Abstract
Interferon-γ (IFN-γ) upregulates expression of certain genes in monocytes, including cell-surface molecules such as HLA class II, B7, and ICAM-1. IFN-γ also potentiates production of cytokines such as tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β) and IL-12. Conversely, IL-10 downregulates expression of many of these same genes and often antagonizes the effects of IFN-γ. IL-10 is known to inhibit TNF-α production in lipopolysaccharide (LPS)-stimulated monocytes; however, the effects of IL-10 on TNF receptor (TNF-R) expression are not well defined. We examined the effects of IL-10 on production of both membrane-associated (m) and soluble (s) TNF-R type II (sTNF-RII) by purified human CD14+ monocytes. We also compared the effects of IFN-γ and IL-10 on production of TNF-α and sTNF-RII by these cells. Monocytes constitutively expressed low levels of TNF-RII mRNA and mTNF-RII protein. LPS stimulation induced rapid, but transient loss (shedding) of mTNF-RII molecules and a delayed, but marked increase in TNF-RII mRNA levels. IL-10 increased expression of both mTNF-RII and sTNF-RII by LPS-stimulated monocytes, whereas IFN-γ decreased their expression. The increased levels of sTNF-RII in cultures of IL-10–treated monocytes correlated directly with increased levels of TNF-RII mRNA and inversely with the levels of TNF-α mRNA. The ability of IL-10 to upregulate TNF-RII gene expression was transcriptionally mediated because actinomycin D blocked this effect. Furthermore, IL-10 treatment did not alter the half-life of TNF-RII mRNA transcripts in LPS-stimulated monocytes. To further examine the mechanism by which IL-10 potentiates TNF-RII gene expression, a 1.8-kb fragment of the human TNF-RII promoter cloned into a luciferase expression vector (pGL2-basic) was transfected into the IL-10–responsive macrophage cell line, RAW264.7. Although IL-10 alone induced only minimal promoter activity in these cells, it markedly increased the LPS-induced response, providing further evidence that the ability of IL-10 to amplify TNF-RII gene expression is transcriptionally controlled. Together, these findings demonstrate that IL-10 coordinately downregulates expression of TNF-α and upregulates expression of TNF-RII, particularly the soluble form of this receptor, in monocytes.
TUMOR NECROSIS FACTOR-α (TNF-α) is one of the primary proinflammatory cytokines produced by mononuclear phagocytes after stimulation with lipopolysaccharide (LPS). TNF-α is initially synthesized as a 26-kD transmembrane protein (mTNF) that is cleaved by a site-specific metalloprotease to generate the mature 17-kD form.1,2 Normally, expression of TNF-α in LPS-stimulated monocytes is short-lived, even in the continued presence of LPS. However, certain cytokines such as interferon-γ (IFN-γ) and granulocyte-macrophage colony-stimulating factor (GM-CSF) markedly upregulate production of TNF-α in LPS-stimulated monocytes.3-5 In contrast, other cytokines, such as interleukin-4 (IL-4), IL-10, and IL-13, suppress TNF-α production in monocytes.6-9
There are two major cell-surface receptors for TNF-α, denoted TNF receptor type I (TNF-RI; p55; CD120a) and TNF-RII (p75; CD120b). The tissue distribution and biological functions of these receptors overlap, but are not identical.10 Most cells express both types of TNF-R; however, the relative ratios of TNF-RI to TNF-RII vary considerably depending on the cell type and tissue of origin. For example, hematopoietic cells such as lymphocytes and monocytes predominantly express TNF-RII, whereas nonhematopoietic cells such as endothelial cells and fibroblasts preferentially express TNF-RI. One study estimated that human monocytes express approximately 230 TNF-RI and approximately 3,800 TNF-RII per cell.11 Activation of monocytes by agents such as LPS or TNF-α induces rapid shedding of membrane TNF-RII molecules12-14 and internalization of TNF-RI molecules.15,16 Soluble (s) TNF-RII molecules are generated by proteolytic cleavage of the peptide bond between amino acids 213 and 214 (Ala-Val) by a sequence-specific endopeptidase that releases a 40-kD extracellular fragment.17 Shed TNF-R molecules often function as TNF antagonists by competing for ligand with membrane-bound (m) TNF-R.17,18 However, when sTNF-R concentrations are low, they may actually enhance TNF activity by stabilizing TNF molecules and prolonging their availability for binding to cell-surface receptors.19 20
IL-10 and IFN-γ differentially regulate multiple macrophage functions. For example, IFN-γ upregulates and IL-10 downregulates expression of major histocompatibility complex (MHC) class II molecules on monocytes.21,22 Similarly, IFN-γ upregulates and IL-10 downregulates expression of ICAM-1 (CD54) and the CD28 counter-receptor, B7, on monocytes.23,24 The precise mechanism by which IL-10 antagonizes the ability of IFN-γ to upregulate expression of ICAM-1 and B7 has not been defined, although in the case of ICAM-1 gene expression, this effect has been shown to be transcriptionally mediated.25 IFN-γ upregulates production of many LPS-inducible cytokines, including TNF-α, IL-1β, IL-6, and IL-12 in monocytes,3-7,26 whereas IL-10 downregulates production of these same cytokines.8,9,27 IFN-γ and IL-10 also differentially regulate production of various reactive oxygen intermediates. For example, IFN-γ and IL-10 inversely regulate nitric oxide synthesis in macrophages.28-30 IL-10 also inhibits IFN-γ–induced superoxide anion production by monocytes.31 Thus, a number of monocyte functional activities are inversely regulated by IFN-γ and IL-10.
Activation of human monocytes with LPS induces rapid, protein kinase C–dependent transmodulation of TNF-R.12-15,32,33 Downregulation of TNF-R expression is transient, and mTNF-R levels are quickly restored as a result of increased receptor biosynthesis.13,14 33 Although mTNF-R are reexpressed on LPS-stimulated monocytes within 3 to 4 hours poststimulation, certain cytokines can affect the rate and magnitude of receptor reexpression, particularly of TNF-RII. These same cytokines also affect production and release of sTNF-R. In the present study, we demonstrate that IL-10 and IFN-γ inversely regulate production of TNF-α and sTNF-RII in LPS-stimulated monocytes. We also show that IL-10 can antagonize the effects of IFN-γ on production of these molecules. Thus, IL-10 regulates monocyte-associated TNF activity at two levels: by decreasing production of TNF-α and by increasing production of sTNF-RII. In contrast, IFN-γ induces the opposite effects.
MATERIALS AND METHODS
Medium and reagents. The complete medium used for monocyte culture consisted of RPMI-1640 medium (GIBCO, Grand Island, NY) supplemented with 10% (vol/vol) heat-inactivated fetal calf serum (Hyclone, Logan, UT), 2 mmol/L L-glutamine, and 50 μg/mL gentamycin. LPS (Escherichia coli 055:B5) was obtained from Sigma Chemical (St Louis, MO). Recombinant human IFN-γ (rhIFN-γ) was provided by Genentech (South San Francisco, CA). Recombinant human IL-10 (rhIL-10) was provided by Dr Kevin Moore (DNAX Research Institute, Palo Alto, CA). The antihuman TNF-RII (CD120b) antibody was a phycoerythrin (PE)-conjugated murine immunoglobulin G1 (IgG1) monoclonal antibody obtained from Caltag Laboratories (South San Francisco, CA).
Cells. Normal human peripheral blood monocytes were isolated by countercurrent centrifugal elutriation in a Beckman JE-6B centrifugal elutriator (Beckman Instruments, Fullerton, CA) as previously described.34 The elutriated monocyte fraction consisted of more than 95% monocytes as determined by histologic staining and FACS analysis after labeling with the anti-CD14 monoclonal antibody (mAb), Leu-M3 (Becton Dickinson, Mountain View, CA). Monocytes were routinely cultured at 5 × 106 cells/mL in complete medium in round-bottom, polystyrene tubes (17 × 100 mm). After incubation for various time periods, cell-free supernatants were harvested and stored at −20°C until assay.
Enzyme-linked immunosorbent assays. TNF-α protein levels in culture supernatants were determined using enzyme-linked immunosorbent assay (ELISA) kits obtained from BioSource International (Camarillo, CA). The levels of sTNF-RII protein were determined using ELISA kits obtained from R & D Systems, Minneapolis, MN. Assays were performed according to the manufacturer's specifications. All samples were assayed in triplicate. Results are expressed in nanograms per milliliter of TNF-α or sTNF-RII. The lower limit of detection for these assays was approximately 5 pg/mL.
Northern blot analysis. Total RNA was isolated from cultured monocytes by the acid guanidinium thiocyanate-phenol-chloroform extraction method as previously described.35 The final RNA precipitate was pelleted by microcentrifugation and redissolved in 100 μL of diethyl pyrocarbonate (DEPC)-treated sterile water. After quantitation by spectrophotometry, equivalent amounts of RNA (10 μg per lane) were size-fractionated by electrophoresis in 1% agarose gels containing 2.2 mol/L formaldehyde. The RNA was then blotted by overnight capillary transfer onto Nytran membranes (Schleicher & Schuell, Keene, NH), and crosslinked by exposure to UV light. The membranes were then prehybridized, hybridized, and washed according to standard procedures. The TNF-α probe was a 1.1-kb Pst I insert of the human TNF-α cDNA, pE4, obtained from American Type Culture Collection (ATCC, Rockville, MD).36 The TNF-RII probe was a 640-bp Not I-EcoRI fragment of the human TNF-RII cDNA,37 kindly provided by Dr Howard Young (National Cancer Institute, Frederick, MD). Gel-purified insert DNA was radiolabeled by the random primer method of Feinberg and Vogelstein38 to a specific activity of ≥108 cpm/μg.
FACS analysis. mTNF-RII expression was measured by FACS analysis after labeling with anti–TNF-RII mAb. After treatment, cells were harvested, washed, and incubated with PE-conjugated anti–TNF-RII mAb (CD120b) for 30 minutes on ice. The cells were then washed three times in buffer consisting of Ca- and Mg-free Dulbecco's phosphate-buffered saline (DPBS), 1% fetal bovine serum (FBS), 1% human AB+ serum, and 0.1% NaN3 . The cells were then analyzed on a Becton Dickinson FACScan. Histograms represent the mean fluorescence intensity of 1 × 104 cells.
Transfections. The human TNF-RII promoter construct, pGL2-1.8-kb HindIII-luc, has been previously described.39 This construct is comprised of 1.8 kb of 5′-flanking sequence immediately upstream of the transcription start site coupled to the luciferase reporter gene, pGL2-basic (Promega, Madison, WI). Transfection of this luciferase reporter gene into RAW264.7 cells was performed as described previously.40 Briefly, RAW264.7 cells were obtained from the ATCC and maintained in medium consisting of RPMI-1640 plus 10% FBS. Cells were cultured under nonadherent conditions in bacterial culture petri dishes. Twenty-four hours before transfection, cells were subcultured at 5 × 105 cells/mL in fresh medium. Transfections were performed using a modified calcium phosphate method. For each transfection, 7.5 × 106 cells were pelleted in polypropylene tubes and resuspended in 1 mL of a calcium phosphate precipitate containing 15 μg of plasmid DNA followed by incubation at room temperature for 20 minutes. Nine milliliters of medium was then added and the cells were transferred to 100-mm bacterial petri dishes and incubated at 37°C for 2 hours. Chloroquine was added to a concentration of 100 μmol/L and the incubation was continued for 3 hours. The cells were removed from the dishes and washed once with serum-free RPMI-1640. The cells were then subjected to a 15% glycerol (in phosphate-buffered saline [PBS]) shock for 1.5 minutes, washed with 10 mL of RPMI-1640, and resuspended in 30 mL of complete medium. The cells were then plated at 5 mL per 60-mm tissue culture dish and incubated at 37°C for 40 hours before stimulation for 6 hours with LPS (100 ng/mL) and/or IL-10 (10 ng/mL). After stimulation, the cells were harvested and assayed for luciferase activity as previously described.40 Results are expressed as relative light units (RLU). In all experiments, luciferase activity was normalized to total protein concentration of the cell lysates determined using the Bio-Rad protein assay kit (Richmond, CA).
RESULTS
IL-10 upregulates and IFN-γ downregulates expression of mTNF-RII in LPS-stimulated monocytes. To compare the effects of IFN-γ and IL-10 on surface expression of TNF-RII, we treated purified CD14+ monocytes with medium alone, IFN-γ, IL-10, or IFN-γ plus IL-10 for 6 hours. The cells were then harvested, washed, and labeled with PE-conjugated mouse antihuman TNF-RII (CD120b) mAb and analyzed by FACS. As shown in Fig 1A, monocytes expressed a basal level of mTNF-RII that was decreased by treatment with IFN-γ and increased by treatment with IL-10. Cotreatment of monocytes with both cytokines together resulted in mTNF-RII levels that were equivalent to the medium control. Thus, IL-10 normalized mTNF-RII expression in IFN-γ–treated cells.
Effects of IFN-γ and IL-10 on membrane expression of TNF-RII (CD120b) in control and LPS-stimulated monocytes. (A) Normal human monocytes (1 × 106 cells/mL) were incubated in medium alone or in medium containing IFN-γ (10 ng/mL), IL-10 (10 ng/mL), or both for 6 hours. Cells were then labeled with PE-conjugated mouse antihuman TNF-RII (CD120b) mAb and analyzed by FACS. (B) Duplicate cultures of normal human monocytes were pretreated for 30 minutes with medium alone or medium containing IFN-γ (10 ng/mL), IL-10 (10 ng/mL), or both. LPS (10 ng/mL) was then added to one of each pair of these cultures and incubated for an additional 6 hours. At the end of the incubation period, cells were labelled with PE-conjugated anti–TNF-RII mAb and analyzed by FACS. Values represent the mean fluorescence intensities of 1 × 104 cells.
Effects of IFN-γ and IL-10 on membrane expression of TNF-RII (CD120b) in control and LPS-stimulated monocytes. (A) Normal human monocytes (1 × 106 cells/mL) were incubated in medium alone or in medium containing IFN-γ (10 ng/mL), IL-10 (10 ng/mL), or both for 6 hours. Cells were then labeled with PE-conjugated mouse antihuman TNF-RII (CD120b) mAb and analyzed by FACS. (B) Duplicate cultures of normal human monocytes were pretreated for 30 minutes with medium alone or medium containing IFN-γ (10 ng/mL), IL-10 (10 ng/mL), or both. LPS (10 ng/mL) was then added to one of each pair of these cultures and incubated for an additional 6 hours. At the end of the incubation period, cells were labelled with PE-conjugated anti–TNF-RII mAb and analyzed by FACS. Values represent the mean fluorescence intensities of 1 × 104 cells.
Previous studies have shown that LPS stimulation of monocytes induces rapid transmodulation of cell-surface TNF-R.12-14,33 Shedding of mTNF-RII is transient, and surface levels are restored to preactivation levels or higher within 4 hours poststimulation.13,14 33 To compare the effects of IFN-γ and IL-10 on expression of mTNF-RII, monocytes were treated with IFN-γ, IL-10, or both in the presence or absence of LPS for 6 hours. At the end of the incubation period, the cells were harvested and analyzed by FACS. As shown in Fig 1B, IFN-γ treatment decreased the relative levels (mean fluorescence intensity) of mTNF-RII in both control (nonstimulated) and LPS-stimulated monocytes. In contrast, IL-10 upregulated mTNF-RII levels in both groups, and normalized mTNF-RII levels in IFN-γ–treated cells. Although IL-10 upregulated mTNF-RII levels in both control (medium) and LPS-stimulated cells, the increase was more pronounced in LPS-stimulated cells. Thus, IL-10 upregulated mTNF-RII levels and antagonized the inhibitory effect of IFN-γ on mTNF-RII expression.
IL-10 and IFN-γ reciprocally regulate production of sTNF-RII and TNF-α in LPS-stimulated monocytes. The ability of IL-10 to increase mTNF-RII levels in LPS-stimulated monocytes suggested that it might upregulate synthesis of TNF-RII. To examine the effects of IL-10 on TNF-RII gene expression, we treated monocytes with LPS or LPS plus IL-10 (10 ng/mL) for 0 to 8 hours and then evaluated TNF-RII mRNA levels by Northern blot analysis. As shown in Fig 2, monocytes constitutively expressed a low basal level of TNF-RII mRNA. LPS stimulation induced a delayed, but pronounced increase in TNF-RII mRNA levels. Costimulation with LPS plus IL-10 resulted in more rapid and higher net levels of expression of TNF-RII mRNA than LPS alone. In contrast to its enhancing effects on TNF-RII expression, IL-10 markedly inhibited expression of TNF-α. Although IL-10 did not prevent the initial induction of TNF-α mRNA in LPS-stimulated monocytes, it greatly accelerated downregulation of TNF-α mRNA levels. Thus, IL-10 reciprocally regulated TNF-α and TNF-RII mRNA levels in these cells.
Temporal analysis of the effects of IL-10 on TNF-RII and TNF-α mRNA levels in LPS-stimulated monocytes. Monocytes (5 × 106 cells/mL) were pretreated with medium alone or rhIL-10 (10 ng/mL) for 30 minutes at 37°C and then stimulated with LPS (100 ng/mL) for 0, 1, 2, 4, or 8 hours. At the designated time points, cells were harvested, RNA isolated, and analyzed by Northern blotting using radiolabeled cDNA probes specific for TNF-α and TNF-RII. Similar results were obtained in 2 separate experiments.
Temporal analysis of the effects of IL-10 on TNF-RII and TNF-α mRNA levels in LPS-stimulated monocytes. Monocytes (5 × 106 cells/mL) were pretreated with medium alone or rhIL-10 (10 ng/mL) for 30 minutes at 37°C and then stimulated with LPS (100 ng/mL) for 0, 1, 2, 4, or 8 hours. At the designated time points, cells were harvested, RNA isolated, and analyzed by Northern blotting using radiolabeled cDNA probes specific for TNF-α and TNF-RII. Similar results were obtained in 2 separate experiments.
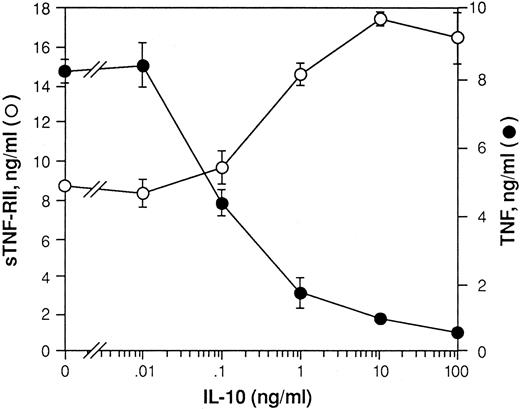
Monocytes constitutively release a low level of sTNF-R, which purportedly contributes to the circulating levels of sTNF-RII present in the sera of all normal individuals.41 42 LPS stimulation induces rapid shedding of mTNF-RII by monocytes. The total amount of sTNF-RII produced by these cultures over time represents a combination of that which is initially shed from the cell surface plus at least a portion of that which is newly synthesized. To determine if the effects of IL-10 on TNF-RII mRNA levels are associated with changes in the levels of sTNF-RII produced, we treated monocytes with LPS or LPS plus increasing concentrations of IL-10. After 24 hours, supernatants were harvested and assayed by ELISA for sTNF-RII protein. As shown in Fig 3, IL-10 induced a dose-dependent increase in the levels of sTNF-RII production. In contrast, IL-10 inhibited production of TNF-α in a dose-dependent manner. Thus, the reciprocal effects of IL-10 on production of sTNF-RII and TNF-α correlated well with its effects on expression of mRNA for these two proteins.
Effect of IL-10 on production of TNF-α and sTNF-RII by LPS-stimulated monocytes. Monocytes (5 × 106 cells/mL) were pretreated with increasing concentrations of rhIL-10 (0-100 ng/mL) for 30 minutes at 37°C. Cells were then stimulated with LPS (10 ng/mL) and cultured overnight. Supernatants were then harvested and assayed by ELISA for TNF-α and sTNF-RII. The levels of TNF-α and sTNF-RII protein in ng/mL are shown. Values represent the mean ± SEM for triplicate determinations of individual culture supernatants. Results are representative of 3 identical experiments.
Effect of IL-10 on production of TNF-α and sTNF-RII by LPS-stimulated monocytes. Monocytes (5 × 106 cells/mL) were pretreated with increasing concentrations of rhIL-10 (0-100 ng/mL) for 30 minutes at 37°C. Cells were then stimulated with LPS (10 ng/mL) and cultured overnight. Supernatants were then harvested and assayed by ELISA for TNF-α and sTNF-RII. The levels of TNF-α and sTNF-RII protein in ng/mL are shown. Values represent the mean ± SEM for triplicate determinations of individual culture supernatants. Results are representative of 3 identical experiments.
We next examined the effects of IFN-γ treatment on TNF-α and TNF-RII expression in LPS-stimulated monocytes. Similar to the experiment shown in Fig 2, monocytes were treated with LPS or LPS plus rhIFN-γ (10 ng/mL) for 0 to 8 hours, and changes in TNF-RII mRNA levels were examined by Northern blot analysis (Fig 4). In contrast to IL-10, IFN-γ downregulated TNF-RII mRNA levels and markedly increased the levels of TNF-α mRNA in LPS-stimulated monocytes. Thus, the effects of IFN-γ on TNF-α and TNF-RII expression were exactly the opposite of those induced by IL-10. Furthermore, in contrast to IL-10, IFN-γ downregulated production of sTNF-RII and upregulated production of TNF-α in a dose-dependent manner (Fig 5).
Temporal analysis of the effects of IFN-γ on TNF-RII and TNF-α mRNA levels in LPS-stimulated monocytes. Monocytes (5 × 106 cells/mL) were pretreated with medium alone or rhIFN-γ (10 ng/mL) for 30 minutes at 37°C and then stimulated with LPS (100 ng/mL) for 0, 1, 2, 4, or 8 hours. At the designated time points, cells were harvested, RNA isolated, and analyzed by Northern blotting using radiolabeled cDNA probes specific for TNF-α and TNF-RII. Similar results were obtained in 2 separate experiments.
Temporal analysis of the effects of IFN-γ on TNF-RII and TNF-α mRNA levels in LPS-stimulated monocytes. Monocytes (5 × 106 cells/mL) were pretreated with medium alone or rhIFN-γ (10 ng/mL) for 30 minutes at 37°C and then stimulated with LPS (100 ng/mL) for 0, 1, 2, 4, or 8 hours. At the designated time points, cells were harvested, RNA isolated, and analyzed by Northern blotting using radiolabeled cDNA probes specific for TNF-α and TNF-RII. Similar results were obtained in 2 separate experiments.
Effect of IFN-γ on production of TNF-α and sTNF-RII by LPS-stimulated monocytes. Monocytes (5 × 106 cells/mL) were pretreated with increasing concentrations of rhIFN-γ (0-100 ng/mL) for 30 minutes at 37°C. Cells were then stimulated with LPS (10 ng/mL) and cultured overnight. Supernatants were then harvested and assayed by ELISA for TNF-α and sTNF-RII. The levels of TNF-α and sTNF-RII protein in ng/mL are shown. Values represent the mean ± SEM of triplicate determinations. Results are representative of 3 identical experiments.
Effect of IFN-γ on production of TNF-α and sTNF-RII by LPS-stimulated monocytes. Monocytes (5 × 106 cells/mL) were pretreated with increasing concentrations of rhIFN-γ (0-100 ng/mL) for 30 minutes at 37°C. Cells were then stimulated with LPS (10 ng/mL) and cultured overnight. Supernatants were then harvested and assayed by ELISA for TNF-α and sTNF-RII. The levels of TNF-α and sTNF-RII protein in ng/mL are shown. Values represent the mean ± SEM of triplicate determinations. Results are representative of 3 identical experiments.
IL-10 antagonizes the effects of IFN-γ on production of TNF-α and sTNF-RII by monocytes. IL-10 has been shown to antagonize a number of IFN-γ–induced responses in human monocytes.8,9,22-24,28-31 IFN-γ also suppresses endogenous IL-10 production in LPS-stimulated monocytes.43 44 We tested whether the effects of IFN-γ on TNF-α and TNF-RII expression could be overcome by cotreatment with IL-10. Duplicate monocyte cultures were incubated overnight (≈18 hours) in medium alone (control) or in medium containing IFN-γ (10 ng/mL), IL-10 (10 ng/mL), or both. On the following day, the cells were stimulated with LPS or medium alone for 6 hours. At the end of the incubation period, supernatants were harvested and RNA extracts were prepared. As shown in Fig 6A, LPS stimulation upregulated mRNA levels for TNF-α and TNF-RII. Pretreatment with IFN-γ suppressed TNF-RII mRNA levels in LPS-stimulated monocytes. Conversely, IFN-γ priming markedly upregulated induction of TNF-α mRNA in LPS-stimulated monocytes. In contrast to the effects of brief (30 minutes) pretreatment with IL-10 on LPS-induced TNF-α and sTNF-RII mRNA levels (Fig 2), pretreatment of monocytes with IL-10 overnight (18 hours) resulted in only modest changes in TNF-α and sTNF-RII mRNA levels following stimulation with LPS. However, IL-10 completely inhibited both the suppression of TNF-RII mRNA levels and the potentiation of TNF-α expression in IFN-γ–primed monocytes. Thus, IL-10 normalized expression of TNF-RII and TNF-α in IFN-γ–treated cells. The inverse effects of IFN-γ and IL-10 on TNF-RII and TNF-α gene expression were associated with similar changes in the levels of TNF-α and sTNF-RII protein production (Fig 6B). Once again, IL-10 antagonized the effects of IFN-γ on production of both TNF-α and sTNF-RII.
IL-10 antagonizes the effects of IFN-γ on production of TNF-α and TNF-RII in monocytes. Monocytes (5 × 106 cells/mL) were cultured overnight (18 hours) in medium alone (control) or in medium containing rhIFN-γ (10 ng/mL), rhIL-10 (10 ng/mL), or both, and then were stimulated or not with LPS (10 ng/mL) for 6 hours. At the end of the incubation period, supernatants were harvested and RNA extracts were prepared. (A) Levels of TNF-α and TNF-RII mRNA determined by Northern blot analysis. (B) Levels of TNF-α and sTNF-RII protein released by LPS-stimulated cells measured by ELISA. Values represent the mean ± SEM for triplicate determinations. Similar results were obtained in 2 separate experiments.
IL-10 antagonizes the effects of IFN-γ on production of TNF-α and TNF-RII in monocytes. Monocytes (5 × 106 cells/mL) were cultured overnight (18 hours) in medium alone (control) or in medium containing rhIFN-γ (10 ng/mL), rhIL-10 (10 ng/mL), or both, and then were stimulated or not with LPS (10 ng/mL) for 6 hours. At the end of the incubation period, supernatants were harvested and RNA extracts were prepared. (A) Levels of TNF-α and TNF-RII mRNA determined by Northern blot analysis. (B) Levels of TNF-α and sTNF-RII protein released by LPS-stimulated cells measured by ELISA. Values represent the mean ± SEM for triplicate determinations. Similar results were obtained in 2 separate experiments.
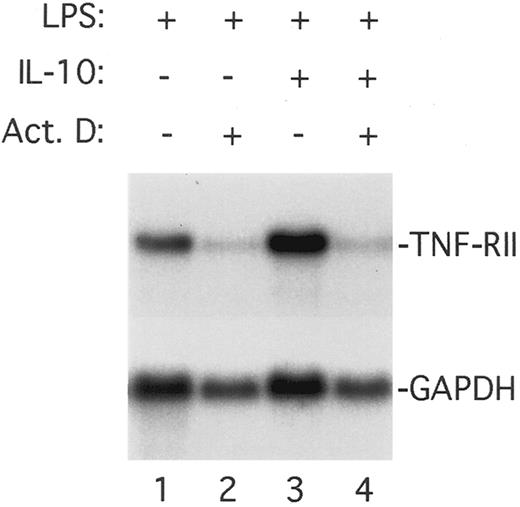
IL-10 upregulates LPS-induced TNF-RII promoter activity. The ability of IL-10 to potentiate TNF-RII gene expression in monocytes may be mediated by a transcriptional and/or posttranscriptional mechanism(s). To determine if the potentiating effects of IL-10 reflect an increase in transcription of the endogenous TNF-RII gene, we stimulated monocytes with LPS or LPS plus IL-10 in the presence or absence of the transcriptional inhibitor, actinomycin D. After incubation for 3 hours, the cultures were harvested, RNA was isolated, and the levels of TNF-RII mRNA were analyzed by Northern blotting. As shown in Fig 7, the ability of IL-10 to upregulate TNF-RII gene expression in LPS-stimulated monocytes was abrogated by treatment with actinomycin D. These results demonstrate that the effects of IL-10 are transcriptionally mediated, and are consistent with our finding (discussed later) that IL-10 potentiates TNF-RII promoter activity in a transiently transfected macrophage cell line.
Effect of actinomycin D on the ability of IL-10 to upregulate TNF-RII mRNA levels in LPS-stimulated monocytes. Monocytes (5 × 106 cells/mL) were preincubated for 30 minutes at 37°C with medium alone or rhIL-10 (10 ng/mL) in the presence or absence of actinomycin D (5 μg/mL), and then stimulated with LPS (100 ng/mL) for an additional 3 hours. At the end of the incubation period, cells were harvested, RNA isolated, and levels of TNF-RII mRNA determined by Northern blotting.
Effect of actinomycin D on the ability of IL-10 to upregulate TNF-RII mRNA levels in LPS-stimulated monocytes. Monocytes (5 × 106 cells/mL) were preincubated for 30 minutes at 37°C with medium alone or rhIL-10 (10 ng/mL) in the presence or absence of actinomycin D (5 μg/mL), and then stimulated with LPS (100 ng/mL) for an additional 3 hours. At the end of the incubation period, cells were harvested, RNA isolated, and levels of TNF-RII mRNA determined by Northern blotting.
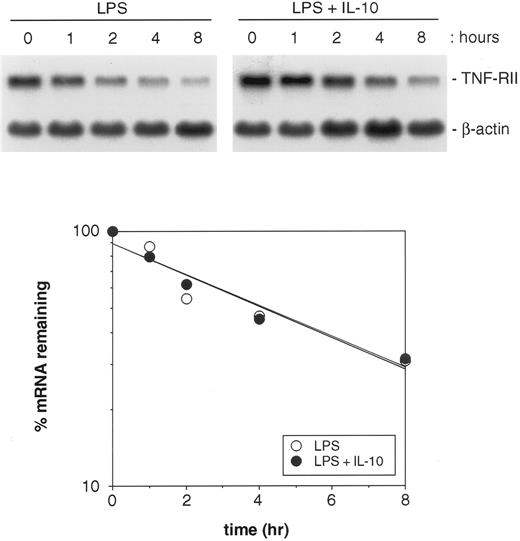
To determine whether IL-10 treatment prolongs the half-life of TNF-RII mRNA, monocytes were stimulated with LPS or LPS plus IL-10 for 4 hours. Actinomycin D (5 μg/mL) was then added, and the cultures were further incubated for up to 8 hours longer. At designated time points, cells were harvested, RNA was extracted, and TNF-RII mRNA levels were evaluated by Northern blotting. As shown in Fig 8, IL-10 treatment did not alter the half-life of TNF-RII mRNA in LPS-stimulated monocytes. The slopes of the mRNA decay curves for these two treatment groups were nearly identical, indicating that IL-10 does not alter the stability of TNF-RII mRNA transcripts.
Effect of IL-10 on the half-life of TNF-RII mRNA in LPS-stimulated monocytes. Monocytes (5 × 106 cells/mL) were cultured in the presence of LPS (100 ng/mL) or LPS + IL-10 (10 ng/mL) for 4 hours. Actinomycin D (5 μg/mL) was then added and the cultures further incubated for up to 8 hours longer. At designated time points, cells were harvested and RNA extracted. Levels of TNF-RII and β-actin mRNA were determined by Northern blotting. Autoradiographic films were analyzed by laser densitometric scanning on an LKB Ultroscan XL-500. Levels of TNF-RII mRNA were normalized to β-actin and then used to plot the percent reduction of TNF-RII mRNA over time for the 2 treatment groups: LPS v LPS + IL-10. Similar results were obtained in 2 separate experiments.
Effect of IL-10 on the half-life of TNF-RII mRNA in LPS-stimulated monocytes. Monocytes (5 × 106 cells/mL) were cultured in the presence of LPS (100 ng/mL) or LPS + IL-10 (10 ng/mL) for 4 hours. Actinomycin D (5 μg/mL) was then added and the cultures further incubated for up to 8 hours longer. At designated time points, cells were harvested and RNA extracted. Levels of TNF-RII and β-actin mRNA were determined by Northern blotting. Autoradiographic films were analyzed by laser densitometric scanning on an LKB Ultroscan XL-500. Levels of TNF-RII mRNA were normalized to β-actin and then used to plot the percent reduction of TNF-RII mRNA over time for the 2 treatment groups: LPS v LPS + IL-10. Similar results were obtained in 2 separate experiments.
The promoter region of the human TNF-RII gene has recently been cloned.39 To determine if IL-10 induces or enhances TNF-RII promoter activity, a 1.8-kb 5′-flanking sequence of the human TNF-RII promoter cloned into a luciferase expression vector (pGL2-basic) was transfected into the IL-10–responsive macrophage cell line, RAW264.7. This cell line was chosen for a number of reasons. First, human monocytes are extremely difficult to transfect and human myeloid leukemic cell lines such as U937, THP-1, and HL-60 do not recapitulate many of the functional effects induced by IL-10 in blood monocytes; second, RAW264.7 cells are responsive to both LPS and IL-10; and third, IL-10-induced functional responses (eg, inhibition of endogenous TNF-α gene expression) in this cell line are similar to those induced by IL-10 in primary monocytes. Therefore, the RAW264.7 cell line provides a relevant model system for assessing IL-10–induced regulation of human TNF-RII promoter activity. Although IL-10 alone induced only minimal promoter activity in RAW264.7 cells, it markedly enhanced the LPS-induced response (Fig 9). Therefore, the ability of IL-10 to amplify TNF-RII gene expression in LPS-stimulated monocytes appears to be transcriptionally mediated through DNA elements present in the promoter of this gene.
Effect of IL-10 on TNF-RII promoter activity in RAW264.7 cells. RAW264.7 cells were transfected with a 1.8-kb human TNF-RII promoter construct (pGL2-1.8-kb HindIII-luc) using calcium phosphate. Forty hours after transfection, cells were treated with either medium alone (control), IL-10 (10 ng/mL), LPS (100 ng/mL), or LPS + IL-10 for 6 hours at 37°C. Cells were then harvested and cell lysates assayed for luciferase activity. Results are expressed as RLU after normalizing to total protein concentration. Results are representative of 3 similar experiments.
Effect of IL-10 on TNF-RII promoter activity in RAW264.7 cells. RAW264.7 cells were transfected with a 1.8-kb human TNF-RII promoter construct (pGL2-1.8-kb HindIII-luc) using calcium phosphate. Forty hours after transfection, cells were treated with either medium alone (control), IL-10 (10 ng/mL), LPS (100 ng/mL), or LPS + IL-10 for 6 hours at 37°C. Cells were then harvested and cell lysates assayed for luciferase activity. Results are expressed as RLU after normalizing to total protein concentration. Results are representative of 3 similar experiments.
DISCUSSION
We have found that IFN-γ and IL-10 reciprocally regulate expression of TNF-α and sTNF-RII in LPS-stimulated monocytes. Specifically, IFN-γ upregulates production of TNF-α and downregulates production of sTNF-RII. In contrast, IL-10 downregulates production of TNF-α and upregulates synthesis and release of sTNF-RII. We also found that IL-10 can antagonize the effects of IFN-γ on production of TNF-α and sTNF-RII by LPS-stimulated monocytes. The ability of IL-10 to amplify TNF-RII gene expression in LPS-stimulated monocytes was not due to stabilization of TNF-RII mRNA transcripts. Rather this effect appears to be transcriptionally controlled because IL-10 markedly increased LPS-induced TNF-RII promoter activity in the IL-10–responsive macrophage cell line, RAW264.7. The ability of IL-10 to concomitantly decrease production of TNF-α and to enhance production of sTNF-RII by monocytes is consistent with its established role as an antiinflammatory cytokine.
By downregulating production and release of sTNF-RII, IFN-γ decreases the levels of a major endogenous TNF antagonist. It also demonstrates that IFN-γ exerts dual regulatory effects on monocytes, those being its ability to upregulate production of TNF-α and to downregulate production and release of sTNF-RII. Our finding that IFN-γ inhibits expression of the TNF-RII gene in human monocytes confirms earlier findings by Tannenbaum et al, which showed that IFN-γ decreases transcription of the TNF-RII gene in murine macrophages.45 However, unlike this earlier study, which did not examine the effects of IFN-γ on production of either membrane or soluble TNF-RII protein, we further demonstrated that decreased TNF-RII mRNA levels correlate with decreased expression of mTNF-RII and sTNF-RII protein. We also showed that, in contrast to IFN-γ, IL-10 upregulates expression and release of sTNF-RII, and that IL-10 can block the ability of IFN-γ to downregulate production of sTNF-RII. Our finding that IFN-γ coordinately inhibits production of sTNF-RII and increases production of TNF-α in cultured monocytes is also compatible with a recent study that showed that treatment with neutralizing anti–IFN-γ antibodies downregulates TNF-α levels and upregulates sTNF-RII levels in the serum of endotoxin-challenged mice.46
Our results regarding the effects of IL-10 on TNF-RII expression are consistent with a related study by Leeuwenberg et al, who reported that, unlike IL-10, other cytokines, such as IL-1, IL-2, IL-4, IL-8, IL-13, transforming growth factor β (TGFβ), TNF-α, and IFN-γ, do not increase production of sTNF-RII by cultured human monocytes.41 Our findings are also compatible with a report by Joyce et al that showed that IL-10 but not IL-4 enhances shedding of sTNF-RI and sTNF-RII by cultured human monocytes.47 IL-4 and IL-13 can induce many of the same functional responses that are induced by IL-10 in monocytes. These include inhibition of production of LPS-inducible cytokines such as TNF-α and IL-1β6,7,9,48 and potentiation of IL-1 receptor antagonist (IL-1ra) production.9,49,50 It is noteworthy, therefore, that unlike IL-10, IL-4 decreases production of both TNF-RI and TNF-RII in monocytes.47 Therefore, although both IL-4 and IL-10 can downregulate production of TNF-α, the ability of IL-10 to upregulate production of sTNF-RII is not shared by IL-4. So far, IL-10 stands alone as the only cytokine shown to both decrease production of TNF-α and to increase production of sTNF-RII.
Studies have shown that sTNF-R can function as TNF antagonists both in vitro and in vivo.17,18 However, under certain conditions, sTNF-R may enhance TNF activity by stabilizing TNF molecules and prolonging their availability for binding to cell-surface TNF receptors.19,20 Clinical trials are now being conducted to explore the potential use of recombinant sTNF-R as therapeutic agents for treating medical conditions characterized by excessive production of TNF-α.51,52 Naturally occurring sTNF-RII are present in normal human sera in the range of 1 to 3 ng/mL.42 However, these levels may be greatly elevated during infection or in patients with certain autoimmune diseases, including rheumatoid arthritis, multiple sclerosis, and systemic lupus erythematosus. It is noteworthy that the levels of expression of TNF-RII, as well as the levels of sTNF-RII production in murine macrophages, increase dramatically following endotoxin stimulation, while the levels of TNF-RI expression remain unchanged.45 Endotoxin challenge studies in both mice and humans have shown that a similar increase in circulating levels of sTNF-RII occurs in vivo.14,18 46 Elevated sTNF-R levels may represent an adaptive host response to infection or inflammation. Presumably, sTNF-R levels increase in vivo to facilitate clearance of excess TNF.
Clinical studies of recombinant human IL-10 in normal volunteers have shown that IL-10 induces many of the same effects in circulating monocytes that were initially demonstrated in vitro.53 54 These include inhibition of production of various LPS-inducible cytokines such as TNF-α, IL-1β, and IL-6, and potentiation of production of IL-1ra. It remains to be shown whether IL-10 therapy increases circulating levels of sTNF-RII, but based on our findings, it is reasonable to predict that at least a transient increase in sTNF-RII levels may occur in patients following IL-10 treatment.
Consistent with earlier studies by others,11-14 we found that normal human monocytes express predominantly TNF-RII. It has been shown previously that myeloid progenitor cells downregulate expression of TNF-RI as they differentiate into mature monocytes.55 In contrast, nonhematopoietic cells such as fibroblasts and endothelial cells, which are highly TNF-responsive, express comparatively high levels of TNF-RI.10 This preferential expression of TNF-RII by monocytes may explain why these cells are only modestly responsive to stimulation by recombinant TNF. Although the precise function of TNF-RII is unclear, one of their primary roles may be to regulate TNF activation via TNF-RI. We have found that induction of nuclear factor-κB (NF-κB) activity by TNF-α in purified monocytes can be blocked by antibodies against TNF-RI, but not by antibodies against TNF-RII (unpublished observations, September 1995). Therefore, primary monocytes must express at least a small number of TNF-RI through which TNF-mediated signaling may occur. Although TNF-RI are the primary signal-transducing TNF-R for sTNF, a recent study suggests that TNF-RII may preferentially signal transduce in response to stimulation by mTNF.56
It is well established that IFN-γ treatment significantly increases the incidence of mortality in endotoxin-challenged mice.57,58 This increased mortality correlates directly with elevated serum levels of TNF-α. In contrast, treatment with IL-10 has been shown to decrease circulating levels of TNF-α and mortality in LPS-challenged mice.59,60 Studies of IFN-γR knock-out mice have shown that these animals are remarkably endotoxin-resistant, and that this resistance correlates with markedly decreased production of TNF-α.61,62 In contrast, mortality can be induced with much lower concentrations of LPS in IL-10 knock-out mice, because these animals lack the inhibitory signal normally provided by IL-10 that limits TNF production.63 These studies underscore the critical opposing roles of IFN-γ and IL-10 in the pathogenesis of endotoxic shock. Whether the changes in serum TNF levels that occur in IFN-γ–treated and IL-10–treated mice correlate with changes in the levels of sTNF-RII is unknown. Regardless, our demonstration that IFN-γ and IL-10 inversely regulate production of sTNF-RII constitutes an important addition to the expanding list of monocyte phenotypic properties that are reciprocally regulated by these cytokines.
ACKNOWLEDGMENT
The authors thank Dr Laurie Owen-Schaub (M.D. Anderson Cancer Center, Houston, TX) for providing the TNF-RII promoter construct, Dr Howard Young (NCI-FCRDC, Frederick, MD) for providing the TNF-RII cDNA, and Claudia Williams for preparing elutriated monocytes. We also thank Drs David Finbloom and Mark Hayes for reviewing the manuscript.
Supported in part by a Postgraduate Research Fellowship Award to H.L.D. from the Oak Ridge Institute for Science and Education (ORISE) through an interagency agreement between the US Department of Energy and the US Food and Drug Administration.
Address reprint requests to Raymond P. Donnelly, PhD, Division of Cytokine Biology, HFM-505, FDA, CBER, 1401 Rockville Pike, Rockville, MD 20852; email: donnelly@a1.cber.fda.gov.








This feature is available to Subscribers Only
Sign In or Create an Account Close Modal