Abstract
Vascular endothelial growth factor (VEGF ), an endothelial cell mitogen, is a potent angiogenic factor produced by several cell types. Whether human neutrophils are potential producers of VEGF has not yet been described. The present work shows that phorbol-12-myristate 13-acetate (PMA), fMet-Leu-Phe, and tumor necrosis factor-α (TNF-α) triggered a time-dependent secretion of VEGF by human neutrophils. Cells incubated with 50 ng/mL of PMA released significant amounts of VEGF after 15 minutes. Because the extracellular content of VEGF in human neutrophils supernatants remained constant over a period of 2 to 24 hours and because PMA is a potent inducer of human neutrophil degranulation, the PMA-induced secretion of VEGF may be due to a pre-existing intracellular pool of this molecule. This hypothesis was reinforced by the absence of cycloheximide effect on the PMA-induced secretion of VEGF. The existence of an intracellular pool of VEGF was confirmed by measuring the intracellular content of VEGF in resting neutrophils. A dosedependent inhibition of PMA-induced VEGF secretion was observed when the cells were incubated in the presence of pentoxifylline, a methylxanthine known to inhibit neutrophil degranulation. To confirm the implication of neutrophil degranulation in VEGF release, the effects of two inducers of physiologic degranulation, fMet-Leu-Phe and TNF-α, were determined. Both agonists induced a release of VEGF in the absence of cytochalasin B, confirming the involvement of neutrophil degranulation and suggesting the intracellular localization of VEGF in the specific granule fraction. In addition, the kinetics of fMet-Leu-Phe– and TNF-α–induced secretion of lactoferrin were similar to those of VEGF release induced by these two both agonists. The subcellular fractionation of human neutrophils showed a granule-specific distribution of the intracellular pool of VEGF in resting neutrophils. The finding that human neutrophils contain an intracellular pool of VEGF, secreted in the extracellular space under PMA-, fMet-Leu-Phe–, and TNF-α–induced degranulation, suggests a role for human neutrophils as cellular effectors of physiologic as well as pathologic angiogenesis.
POLYMORPHONUCLEAR neutrophils (neutrophils) are nonproliferative circulating phagocytes that actively participate in the first line of self-defense. They are called into function by and respond to various soluble and particulate agonists. Human neutrophils also secrete a limited repertoire of soluble molecules, such as cytokines, that regulate intercellular signaling events involved in several host responses and inflammatory processes.1-5
Vascular endothelial growth factor (VEGF )/vascular permeability factor (VPS), which was originally identified as a tumor cell-derived VPS,6 plays a critical role in angiogenesis. Angiogenesis is defined as the formation of new capillary blood vessels from existent microvessels. It has a major role in the evolvement of vascular supply, in adult tissue remodeling, and in disease.7 VEGF is expressed in a limited number of sites in normal tissues and is upregulated in keratinocytes during wound healing, a process that implies microvascular hyperpermeability and angiogenesis. Accordingly, angiogenesis is critical in normal physiologic conditions such as reproduction, wound healing, and bone repair and in pathologic conditions such as rheumatoid arthritis (RA), ischemic heart disease, ischemic peripheral vascular disease, diabetic retinopathy, tumor growth, and metastazation.6-13 Several cell types, including monocytes, macrophages, T lymphocytes, keratinocytes, and fibroblasts, can produce VEGF.6,8,12,14-16 VEGF production by human neutrophils has not yet been described despite their involvement in numerous physiologic and pathologic processes that involve angiogenesis and vascular permeability.17-20 Besides, it is also well established that neutrophils express proteolytic enzymes that are essential for new blood vessels formation21 22 and that neoangiogenesis arises from microvessels, capillaries, and small vesicles that are in contact with the marginated pool of human neutrophils.
In this study, we show for the first time that adherent human neutrophils from normal donors secrete VEGF that is stored in specific granules. These findings suggest that neutrophil infiltrates may promote vasculogenesis and microvascular remodeling in vivo under both normal physiologic conditions, such as wound healing, and pathologic conditions, such as tumors and RA.
MATERIALS AND METHODS
Materials
Phorbol-12-myristate 13-acetate (PMA), fMet-Leu-Phe, and cycloheximide were purchased from Sigma Chemical Co (St Louis, MO). Tumor necrosis factor-α (TNF-α) was from Genzyme (Cambridge, MA). The extracellular matrix was obtained from Harbor Bioproducts (Norwood, MA). The human VEGF immunoassay for the quantitative determination of human VEGF concentrations was obtained from R&D Systems (Minneapolis, MN). The immunoassay kit for lactoferrin quantification was from Oxis International, Inc (Portland, OR). Dynabeads M-450 Pan Human HLA class II were purchased from Dynal (Oslo, Norway). Pentoxifylline (PTX) was obtained from Hoechst (Paris-La Defense, France). The rhVEGF and the polyclonal anti-VEGF were purchased from R&D Systems and Santa Cruz Biotechnology, Inc (Santa Cruz, CA), respectively.
Methods
Neutrophil preparation and cultures. Neutrophils were obtained from healthy adults volunteers. After 2% dextran sedimentation, neutrophils were purified under sterile conditions by centrifugation on Ficoll-Paque cushions. Contaminating erythrocytes were removed by hypotonic lysis and the cells were resuspended in RPMI 1640 (GIBCO Life Technologies, Grand Island, NY), supplemented with 5% fetal calf serum, penicillin/streptomycin, and glutamine at a final concentration of 10 × 106 cells/mL. To deplete the neutrophil suspension of contaminating HLA class II-positive cells (B cells, monocytes, macrophages, and activated T cells), 50 μL of Dynabeads M-450 was added to 1 mL of the cell suspension. After 60 minutes at 4°C, the purified neutrophil suspension was collected for further studies. The absence of contaminating monocytes was evaluated by the classical esterase coloration and more than 99% depletion was achieved by this method. Neutrophils were then cultured in 24-well (3 × 106/well) plates (Costar, Cambridge, MA), precoated with diluted (1/12) extracellular matrix (Harbor Bioproducts). After stimulation with PMA, fMet-Leu-Phe (10−7 mol/L), or TNF-α (20 ng/mL) for various periods of time, the culture supernatants were removed, centrifuged at 3,000 rpm for 7 minutes at 4°C, and stored at −80°C for further VEGF and lactoferrin quantification.
Enzyme immunoassay for VEGF and lactoferrin. VEGF and lactoferrin measurements were performed using commercially available enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems and Oxis International, respectively) according to the manufacturers' recommendations.23 The VEGF assay had a minimum detectable concentration of 5 pg/mL and did not cross-react with other known cytokines, in particular platelet-derived growth factor (PDGF). For lactoferrin, the detection limit was 1 ng/mL.
Cycloheximide treatment. Neutrophils (107/mL) were preincubated with or without 10 μg/mL of cycloheximide for 30 minutes at 37°C and then further incubated with 50 ng/mL of PMA, 10−7 mol/L fMet-Leu-Phe, or 20 ng/mL of TNF-α for the indicated times. Secreted VEGF and lactoferrin were assessed by specific ELISAs.
T-lymphocyte preparation. After dextran sedimentation, the removed fraction containing mononuclear cells and neutrophils was layered on Ficoll-Paque cushions. The mononuclear fraction was then submitted to depletion of B cells, monocytes, and activated T cells by incubation with Dynabeads M-450. Various concentrations of highly purified resting T cells were then stimulated with either 50 ng/mL of PMA or coincubated with 3 × 106 neutrophils and stimulated with 50 ng/mL of PMA.
Subcellular fractionation. Specific and azurophilic granules were purified as previously described.24 Briefly, purified neutrophils (100 × 106) in 5 mL of ice-cold relaxation buffer supplemented with EGTA were pressurized with N2 for 20 minutes at 450 psi with constant stirring in a nitrogen bomb. The cavitate was then collected dropwise into EGTA, pH 7.4, sufficient for a final concentration of 1.25 mmol/L. Nuclei and unbroken cells were pelleted by centrifugation of the cavitate. The supernatant was decanted, loaded onto percoll gradients precooled at 4°C, and spinned at 4°C for 20 minutes at 20,000 rpm in a SW50 rotor. For higher purity of the specific and azurophilic granules, the respective fractions were subjected to an additional centrifugation on percoll. Percoll was removed from pooled fractions by spinning at 40,000 rpm for 60 minutes in an SW41 rotor (Beckman Instruments, Inc, Fullerton, CA).24 The specific and azurophilic granules fractions were then removed and diluted with an equal volume of 0.68 mol/L sucrose. The purity of specific and azurophilic granules fractions was assessed by measurements of intragranular enzymes, lactoferrin, and β-glucuronidase, respectively. β-Glucuronidase was assayed as described24 by release of phenolphthalein from 1 mmol/L phenolphthalein β-monoglucuronic acid in 100 mmol/L sodium acetate buffer, pH 7.4, at 37°C for 4 hours. The assay was terminated by adding 120 mmol/L glycine, pH 10.5. The activity was calculated by measuring the absorbance at 540 nm. The assessement of lactoferrin was performed using an ELISA.
Immunoblotting. After subcellular fractionation, a cellular equivalent of each subcellular fractions of specific and azurophilic granules preparations were added to 2× Laemmli's sample buffer and proteins were separated in 12% sodium dodecyl sulfate-polyacrylamide gels under nonreducing conditions. The transferred proteins to Immobilon polyvinylidene difluoride (PVDF) membranes were then immunoblotted. Nonspecific sites were blocked using 2% gelatin in TBS-Tween as previously described25 and then probed with the polyclonal anti-VEGF antibody (1/1,000 dilution) in fresh blocking solution. The second antibody used was an antirabbit Ig used at a 1/10,000 dilution in blocking solution for 45 minutes at 37°C. The immunoblots were developed using the chemiluminescence method (ECL; Amersham Life Sciences, Arlington Heights, IL) following the manufacturer's guidelines.
Statistics. Results were expressed as the mean ± SEM. For PMA-, fMet-Leu-Phe–, and TNF-α–induced VEGF and lactoferrin secretion, the data were analyzed by the two-tailed, Student's t-test. Significance was considered attained when P < .05.
RESULTS
PMA Treatment Induces VEGF Secretion by Adherent Human Neutrophils
Numerous agents have been shown to induce VEGF synthesis, among which is PMA.6 The secretion of VEGF by PMA-stimulated adherent human neutrophils was then evaluated using VEGF-specific ELISA. As shown in Fig 1, a time-dependent release of VEGF was observed. Significant amounts of VEGF were detected after 15 minutes of stimulation with 50 ng/mL of PMA as compared with control (161 ± 18 and 61 ± 27 pg/mL, respectively, n = 4, P < .05). The extracellular content of VEGF in the supernatants of neutrophils stimulated with 50 ng/mL of PMA increased from 15 minutes to 2 hours and remained constant over a period of 2 to 24 hours, with a slight decrease for this last point. This decrease was probably due to a degradation of VEGF by proteases released into the culture medium as cells viability was not affected (data not shown). For this reason, the experiments described below were performed over a period of 15 minutes to 120 minutes.
Kinetics of PMA-induced VEGF secretion in human adherent neutrophils. Neutrophils (107/mL) were incubated with 50 ng/mL of PMA for the indicated periods of times at 37°C. Secreted VEGF was assessed by a specific ELISA. Each point represents the mean ± SEM of at least three different determinations.
Kinetics of PMA-induced VEGF secretion in human adherent neutrophils. Neutrophils (107/mL) were incubated with 50 ng/mL of PMA for the indicated periods of times at 37°C. Secreted VEGF was assessed by a specific ELISA. Each point represents the mean ± SEM of at least three different determinations.
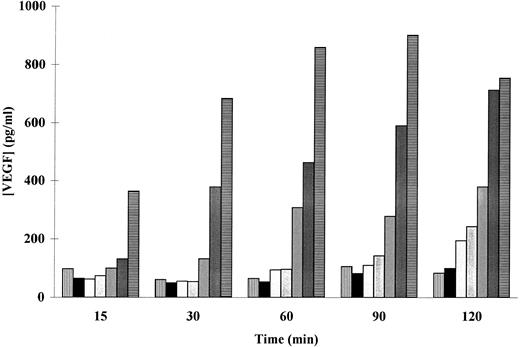
The patterns of PMA-induced secretion of VEGF by adherent human neutrophils were thus studied. As shown in a representative experiment (Fig 2), a dose- as well as a time-dependent PMA-induced secretion of VEGF was observed. Cells incubated with 50 ng/mL of PMA released significant amounts of VEGF after 15 minutes, whereas 120 minutes were needed to induce a significant release of this factor with 5 ng/mL PMA (169 ± 16 pg/mL, n = 3, P < .05). The maximal VEGF extracellular level was obtained at 60 minutes with 100 ng/mL PMA (668 ± 79 pg/mL, n = 5, P < .05) and at 120 minutes with 50 ng/mL PMA (698 ± 38 pg/mL, n = 7, P < .01). A spontaneous secretion of VEGF was observed (Figs 1 and 2), probably due to an activation of the neutrophils induced by the adherence to the extracellular matrix (ECM).
Effect of PMA on the secretion of VEGF in adherent human neutrophils. Neutrophils (×107/mL) were incubated without (▥) or with (▪) 1, (▧) 5, () 10, (▨) 25, () 50, and (▤) 100 ng/mL of PMA at 37°C. Secreted VEGF was assessed by a specific ELISA. Data are from a representative experiment.
Effect of PMA on the secretion of VEGF in adherent human neutrophils. Neutrophils (×107/mL) were incubated without (▥) or with (▪) 1, (▧) 5, () 10, (▨) 25, () 50, and (▤) 100 ng/mL of PMA at 37°C. Secreted VEGF was assessed by a specific ELISA. Data are from a representative experiment.
VEGF Expression in Adherent Human Neutrophils Is Not Due to Contamination by T Cells
It has been shown that T lymphocytes, in the presence of interleukin-2 (IL-2) and under hypoxic conditions, synthetize VEGF. Both CD4+ and CD8+ T cells as well as Jurkat cells secrete VEGF after stimulation with IL-2. These data indicate that T lymphocytes are able of exporting bioactive concentrations of VEGF into the extracellular space.12 Therefore, it was important to verify that the VEGF secretion observed that PMA-stimulated purified neutrophils was not due to a possible contamination with resting T cells that cannot be depleted by Dynabeads because they are class II negative.
To this end, various concentrations of purified adherent T lymphocytes were stimulated with 50 ng/mL of PMA for 2 and 24 hours. Stimulation of 0.1 to 5 × 106 T lymphocytes with PMA for 2 hours did not induce any significant VEGF production (0 to 10 pg/mL, respectively), whereas 3 × 106 adherent neutrophils secreted a sustained 500 to 600 pg/mL of VEGF. Culturing T lymphocytes with or without PMA for 24 hours led to a significant increase of secreted VEGF that correlated with the number of T lymphocytes per well (9 to 39 pg/mL for 0.1 to 5 × 106 T cells, respectively). The T cells' VEGF secretion rate was 4 to 5 times lower than that secreted by stimulated neutrophils and did not influence the level of PMA-induced VEGF secretion by human neutrophils. The level of secreted VEGF by cocultures of neutrophils and 5 × 106 T lymphocytes was indeed the sum of what was secreted by each cell type alone. These results lead us to conclude that the observed VEGF secretion by adherent human neutrophils was not due to contaminating T lymphocytes.
Human Neutrophils Contain an Intracellular Pool of VEGF
PMA is a potent inducer of human neutrophils degranulation.26 Accordingly, the release of VEGF by adherent human neutrophils after PMA stimulation may be a result of PMA-induced degranulation (Fig 1), suggesting the existence of an intracellular pool of VEGF in human neutrophils. To investigate this possibility, 3 × 106 resting neutrophils were sonicated and the intracellular content of VEGF was determined. The intracellular content of VEGF (730 ± 83 pg/mL, n = 6) was similar to the maximal extracellular level obtained under PMA-induced stimulation (Figs 1 and 2). Furthermore, a parallel decrease of the intracellular content of VEGF was observed after PMA-induced degranulation (data not shown).
Pretreatment of adherent neutrophils with cycloheximide over a period of 15 minutes to 2 hours did not affect PMA-induced VEGF secretion, reinforcing the hypothesis that the observed secretion is due to the release of a pre-existing intracellular pool of VEGF instead of a de novo synthesis (data not shown).
Effect of Pentoxifylline on PMA-Induced VEGF Secretion in Adherent Human Neutrophils
Because the PMA-induced secretion of VEGF by adherent neutrophils seems to result from release of a pre-existing intracellular pool, we investigated whether neutrophil degranulation is implicated in this release. We first tested the effect of PTX [3,7-dimethyl-1-(5-oxohexyl)-xanthine], a methylxanthine derivative known to inhibit neutrophil degranulation27 on PMA-induced VEGF secretion. Incubation of adherent neutrophils with various concentrations of PTX induced a dose-dependent inhibition of the PMA-induced VEGF secretion (Fig 3). The maximal inhibition rate was obtained with 500 μmol/L of PTX for all the incubation times tested. On the other hand, an inhibitory effect was observed with all assayed PTX concentrations after 60 minutes of incubation (Fig 3). These data suggest that neutrophil degranulation is involved in VEGF secretion by adherent neutrophils after their stimulation with PMA.
Effect of PTX on the PMA-induced VEGF secretion in adherent neutrophils. Neutrophils (107/mL) were incubated (▤) without or with (▪) 100 or (▥) 500 μmol/L of PTX for the indicated periods of times at 37°C with 50 ng/mL of PMA for the indicated period of times at 37°C. Secreted VEGF was assessed by a specific ELISA. Data are from a representative experiment.
Effect of PTX on the PMA-induced VEGF secretion in adherent neutrophils. Neutrophils (107/mL) were incubated (▤) without or with (▪) 100 or (▥) 500 μmol/L of PTX for the indicated periods of times at 37°C with 50 ng/mL of PMA for the indicated period of times at 37°C. Secreted VEGF was assessed by a specific ELISA. Data are from a representative experiment.
fMet-Leu-Phe and TNF-α Induced VEGF Secretion in Adherent Human Neutrophils
The entererity of our above-noted results indicated that the secretion of VEGF by neutrophils after PMA stimulation is indeed a release of a pre-existing pool of VEGF, after degranulation, instead of a de novo synthesis. Accordingly, the effect of two known inducers of physiologic degranulation in adherent neutrophils, fMet-Leu-Phe and TNF-α, on VEGF secretion by neutrophils was thus studied.26 As shown in Fig 4A, fMet-Leu-Phe (10−7 mol/L) and TNF-α (20 ng/mL) induced a time-dependent secretion of VEGF in adherent human neutrophils. Significant amounts of VEGF were detected after 15 minutes of stimulation with fMet-Leu-Phe as compared with control (140 ± 10 pg/mL and 70 ± 8 pg/mL, respectively, n = 5, P < .05). The fMet-Leu-Phe–induced VEGF secretion in the extracellular medium exhibited a slight, but not statistically significant increase over a period of 15 to 120 minutes (Fig 4A). The maximal fMet-Leu-Phe–induced VEGF secretion was 28% ± 2% as compared with control (12% ± 1%, n = 5, P < .05), whereas a total release of the intracellular content was observed with PMA (Fig 1).
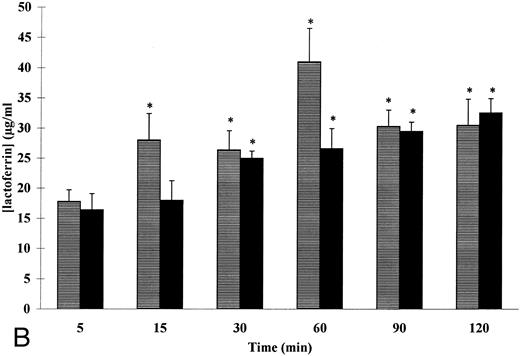
Kinetics of fMet-Leu-Phe– and TNF-α–induced VEGF (A) and lactoferrin (B) secretion in adherent human neutrophils. Neutrophils (107/mL) were incubated with (▤) 10−7 mol/L of fMet-Leu-Phe and (▪) 20 ng/mL of TNF-α for the indicated periods of times at 37°C. Secreted VEGF and lactoferrin were assessed by specific ELISAs. Each point represents the mean ± SEM of five different determinations. * P < .05 v unstimulated neutrophils.
Kinetics of fMet-Leu-Phe– and TNF-α–induced VEGF (A) and lactoferrin (B) secretion in adherent human neutrophils. Neutrophils (107/mL) were incubated with (▤) 10−7 mol/L of fMet-Leu-Phe and (▪) 20 ng/mL of TNF-α for the indicated periods of times at 37°C. Secreted VEGF and lactoferrin were assessed by specific ELISAs. Each point represents the mean ± SEM of five different determinations. * P < .05 v unstimulated neutrophils.
Stimulation of adherent neutrophils with 20 ng/mL of TNF-α induced, as with fMet-Leu-Phe, a time-dependent release of VEGF in the extracellular medium (Fig 4A). Significant amounts of VEGF were detected after 30 minutes of stimulation (149 ± 16 pg/mL) as compared with control (79 ± 11 pg/mL, n = 5, P < .05). The maximal VEGF extracellular level was obtained at 60 minutes as compared with control (185 ± 17 and 65 ± 13 pg/mL, respectively, n = 5, P < .05) and remained constant over a period of 60 to 120 minutes. As with fMet-Leu-Phe, a partial degranulation was also observed with TNF-α and the maximal release observed was 31.4% ± 0.5% as compared with control (11.2% ± 1.2%, n = 5, P < .05).
In contrast to PMA, which induces both specific and azurophilic degranulation, fMet-Leu-Phe and TNF-α are secretagogues of specific granules only.26 The kinetics of fMet-Leu-Phe– and TNF-α–induced VEGF release were thus compared with that of lactoferrin, a granule-specific marker. As shown in Fig 4B, 10−7 mol/L of fMet-Leu-Phe and 20 ng/mL of TNF-α induced a time-dependent release of lactoferrin with no detectable release of β-glucuronidase, a marker of azurophilic granules (data not shown). Significant amounts of lactoferrin were detected for 15 minutes of stimulation with fMet-Leu-Phe as compared with control (28 ± 4.4 and 16.1 ± 1.95 μg/mL, respectively, n = 5, P < .05). This fMet-Leu-Phe–induced lactoferrin secretion did not increase significantly over a period of 15 to 120 minutes (Fig 4B). The kinetics of fMet-Leu-Phe–induced lactoferrin secretion were parallel to that of fMet-Leu-Phe–induced VEGF secretion. The maximal fMet-Leu-Phe–induced lactoferrin secretion was 35% ± 2.4% as compared with control (19.5% ± 2.2%). This percentage was consistant with the data obtained for the fMet-Leu-Phe–induced VEGF secretion.
Similar results were obtained when neutrophils were stimulated with 20 ng/mL of TNF-α (Fig 5B). Significant amounts of lactoferrin were observed for 30 minutes of stimulation as compared with control (25 ± 1.2 and 16.1 ± 1.95 μg/mL, respectively, n = 5, P < .05). The maximal TNF-α–induced lactoferrin secretion was 36% ± 1.7% as compared with control (19.5% ± 2.2%). This result was similar to the percentage of the TNF-α–induced secretion of VEGF.
Immunoblotting of VEGF in human neutrophils' subcellular compartments. Specific and azurophilic granules membranes were prepared on Percoll gradients. The cavitate was recovered after neutrogen cavitation. Western blots were probed with a polyclonal anti-VEGF antibody. Human recombinant VEGF (R&D Systems) was loaded and blotted in parallel. The molecular masses of protein standards are indicated in kilodaltons.
Immunoblotting of VEGF in human neutrophils' subcellular compartments. Specific and azurophilic granules membranes were prepared on Percoll gradients. The cavitate was recovered after neutrogen cavitation. Western blots were probed with a polyclonal anti-VEGF antibody. Human recombinant VEGF (R&D Systems) was loaded and blotted in parallel. The molecular masses of protein standards are indicated in kilodaltons.
Pretreatment of adherent neutrophils with cycloheximide over a period of 5 to 120 minutes was found without any effect on the fMet-Leu-Phe– and TNF-α–induced VEGF and lactoferrin releases reinforcing the hypothesis of a pre-existing pool of VEGF in human neutrophils (data not shown).
Taken together, these results suggest a fMet-Leu-Phe– and TNF-α–induced VEGF secretion that occur in parallel to the lactoferrin release mediated by these two both agonists.
Subcellular Localization of VEGF in Specific Granules
To further localize the human neutrophils intracellular VEGF pool, specific and azurophilic granules fractions were prepared. The content of VEGF as well as lactoferrin and β-glucuronidase, markers of specific and azurophilic granules, respectively, were determined in each fraction. The results shown in Table 1 indicate that VEGF is located mainly in the specific granule fraction. More than 70% of the VEGF was recovered in the specific granule fraction, whereas the contamination of this fraction by azurophilic granules was undetectable (<0.5%). The markers for the specific granules followed closely the VEGF distribution between the subcellular fractions. Approximately 16% of the recovered β-glucuronidase was in the specific granule fraction. The subcellular localization of VEGF in the specifc granules was confirmed by Western blot analysis. Indeed, Fig 5 showed the presence of a 43-kD band in the specific granule fraction and the cavitate, migrating at the same level than human recombinant VEGF, ran in parallel. In contrast, no detectable VEGF was observed in the azurophilic granules and in the membrane fractions.
Distribution of Markers and VEGF Among Subcellular Fractions From Nitrogen-Cavitated Human Neutrophils
| . | VEGF . | Lactoferrin . | β-Glucuronidase . |
|---|---|---|---|
| Cavitate | 100 | 100 | 100 |
| Plasma membrane | 16 | 17 | <5 |
| Cytosol | 11 | <0.5 | 27 |
| Specific granules | 73 | 81 | 16 |
| Azurophilic granules | <0.5 | <0.5 | 53 |
| . | VEGF . | Lactoferrin . | β-Glucuronidase . |
|---|---|---|---|
| Cavitate | 100 | 100 | 100 |
| Plasma membrane | 16 | 17 | <5 |
| Cytosol | 11 | <0.5 | 27 |
| Specific granules | 73 | 81 | 16 |
| Azurophilic granules | <0.5 | <0.5 | 53 |
Results are expressed as the percentage of the cavitate content of each marker and VEGF. Data are from a representative experiment.
DISCUSSION
This is the first report describing an intracellular pool for the potent angiogenic factor VEGF in human neutrophils and its extracellular secretion after PMA-, fMet-Leu-Phe–, and TNF-α–induced degranulation in an experimental model of adherent cells on extracellular matrix. This model was established to reproduce the most physiologic conditions for examining the effects of neutrophils that may be displayed in tissues. The absence of effect of cycloheximide on stimuli-induced secretion of VEGF by adherent neutrophils strongly suggested a release of a pre-existing pool instead of a de novo synthesis of this agent by these cells. The observed inhibitory effect of PTX reinforced this possibility and indicated the implication of neutrophil degranulation in their VEGF release. The two known inducers of physiologic degranulation in adherent cells, fMet-Leu-Phe and TNF-α, that induced a time-dependent release of VEGF to the extracellular medium confirmed this possibility.
In subcellular fractions, we showed that VEGF was present in the specific granule fraction containing lactoferrin, but not in the azurophilic granule fraction containing β-glucuronidase. The fMet-Leu-Phe– and TNF-α–induced VEGF release in adherent cells in the absence of cytochalasin B provided further evidence that VEGF was present in the specific granules and/or gelatinase-containing vesicles but not in the azurophilic granules. A common protocol was used for the preparation of the subcellular fractions.24 This procedure did not separate the secretory vesicles from the plasma membrane fraction and the gelatinase-containing vesicles from the specific granules. However, the kinetics of VEGF release were similar to those of lactoferrin, and the majority of VEGF colocalized on the Percoll gradient with the specific granule fraction that is devoid of secretory vesicles. Under PMA stimulation, the maximal level of VEGF secreted was similar to the intracellular content, although in the literature the maximum level of lactoferrin released with this agonist was about 50%.28 The coexistence of differentially mobilizable subpopulations within the specific granule compartment, depending of the secretagogue used, was previously demonstrated. This could account for the discrepancies observed between PMA and fMet-Leu-Phe or TNF-α in our study. Although neutrophil gelatinase-associated lipocalin (NGAL) mainly colocalized with lactoferrin, this marker was liberated from neutrophils by potent stimuli such as PMA and poorly secreted under fMet-Leu-Phe stimulation. In addition, stimulation of neutrophils with PMA induced the secretion of VitB12 binding protein as well as a high level of gelatinase in contrast to fMet-Leu-Phe.28 These overall data suggest that the maximal level of VEGF released induced by PMA may be the result of the concommitant degranulation of lactoferrin- and/or NGAL- and/or VitB12BP- and/or gelatinase-contaning granules.
VEGF can be produced by several cell types6,8,12,14-16; however, most of the studies on the induction of VEGF synthesis are based on reverse transcriptase-polymerase chain reaction (RT-PCR) analysis, Northern blot, and in situ hibridization and few data concerning the levels of secreted VEGF are available. Analysis of levels of VEGF in IL-2–stimulated T cells showed the presence of soluble VEGF at concentration of approximately 10 ng/mL,12 but without any indication on the number of cells used for this determination. The levels of the intracellular content of VEGF in human neutrophils may be compared with those shown in keratinocytes and macrophages. In epidermal keratinocytes, VEGF expression and secretion of bioactive VEGF were shown to be induced by transforming growth factor-α (TGF-α)29 and a statistically significant secretion of VEGF of 200 pg/mL per 105 cells was reported. VEGF was also found to be secreted by human peritoneal fluid macrophages at concentrations of 1 to 2 ng/mL.30 Because of the discrepancies observed between the amounts of VEGF secreted by various cell types, the comparison of the intracellular content of VEGF in human neutrophils with the physiologic bioactive concentrations of VEGF may thus be more relevant. Nevertheless, the findings presented hereby extend previous observations that human neutrophils synthetize and secrete angiogenic peptides such as IL-8,1,31 Gro-α,4 TNF-α,2 and TGF-β5 and thus may play an important role in angiogenesis.
VEGF, which is also known as vascular permeability factor, is a dimeric, heparin-binding glycoprotein that induces endothelial growth factor and migration by binding and activation of at least two distinct tyrosine kinase receptors, KDR/flk and flt-1.6 Unlike other secreted proteins with angiogenic activity, VEGF exhibits a highly restricted target cell specificity; biologic effects are evoked largely by endothelial cells in vitro. A significant effect of VEGF on endothelial proliferation was observed for 1 ng/mL.32 A class of high-affinity binding sites has been characterized on endothelial cells with a kd of approximately 40 pg/mL to 2 ng/mL. When examining the potential role of VEGF in mediating chemotaxis and proliferation of endothelial cells in rheumatoid arthritis, it was found that human recombinant VEGF was chemotactic for human umbilical vein endothelial cells (HUVECs) at concentrations greater than 0.8 ng/mL.33 These observations support a physiologic role of the described intracellular pool of VEGF in human neutrophils during the angiogenic process.
Experiments with a variety of in vitro and in vivo systems support a role for VEGF as an important regulator of vascular endothelial cell function during vasculogenesis, neovascularization, tumor growth, and tissue damage occurring in multiple sclerosis RA and atherosclerosis. Angiogenesis is one of the most pervasive and essential biologic events encountered in the mammalian organism. Normally, physiologic angiogenesis occurs infrequently, yet can be rapidly induced in response to a number of diverse physiologic stimuli. Among the most extensively studied angiogenesis-dependent physiologic processes is normal wound repair.6 34 The present evidence for an intracellular pool of VEGF in human neutrophils that can be released to the extracellular medium by degranulation may suggest an active role of these cells in wound healing repair.
During neoplastic transformation and inflammatory disorder, neovascularization is exaggerated.35 In breast tumors, for example, an heterogeneity with varying tumor cellularity and varying amounts of stroma with infiltration of macrophages, lymphocytes, neutrophils, and fibroblasts were observed. There is evidence that inflammatory cell infiltration is associated with poor prognosis in human breast cancer. Although the precise role of neutrophils in tumor development remains uncertain, neutrophils are nevertheless capable of secreting vascular mitogens and might actually promote tumor growth by direct remodeling of the tumor microvasculature. Our results suggest that neutrophils may play a role in tumor angiogenesis by secreting angiogenic factors such as VEGF directly into the tumor stroma.
Autoimmune diseases, such as RA and inflammatory bowel disease, are characterized by chronic inflammatory responses resulting in tissue damage.17 RA is characterized by infiltration of leukocytes into the synovial tissue and synovial fluid of joints8 and the development of a highly vascularized tissue.9 Immunofluorometric analysis revealed detectable VEGF in synovial fluids from patients with various forms of disease.11 These facts taken together suggest that angiogenesis may have a role in the pathogenesis of arthritis. The results obtained in the present study reinforce the role for human neutrophils in the pathogenesis of joint destruction. The rapid mobilization of an intracellular pool of VEGF suggest that neutrophils could play a crucial role, because VEGF is a potentially key mediator of the changes in the microvasculature that are observed as part of the early pathogenesis of RA.8
In general, the role of neutrophils in tissue-degradative diseases has been proposed to be a secretion of proteolytic enzymes and the secretion of a relatively limited number of cytokines, which regulate lymphocytes, monocyte-derived macrophages, and other inflammatory cells capable of tissue destruction or remodeling. The finding of an intracellular pool of VEGF located in specific granules that could be rapidly mobilized by degranulation, besides their capacity of synthesizing angiogenic proteins such as IL-8, Gro-α, TNF-α, and TGF-β, suggests that neutrophils, independently from other inflammatory cells, might play a very early role in tissue remodeling.
In summary, this study presents a novel observation on the intracellular protein content of human neutrophils. Further studies will be needed to clarify the function of this specific granule pool of VEGF in physiologic inflammatory responses.
ACKNOWLEDGMENT
The authors are grateful to R. Al-Daccak (CHUL, Ste-Foy, Quebec, Canada) for critical review of the manuscript and to O. Bakouche, V. Ollivier, and C. Pasquier for helpful discussions and advice.
Address reprint requests to Murielle Gaudry, PhD, Unité INSERM U294, Faculté X. Bichat, 16 rue H. Huchard, 75018 Paris, France.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal