a dose-dependent manner. Ba/F3 cells expressing only the GMRα chain (Ba/F3-Lx) do not proliferate or survive in response to the concentrations of human GM-CSF used in this study. We looked for any defects in biologic responses that could be correlated with the signaling abnormalities described above. At 10 ng/mL, GM-CSF induced proliferation of Ba/F3 cells expressing GMRβ-F8 mutant receptors, although it was minimally, but reproducibly, less effective than GMRβ-WT (Fig 6A). Also, viability of Ba/F3-GMRαβ-F8 cells was very high in cell lines at this concentration of GM-CSF (Fig 6B). As we have previously shown,10 FCS provides an important viability signal for Ba/F3 cells in addition to GM-CSF, and neither the wild-type receptor nor the F8 mutant could maintain long-term viability in the absence of FCS (Fig 6B). At 1 ng/mL of GM-CSF, the GMRβ-F8 mutant was significantly less effective than the wild-type receptor in supporting proliferation or maintaining viability in three separate experiments (Fig 6C and D). However, the reason for this was not clear. The most likely explanation is that the GMRβ-F8 mutant has an intrinsic signaling defect, possibly related to its inability to activate the SHC or SHP2 pathways. However, it is also possible that the GMRβ-F8 receptor is somewhat unstable or not expressed at as high a level on the surface as the other mutants in this study. Interestingly, replacement of any one of the distal 6 tyrosines in the βc chain (mutants Y3,4,5,6,7, or 8 ) improved viability of Ba/F3 cells to near wild-type responses (data not shown).
Proliferation of Ba/F3-GMRαβ-WT, Ba/F3-GMRαβ-F8 , and Ba/F3-Lx (GMR-α chain alone) cells in response to 10 ng/mL GM-CSF (A) or 1 ng/mL (C). Viability (trypan blue exclusion) was monitored in the same cell lines cultured with 10 ng/mL GM-CSF (B) or 1 ng/mL (D) in the presence and absence of FCS (10% vol/vol) as indicated.
Proliferation of Ba/F3-GMRαβ-WT, Ba/F3-GMRαβ-F8 , and Ba/F3-Lx (GMR-α chain alone) cells in response to 10 ng/mL GM-CSF (A) or 1 ng/mL (C). Viability (trypan blue exclusion) was monitored in the same cell lines cultured with 10 ng/mL GM-CSF (B) or 1 ng/mL (D) in the presence and absence of FCS (10% vol/vol) as indicated.
GM-CSF is known to induce a transient increase in binding of Ba/F3 cells to fibronectin-coated surfaces.12 18 When stimulated with 10 ng/mL GM-CSF, there was an increase in the short-term adhesion to fibronection of Ba/F3-GMRαβ-F8 cells that tended to be about twofold less than Ba/F3-GMRαβ-WT cells (Fig 7), suggesting that β chain tyrosine residues contribute to, but are not required for, transient upregulation of integrin function.
Adhesion to fibronectin after GM-CSF stimulation in mutant cell lines. Cells were incubated for 6 hours in the absence or in the presence of GM-CSF (10 ng/mL) and tested for adhesion to wells previously coated with fibronectin (10 μg/mL). The figure shows a photograph of the plate with each well stained for adherent cells with Wright-Giemsa stain.
Adhesion to fibronectin after GM-CSF stimulation in mutant cell lines. Cells were incubated for 6 hours in the absence or in the presence of GM-CSF (10 ng/mL) and tested for adhesion to wells previously coated with fibronectin (10 μg/mL). The figure shows a photograph of the plate with each well stained for adherent cells with Wright-Giemsa stain.
DISCUSSION
GM-CSF has a number of important biologic effects on myeloid lineage cells, including induction of proliferation of immature cells and activation of mature neutrophils in vitro. Disruption of the GM-CSF gene in mice results in a lethal pulmonary defect associated with a defect in macrophage function.19,20 The GM-CSF receptor is expressed on hematopoietic cells, endothelial cells, and certain tumor cell lines. The receptor is believed to contain two transmembrane chains, but the stoichiometry of the receptor complex is not known. The role of the cytoplasmic domain of the GMRα in signal transduction is unclear, although it has been reported to be important in regulating glucose transport.21 If the cytoplasmic domain of the GMRα is deleted, the receptor is nonfunctional. However, it can be replaced with the cytoplasmic domain from the erythropoietin or IL-2 receptor, and even with the cytoplasmic domain of the β chain, and will then support GM-CSF–dependent proliferation and viability of hematopoietic cell lines.22 The GMRβ is believed to be required for GM-CSF signal transduction and specifically for activation of JAK2, STAT5, SHC, p21ras, SHP2, and several other signaling molecules.23-25
One of the first events detected after stimulation by GM-CSF is tyrosine phosphorylation of GMRβ (GMRα is not tyrosine phosphorylated after stimulation).6 There are 8 tyrosine residues in the β chain, but the sites of phosphorylation have not been identified.26 Previous studies with deletion mutants of the GMRβ have identified a number of functional domains with the receptor, and it has been suggested that tyrosine residues within these functional domains may be involved in attracting signaling molecules with SH2 domains.6,7,10 For example, we have shown that a GMRβ truncated at amino acid 626 supports short-term proliferation, but not long-term proliferation in serum-free medium, whereas a GMRβ truncated at amino acid 763 has no detectable defect in proliferation or viability.10 The presence of a viability domain distal to amino acid 544 is further supported by recent work that shows that Ba/F3-GMRαβΔ544 cell lines died by apoptosis in the absence of serum despite a transient mitogenic response to GM-CSF. Interestingly, expression of an activated Ras protein in Ba/F3-GMRαβΔ544 cell lines complemented the viability defect.27 Because Ras activation maps to amino acids 627-763, the Ras pathway may account for part or all of the viability signal.27 Also, there is evidence that tyrosine 577 is involved in signaling, because mutation of this amino acid to phenylalanine results in loss of tyrosine phosphorylation of SHC, although this mutant does not have a defect in proliferation or viability.9,13 Similarly, analysis of GMRβ with a Y-F mutation at tyrosine 750 suggested that this tyrosine residue might be a site of phosphorylation.10
In this study, we aimed to define the contributions of the tyrosine residues of the β chain to signal transduction and biologic effects of GM-CSF in the Ba/F3 cell line. By analogy to other growth factor receptors, we anticipated that tyrosine phosphorylation of specific residues would be required to attract SH2-containing signaling molecules, such as STATs, SHP1, SHP2, SHC, and others, and that defects in proliferation or other biologic events would be associated with β chain mutants lacking sites of tyrosine phosphorylation.28 Because the sites of tyrosine phosphorylation have not been mapped, a mutant GMRβ was generated by polymerase chain reaction mutagenesis in which all 8 cytoplasmic tyrosine residues were converted to phenylalanine residues. Interestingly, this mutant was still capable of activating JAK2 and also induced tyrosine phosphorylation of STATs and many other cellular proteins. The only signaling defects in Ba/F3-GMRαβ-F8 cells observed in this study were significant reductions in the phosphorylation of SHP2 and SHC.
The reduced tyrosine phosphorylation of SHC in the Ba/F3-GMRαβF8 cells is expected. Previous studies have shown that SHC tyrosine phosphorylation was absent in cell lines containing GMRβ truncated at amino acid 544, and we and others have previously shown that mutation of tyrosine 577 to phenylalanine reduces SHC phosphorylation.9,13 SHC is an SH2-containing protein that is tyrosine phosphorylated in response to several growth factors and cytokines, as well as by oncogenic protein tyrosine kinases.16 Furthermore, overexpression of SHC has been shown to make cells hypersensitive to GM-CSF.14 Tyrosine phosphorylation of SHC is thought to be required for binding of Grb2-Sos via the SH2 domain of Grb2, thus activating p21ras nucleotide exchange. However, we have previously shown that Ba/F3 cells expressing the β chain tyrosine 577 mutant are phenotypically normal and do not have any detectable defect in proliferation or viability.13 The results presented here extend these previous studies by showing that Y577 is both necessary and sufficient, by itself, for SHC tyrosine phosphorylation.
The reduced tyrosine phosphorylation of SHP2 in response to GM-CSF in Ba/F3-GMRαβF8 cells is of interest. SHP2 has been shown to be phosphorylated after stimulation of several different mammalian growth factor receptors29 and is closely related to the product of the Drosophila corkscrew gene, previously shown to be required for signaling downstream of the Torso receptor tyrosine kinase. Because tyrosine phosphorylated SHP2 can bind GRB2-SOS, it is likely to induce activation of the p21ras pathway in some cells. In the case of the GM-CSF receptor, SHP2 is known to be a prominent tyrosine phosphoprotein after stimulation and may well provide a link between the β chain and p21ras, in addition to the SHC-GRB2-SOS pathway. SHP2 also binds to at least two other tyrosine phosphoproteins after GM-CSF stimulation, pp95 and pp140.30 It is possible that the pp140 protein is composed in part or in whole of SHIP, the recently cloned inositol 5 phosphate phosphatase, which can also interact with SHC.31 32 If so, both SHP2 and SHC may activate other signaling pathways in addition to p21ras.
Three different tyrosine residues were found to be sufficient to induce tyrosine phosphorylation of SHP2 in these studies (Y577, Y612, or Y695), although Y695 by itself did not lead to binding of GRB2, p95, or p140 to SHP2. These results suggest that the interactions of SHP2 and its binding molecules with the β chain may be complex, and it is quite possible that it is one or more of the binding proteins (such as pp95 or pp140) that bind directly to the β chain, rather than SHP2 itself.
Interestingly, the biologic activities of the GMRβ-F8 receptor in Ba/F3 cells were dependent on GM-CSF dose. At a concentration of GM-CSF of 10 ng/mL, Ba/F3-GMRαβ-F8 cells proliferated readily, but were slightly slower than cells expressing wild-type receptors. However, at concentrations of GM-CSF that were suboptimal, such as 0.1 to 1 ng/mL, Ba/F3 cells expressing GMRβ-F8 more rapidly lost viability than did cells expressing wild-type GMRβ receptors. This was true for two different, independently derived clones of Ba/F3-GMRαβ-F8 cells, but it is possible that the F8 receptor is expressed at lower levels on the surface of the cells compared with wild-type. It is worth noting that Ba/F3 cells do express endogenous murine βc chains. It is possible that, at higher concentrations of GM-CSF, the murine and human receptors can interact in a receptor complex and that both chains participate in signaling. It may be of interest to compare the functions of β chain mutants in Ba/F3 cells with those in other cell lines that do not have endogenous GM-CSF receptor components.
The results presented here extend our understanding of the signaling functions of the βc chain. We have mapped the tyrosines involved in two signaling pathways, SHP2 and SHC, to specific tyrosine residues. Indirectly, our results suggest that tyrosines 577, 612, and 696 are each potential sites of tyrosine phosphorylation, but direct proof has not been obtained. The Ba/F3 cell lines described here will be useful in determining the biologic functions associated with SHP2 and SHC signaling. Finally, it is of interest that, although tyrosine phosphorylation of the βc chain in Ba/F3 cells appears to be important for proliferation and viability at low concentrations of GM-CSF, it is not as important at higher concentrations. It is anticipated that a more comprehensive model of GM-CSF signaling will emerge as the present data are combined with results obtained from studies of internal deletion mutants of this receptor.
Supported by Public Health Services Grants No. CA 36167 and CA 34183.
Address reprint requests to James D. Griffin, MD, Division of Hematologic Malignancies, Dana-Farber Cancer Institute, 44 Binney St, Boston, MA 02115.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal