Abstract
Malaria-parasitized erythrocytes have increased endothelial adherence due to exposure of previously buried intramembranous sites of band 3. Because sickle erythrocytes also show increased adhesiveness and because the membrane portion of band 3 is aggregated in both types of cells, we examined the role of band 3 in sickle cell adhesiveness. Synthetic peptides derived from the second and third exofacial, interhelical regions of band 3 completely inhibited the abnormal adherence of sickle cells to an endothelial monolayer in a static assay. This effect was observed independently of plasma factors, required micromolar levels of peptide, was sequence-specific, and was found with both L- and D-isomers. The active peptides also inhibited the increased adherence induced by low-dose calcium loading of normal red blood cells. Finally, a monoclonal antibody against an active peptide specifically immunostained a fraction of sickle cells. These findings implicate a role for band 3 in at least one type of sickle cell adhesiveness via the exposure of normally cryptic membrane sites.
THE MOLECULAR ABNORMALITY of sickle cell hemoglobin and its consequent tendency to polymerize in vivo is the essential basis of sickle cell disease. However, the vaso-occlusive clinical symptoms of sickle cell disease are not conclusively correlated with either the concentration of sickle hemoglobin or its polymerization state. Accordingly, multiple factors have been proposed to have an adjuvant role in intensifying local sickling that results in the ischemia and intermittent crises of this disease.1-3 These include metabolic effects, which can influence the cells' hydration state or pH; whole blood viscosity effects, which can influence the cells' duration of hypoxic exposure; and cellular and humoral factors, which can influence the cells' adhesion to the endothelial lining of blood vessels.4 In the hypoxic venous microcirculation the adhesive effects are particularly attractive candidates for modulating local vaso-occlusion, as there is ample anatomic opportunity for adhesive red blood cells lining small vessels to reduce flow and initiate stasis of poorly-deformable cells behind which more cells may accumulate to amplify the blockage and lead to the vicious cycle of sickling classically proposed by Ham and Castle5 and subsequently iterated by Kaul et al.6
Human red blood cells parasitized by mature stages of the malarial parasite, Plasmodium falciparum, also show increased endothelial adherence.7 Two types of adhesion-related surface proteins of the malaria-infected red blood cell have been described: (1) a parasite-encoded, antigenically variable family of proteins, named P. falciparum erythrocyte membrane protein 1 or PfEMP-1 and sequestrin8-12; and (2) alterations in the erythrocyte band 3 protein. PfEMP-1 is a strain-specific, high molecular weight (>200 kD), Triton X-100 insoluble antigen encoded by the var gene complex.8,11,12 Baruch et al9 have shown that different domains of PfEMP-1 can act as receptors for both CD369 and thrombospondin, whereas sequestrin is a conserved antigenically invariant parasite protein that binds only to CD36.10 Immunologic findings have provided evidence that the adhesiveness mediated by band 3 involves a membrane conformational change, which exposes previously buried interhelical regions of band 3, providing novel sites for endothelial adhesion.13-15 Important additional support for the importance of band 3 has been collected both in vitro and in vivo. For example, synthetic peptides patterned on these abnormally exposed sequences effectively inhibit the increased adherence of malaria-infected red blood cells in vitro.16,17 Also, in vivo, infusion of such band 3 peptides into monkeys infected with falciparum malaria resulted in the marked accumulation of parasitized red blood cells in the peripheral circulation rather than the deeper tissues, presumably due to a block in the adhesion of these cells in the microcirculation.16
In work on normal red blood cells, it has also been shown that a rise in intracellular calcium induces increased cell adhesiveness, as well as clustering of surface charges as found in sickle cells.18 This opened the possibility of a role for the rearrangement of membrane proteins in the increased adherence of sickle cells. Because both parasitized red blood cells and sickle cells show increased endothelial adherence and because both show aggregation of band 3 in the membrane bilayer,19 20 we chose to determine whether structural changes in band 3 were also involved in sickle cell adhesiveness, and to see if band 3-derived peptides could inhibit that adhesiveness.
MATERIALS AND METHODS
Peptides. Peptides were synthesized using the t-butoxycarbonyl method followed by HF release and were obtained from Coast Scientific (San Diego, CA). All peptides were >97% pure as determined by high-performance liquid chromatography (HPLC) and mass spectrometry and were stored as dry powders under refrigeration at 4°C.
Immunostaining. Thin blood films were first incubated with the 1F4 hybridoma supernatant, then incubated with biotin-conjugated goat antimouse antibody and finally stained with fluorescein-conjugated avidin, as previously described.21
Endothelial cell culture. Human umbilical vein endothelial cells (HUVECs) were obtained from Clonetics (San Diego, CA) and used for up to six additional passages. Two days before the assay HUVECs were transferred and grown to confluency in 24-well polystyrene plates (Nunc, Inc, Naperville, IL) using endothelial basal medium (MCDB 131) containing 10 ng/mL human recombinant epidermal growth factor, 1 μg/mL hydrocortisone, 5% fetal bovine serum, 12 μg/mL bovine brain extract, and 10 μg/mL heparin (Clonetics) at 37°C in a 95% air/5% CO2 incubator.
Red blood cell preparation. Blood from five normal and nine sickle (homozygous) volunteer donors was collected in citrated vacutainer tubes (ACD solution; Becton Dickinson, Rutherford, NJ) and stored on ice. Before use, red blood cells were washed three times with 10 vol of Hanks' Balanced Salt Solution (HBSS; 138 mmol/L NaCl, 5 mmol/L KCl, 0.3 mmol/L Na2HPO4 , 0.3 mmol/L KH2PO4 , 0.4 mmol/L MgSO4 , 1.3 mmol/L CaCl2 , 0.5 mmol/L MgCl2 , 4 mmol/L NaHCO3 , 5.6 mmol/L glucose, pH 7.4 containing 0.5% bovine serum albumin (BSA) with removal of plasma and buffy coat after each wash by aspiration. For experiments with calcium-loaded cells, normal red blood cells, washed and suspended in calcium and magnesium-free HBSS-BSA, were incubated for 30 minutes at 37°C with 10 μmol/L calcium chloride and 10 μmol/L A23187 (final concentrations) as described earlier,18 and then washed three times in HBSS-BSA just before use in the adherence assay.
Endothelial adherence assay. Studies of endothelial adherence were conducted using minor modifications of Hebbel's original gravity sedimentation assay.18 Washed red blood cells were resuspended to 2.5% hematocrit in HBSS-BSA (± peptide), and 2.6 mL of this cell suspension was gently layered over the confluent HUVECs in each well and incubated without agitation in air for 40 minutes at 37°C. For plasma experiments, red blood cells were instead resuspended in citrated autologous plasma. Then, for all experiments, 0.75 mL of HBSS-BSA (± peptide) was added to the wells to create a slight convex meniscus over each well, and a sheet of Saran wrap (Dow Brands, Inc, Indianapolis, IN) was laid down so as to displace the meniscus and seal all wells with gentle pressure before inverting the plate as described.22 After incubation in this upside down position for an additional 30 minutes at 37°C, the wells were gently washed three times with 1.5 mL HBSS-BSA (± peptide) followed by aspiration; finally, 0.15 mL of HBSS-BSA (± peptide) was added to each well, and all red blood cells bound to a central 0.25 cm2 region of the endothelial layer were counted by phase microscopy using a 25-field grid. Occasionally, red blood cells were found to adhere to the plastic in atypical small spaces between endothelial cells; these were not counted.
Adherence of normal red blood cells and sickle cells was always assayed simultaneously and multiple independent experiments were conducted with each patient. Initially, we examined the influence of sample storage by comparing the adherence of fresh blood (4 to 24 hours old) versus blood stored for 7 to 14 days from the same four patients. We found no statistical difference in adherence either in absolute terms or in the ratio of adherence between sickle and normal cells. Further, previous investigators had found no effect of shipment storage for 4 days.23 24 Accordingly, for the most part, we used samples that were 1 to 7 days old and occasionally up to 14 days old.
Statistical methods. Each separate experiment assaying the paired adherence of normal (control) red blood cells and sickle (or calcium-loaded) cells with and without peptide was conducted on a single plate with four or more replicate wells for each sample. Replicates were always distributed into different wells in each plate so as to minimize potential experimental and counting biases. The mean number of cells bound per cm2 in replicate wells was determined for each sample. All statistical comparisons between the adherence of normal and sickle cells were directly performed on these raw adherence values for the indicated number of experiments according to the Student's t-test. However, because of substantial day-to-day, plate to plate variability in the number of bound red blood cells (which correlated neither with specific blood samples nor their age), the adherence data from each paired experiment was presented as a relative adherence ratio between sickle and normal cells. This relative adherence of sickle cells was defined as follows:
Abnormal adherence was defined as the adherence of sickle cells above that of normal cells as follows:
For the presentation of peptide experiments, we chose to express the effect of peptide on the abnormal adherence data from each experiment as an abnormal adherence ratio for sickle cells between the presence and the absence of peptide. This relative abnormal adherence of sickle cells in the presence of peptide with respect to their abnormal adherence in the absence of peptide was defined as follows:
We chose to use ratios to simplify presentation of the data. However, all statistical analyses were performed by direct comparison of the raw adherence values of sickle cells obtained in the absence or in the presence of peptide for the indicated number of experiments according to the Student's t-test.
RESULTS
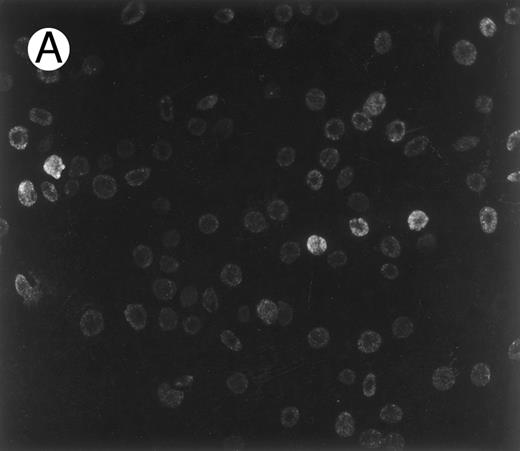
First, to investigate the possibility that sickle cells might expose normally buried epitopes as do malaria-infected cells, we stained sickle cells with 1F4, a monoclonal antibody against a synthetic peptide based on an adhesive band 3 sequence exposed in malaria-infected cells (residues 547-553).15 The immunostaining result presented in Fig 1 shows that a small, but distinct, fraction of sickle red blood cells exposes this band 3 epitope, which is inaccessible in normal cells. As seen in Fig 1A, the extent of this specific exposure of a band 3 sequence was not uniform. The majority of cells are lightly stained, and a small subpopulation shows heavy staining. Consistent with the findings for malaria obtained with other antibodies against the same band 3 motif,13 14 normal red blood cells showed virtually no staining with the 1F4 antibody (Fig 1B). Importantly, the control in Fig 1C shows that sickle cells are not immunostained in the absence of the primary antibody, 1F4.
Immunofluorescence staining of sickle cells by an antibody to an adhesive band 3 sequence in malaria infected cells. Sickle cells (A) and normal cells (B) were immunostained with monoclonal antibody (1F4) prepared against peptide 3d (band 3 residues 547-553), which inhibits cytoadherence in malaria infected cells.15 (C) Control immunostaining of sickle cells in the absence of primary antibody, 1F4.
Immunofluorescence staining of sickle cells by an antibody to an adhesive band 3 sequence in malaria infected cells. Sickle cells (A) and normal cells (B) were immunostained with monoclonal antibody (1F4) prepared against peptide 3d (band 3 residues 547-553), which inhibits cytoadherence in malaria infected cells.15 (C) Control immunostaining of sickle cells in the absence of primary antibody, 1F4.
To determine whether this abnormal epitope exposure could play a role in the increased cytoadherence of sickle cells, we sought to determine if this adhesiveness could be inhibited by specific band 3 peptides derived from the epitopes of monoclonal antibodies previously shown to inhibit the increased adhesiveness of malaria-infected cells.13 This approach was felt to be preferable to using monoclonal antibodies because their adhesion-blocking effects could be indirect (eg, due to steric hindrance) and the monoclonal antibodies, although very specific for band 3 motifs, exhibited some cross-reactivity with endothelial cells (Prudhomme and Sherman, unpublished results).
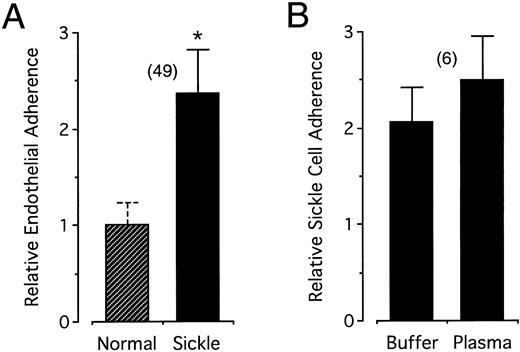
To measure the adherence of sickle cells, we used a simple static assay involving binding of gravity-sedimented sickle cells to a cultured endothelial monolayer, gentle washing steps, and visual counting of the bound cells. As shown in Fig 2A, this assay was able to reproduce the twofold to threefold increase in the endothelial adherence of sickle cells compared with normal cells found previously by others.18,24-26 Also, consistent with the findings of others,27-29 irreversibly sickled cells were not observed among the adhesive sickle cells. The increased adhesiveness of sickle cells was found in the absence of plasma (mean relative adherence ± standard deviation [SD] = 2.36 ± 0.46). Further, as seen in Fig 2B, when parallel measurements were conducted on the same samples with and without plasma, the increased adherence of sickle cells was not significantly different (respective adherence values for normal and sickle cells were 97 ± 25 and 197 ± 42 cells/cm2 in buffer, and 87 ± 38 and 219 ± 109 cells/cm2 in autologous plasma). Subsequent studies were conducted without added plasma.
(A) Increased endothelial adherence of sickle cells in buffer. Sickle cell and normal cell endothelial adhesiveness was measured in HBSS-BSA buffer and the figure presents the relative adherence with respect to normal controls (see Materials and Methods). (B) Direct paired measurement of the relative adherence of sickle cells in buffer and in autologous plasma. Error bars associated with the relative adherence of sickle cells represent standard deviations for the number of experiments indicated in the parentheses. By definition, there is no standard deviation associated with the normalized relative adherence of normal cells; however, in (A) and also in Figs 4 and 6 below, a dashed error bar representing the average standard deviation of the number of bound control cells in replicate wells within each experiment is provided as an additional index of internal variability independent of the statistical analysis. The asterisk in (A) indicates statistical significance at P < .001 (see Materials and Methods). In (B), the relative adherence of sickle cells in buffer and in plasma did not differ at the .05 level (sickle cell adherence in buffer or in plasma was nonetheless significantly different from the unitary adherence of control normal cells at P < .001).
(A) Increased endothelial adherence of sickle cells in buffer. Sickle cell and normal cell endothelial adhesiveness was measured in HBSS-BSA buffer and the figure presents the relative adherence with respect to normal controls (see Materials and Methods). (B) Direct paired measurement of the relative adherence of sickle cells in buffer and in autologous plasma. Error bars associated with the relative adherence of sickle cells represent standard deviations for the number of experiments indicated in the parentheses. By definition, there is no standard deviation associated with the normalized relative adherence of normal cells; however, in (A) and also in Figs 4 and 6 below, a dashed error bar representing the average standard deviation of the number of bound control cells in replicate wells within each experiment is provided as an additional index of internal variability independent of the statistical analysis. The asterisk in (A) indicates statistical significance at P < .001 (see Materials and Methods). In (B), the relative adherence of sickle cells in buffer and in plasma did not differ at the .05 level (sickle cell adherence in buffer or in plasma was nonetheless significantly different from the unitary adherence of control normal cells at P < .001).
In buffer, red blood cell adherence values were 106 ± 39 (range, 53 to 267) and 241 ± 61 (range, 114 to 415) cells/cm2 for normal and sickle cells, respectively. These absolute binding values are substantially lower than those reported by Sugihara et al22 using similar binding conditions. They are nevertheless consistent with this previous work, as our protocol included three washes, which may well account for the lower number of bound cells. In addition, our visual counting method, which is limited to the center of the well, avoids a large artifactual increase in red blood cells near the periphery of the wells. The mean relative cytoadherence obtained for each patient in multiple experiments ranged from 2.05 to 2.69, and these values differed significantly between some individuals (P < .05).
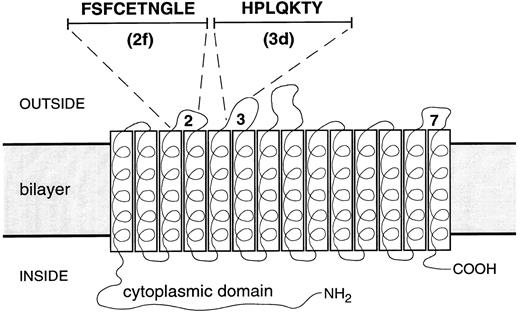
To examine the effect of band 3 peptides on this increased adhesiveness, synthetic peptides were prepared with amino acid sequences found in connecting “loop” regions between transmembrane helices of band 3. Figure 3 indicates the location of these regions and specifies the amino acid composition of peptide 2f from loop 2, which connects helices 3 and 4, and peptide 3d from loop 3, which connects helices 5 and 6. The adherence of sickle cells was then assayed in the presence and in the absence of these peptides which, when used, were present in all incubation and washing steps at micromolar levels.
Sequence and location of band 3 peptides. The membrane domain of the band 3 monomer is depicted as 14 transbilayer helices connected by loop sequences. The position and sequence of peptides 2f (residues 476-485) from extracellular loop 2 connecting helices 3 and 4, and 3d (residues 547-553) from extracellular loop 3 connecting helices 5 and 6, are shown. Extracellular loop 7, the putative position of peptide 7e (residues 814-820: KPPKYHP), is also indicated.
Sequence and location of band 3 peptides. The membrane domain of the band 3 monomer is depicted as 14 transbilayer helices connected by loop sequences. The position and sequence of peptides 2f (residues 476-485) from extracellular loop 2 connecting helices 3 and 4, and 3d (residues 547-553) from extracellular loop 3 connecting helices 5 and 6, are shown. Extracellular loop 7, the putative position of peptide 7e (residues 814-820: KPPKYHP), is also indicated.
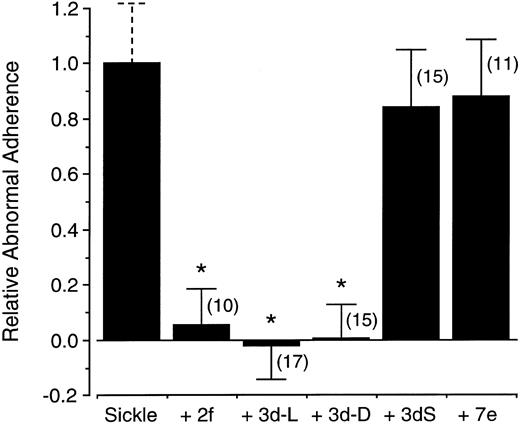
The effects of band 3 peptides on the abnormal adherence of sickle cells, which is defined as the adherence of sickle cells above that of normal cells, are summarized in Fig 4. Micromolar concentrations of peptide 2f or 3d completely abrogated the increased endothelial adherence of sickle cells found in the present assay system. Remarkably, when peptide 3d was composed of all D-amino acids (3d-D), it, too, completely inhibited the abnormal sickle cell adherence. In contrast, 3dS, a peptide using the same amino acids as those in the active peptide 3d, but scrambled in order, did not significantly inhibit the adherence of sickle cells. Further, peptide 7e, derived from the region encompassing interhelical loop 7 and distant from the region encompassing peptides 2f and 3d in the primary sequence, also had no effect.
Inhibition of sickle cell adherence by band 3 peptides. The increased adherence of sickle cells above that of normal red blood cells, referred to as “abnormal adherence,” is arbitrarily normalized in the first bar. Shown in the following bars are the relative effects on this abnormal adherence of peptides 2f, 3d, 3d-D (an all D- isomer of 3d), 3dS (a scrambled sequence of 3d: LYPQHKT, and 7e). Concentrations were 25 μg/mL for 3d, 3d-D, and 2f, and 42 μg/mL for 3dS and 7e. Error bars represent corresponding standard deviations for the number of experiments indicated in the parentheses and asterisks indicate statistical significance at P < .001 (see Materials and Methods).
Inhibition of sickle cell adherence by band 3 peptides. The increased adherence of sickle cells above that of normal red blood cells, referred to as “abnormal adherence,” is arbitrarily normalized in the first bar. Shown in the following bars are the relative effects on this abnormal adherence of peptides 2f, 3d, 3d-D (an all D- isomer of 3d), 3dS (a scrambled sequence of 3d: LYPQHKT, and 7e). Concentrations were 25 μg/mL for 3d, 3d-D, and 2f, and 42 μg/mL for 3dS and 7e. Error bars represent corresponding standard deviations for the number of experiments indicated in the parentheses and asterisks indicate statistical significance at P < .001 (see Materials and Methods).
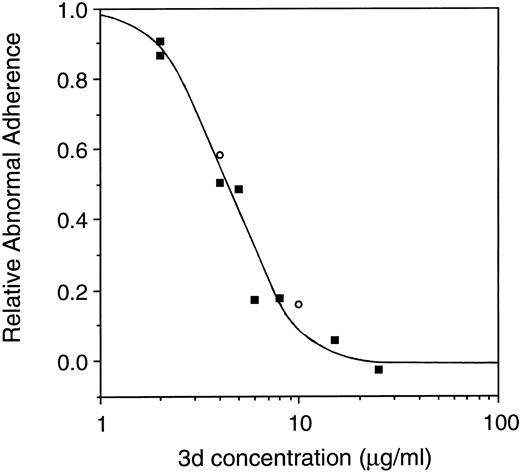
The peptide concentration required to maximally inhibit sickle cell adhesiveness was examined. As shown in Fig 5, for peptide 3d, there was a distinct concentration dependence, with 50% inhibition of adherence occurring at approximately 4 μmol/L, and 100% inhibition occurring at approximately 20 μmol/L. Supporting the result of Fig 4, the potency of the L- and the D-isomer was equivalent. Very similar results (not shown) were obtained with peptide 2f.
Concentration dependence of inhibition of the abnormal adherence of sickle cells by peptide 3d. All-L or all-D isomers of peptide 3d were added to the adherence assay at final concentrations between 2 and 25 μg/mL, as indicated (▪ = L-isomer; ○ = D-isomer). The data presented represent the pool of multiple experiments.
Concentration dependence of inhibition of the abnormal adherence of sickle cells by peptide 3d. All-L or all-D isomers of peptide 3d were added to the adherence assay at final concentrations between 2 and 25 μg/mL, as indicated (▪ = L-isomer; ○ = D-isomer). The data presented represent the pool of multiple experiments.
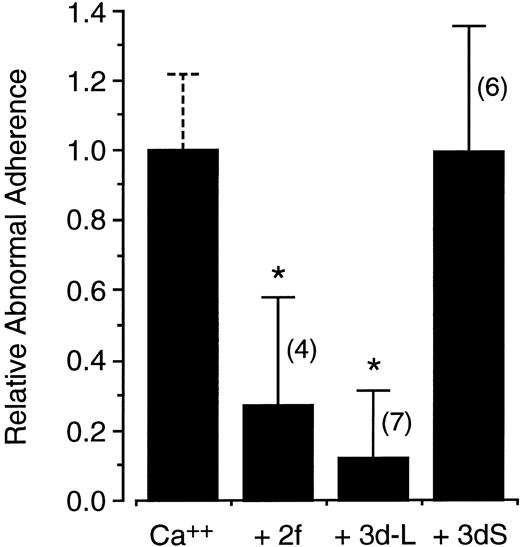
Because very low dose calcium loading of normal cells has been shown to reproduce both the abnormal adherence and clumped surface charge distribution of sickle cells18 and because band 3 is abnormally aggregated in the sickle membrane,20 the effect of the band 3 peptides on the adherence of these modified cells was also evaluated. Prior loading of normal cells with 10 μmol/L calcium increased endothelial adherence by 3.40 ± 1.39-fold in 11 paired adherence experiments conducted with and without peptide. As shown in Fig 6, the abnormal adherence of calcium-loaded cells was strongly inhibited by micromolar concentrations of peptides 3d or 2f. In contrast, no effect was observed with the scrambled peptide 3dS.
Peptide inhibition of increased adherence of calcium-loaded normal red blood cells. Red blood cells were loaded with 10 μmol/L calcium using the ionophore A23187. The increased adherence of calcium-loaded cells above that of control cells is defined as abnormal adherence and is arbitrarily normalized in the first bar. Shown in the following bars are the relative effects on this abnormal adherence of peptides 2f, 3d-L, and 3dS at 10, 25, and 42 μg/mL, respectively. Error bars represent corresponding standard deviations for the number of experiments indicated in the parentheses and asterisks indicate statistical significance at P < .05 (see Materials and Methods).
Peptide inhibition of increased adherence of calcium-loaded normal red blood cells. Red blood cells were loaded with 10 μmol/L calcium using the ionophore A23187. The increased adherence of calcium-loaded cells above that of control cells is defined as abnormal adherence and is arbitrarily normalized in the first bar. Shown in the following bars are the relative effects on this abnormal adherence of peptides 2f, 3d-L, and 3dS at 10, 25, and 42 μg/mL, respectively. Error bars represent corresponding standard deviations for the number of experiments indicated in the parentheses and asterisks indicate statistical significance at P < .05 (see Materials and Methods).
DISCUSSION
Although several different assays have been used to measure sickle cell adhesiveness, the one method that has provided data showing a correlation between the extent of adhesiveness and the clinical severity of sickle cell disease is the static gravity sedimentation assay with gentle washing developed by Hebbel et al.18 23 Because of this, we elected to use this assay with only a minor modification: substituting microscopic visualization for chromium labeling. We recognize that the use of washes in this assay selects a fraction of adhesive cells, which are comparatively firmly bound, and thus reduces the total number of bound cells detected. However, the fraction of bound cells that is physiologically important has not been established for any assay. Importantly, the present assay closely reproduced the increased relative adherence of sickle cells with respect to normal cells found with previous static assays.
With this assay, we found no significant difference in adherence values when autologous plasma was present or absent. In early work, Hebbel et al30 reported a substantial increase in the number of adherent sickle cells suspended in autologous plasma. However, in subsequent studies,22 this effect was markedly reduced and of uncertain statistical significance. In view of the fact that our method did not employ plasma in all the washing steps as in Hebbel's early work, and the absence of a plasma effect in other studies where plasma was absent during washing,26,31 it is possible that the difference in response to plasma depends on its presence in the washes. Other demonstrations of a plasma effect have used very different assay systems (eg, using micropipettes or flow chambers), which are presumably not comparable to static assays because they are likely to emphasize different binding characteristics or subpopulations.27 32-35
The present in vitro experiments indicate that specific peptides derived from segments of the membrane domain of band 3 effectively block at least one form of increased endothelial adherence of sickle cells and implicate a role for band 3 in that adherence. That peptides derived from the neighboring connecting regions between helices 3 and 4 and 5 and 6 were active, whereas a peptide from a distinct region of the molecule next to helix 13 was inactive, suggests that a specific local region at the surface of band 3 has become exposed to serve an adhesive role. The immunostaining of a fraction of sickle cells with a monoclonal antibody recognizing the interhelical sequence comprising peptide 3d (Fig 1) further supports this assertion (immunostaining of a fraction of sickle cells with antiband 3 antibodies has been confirmed by the finding of <1% to 8% reactive cells by fluorescence-activated cell sorting [FACS] analysis in a separate study36 ). The results with the calcium-loaded cells indicate that even normal cells can be induced to expose adhesive segments of band 3 and suggest the possibility of a common intermediate (band 3 rearrangement) for increased adhesiveness in various red blood cell disorders.
The surprising finding that a peptide composed of all D-amino acids was just as active as one composed of all L-amino acids is not without precedent. Recently, D-isomers of small peptides have been found to be biologically active in immunologic,37 neurologic,38 cell attachment,39 and inflammatory systems.40 Further, in a direct parallel to the present study, full activity of the D-peptides in inhibiting cytoadherence was also observed in malaria-infected red blood cells.17 Perhaps, if the peptide is short enough and flexible enough, the structural constraints of “handedness” are less important than usually assumed.37
The abnormally increased adhesiveness of sickle cells is likely to have a pathophysiologic role in the morbidity of sickle cell disease. Indeed, despite extensive and long-term clinical observations,41-43 the only laboratory parameters found thus far to positively correlate with the vaso-occlusive severity of sickle cell disease are higher hematocrit levels and increased endothelial adherence.23,42 43 Thus, although the intracellular concentration of sickle hemoglobin, the concentration of polymer, or the number of nondeformable cells, might all be reasonably expected to have some role in what is likely a multifactorial vaso-occlusive process, endothelial adhesiveness appears to play a comparatively important role.
Perhaps because of its importance, many studies have focused on the possible mechanism for the endothelial adherence of sickle cells. Because of their accepted role in cell/cell interactions, several plasma factors have been considered as possible mediators.22,29,35 These include fibrinogen, von Willebrand's factor, and thrombospondin, which can potentiate sickle cell adhesion to larger and smaller vessels, respectively33,34 and to human dermal microvascular cells.44 However, the inhibition of sickle cell adhesion by specific synthetic peptides suggests that the abnormal sickle cell adherence observed here is primarily mediated by a cellular factor–likely a change in the disposition of connecting regions between intramembrane helices of band 3. Certainly in other systems, which likely examine different cells or different adhesion characteristics,35 substantial effects of plasma proteins have been detected and our observations do not preclude an adhesive role for these factors. In particular, the current in vitro experiments were not designed to establish the relative importance of the cellular factor described here vis-à-vis plasma factors in mediating sickle cell adhesiveness. Further, we have not examined possible effects of residual plasma proteins bound to the membrane, which were not removed by washing procedures. Nevertheless, the current in vitro findings may imply in vivo relevance, because without the addition of plasma, they show inhibition of adhesiveness in an assay closely analogous to that used to show the correlation of adhesiveness with clinical severity.23
Sickle cells, unlike normal red blood cells, adhere using multiple attachment sites.2 Sickle reticulocytes express the molecules CD36,45 as well as the very late antigen-4 (VLA-4) or α4β1 integrin,46 and these may play a role in binding to the endothelium via thrombospondin47 and vascular cell adhesion molecule-1,48 respectively. In addition, there is evidence for an interaction between thrombospondin and von Willebrand factor in influencing the adhesion of sickle cells.49 The present findings imply yet another adhesin, namely altered band 3. The mechanism for exposure of band 3 adhesive segments remains undetermined. Abnormal distribution of intramembranous particles is a common denominator in malaria-infected cells19 and sickle cells,20,50 which both show band 3-dependent adhesion. Therefore, we propose that exposure of adhesive band 3 sites may be a common result of band 3 aggregation. That aggregation could be, for example, due to oxidative damage from the increased reactive oxygen species found in both malaria-infected and sickle cells,51 52 or to calcium influx in normal red blood cells. The band 3 aggregation in turn, would distort the conformation of the membrane domain to expose the adhesive sites.
In parallel studies, no correlation was found between the immunostaining of sickle cells with various monoclonal antibodies to the putative band 3 adhesin and the degree of reticulocytosis36 (Swerlick personal communication, June, 1995). This suggests that, in contrast to some other forms of sickle cell adhesiveness, the adherence observed here, which appears to involve a structural change in band 3, is not dependent on the immaturity of the red blood cell.
Finally, the in vitro experiments described here with synthetic peptides derived from band 3 protein provide for the possibility of a novel approach to the therapy of sickle cell disease. Both the low concentration of peptides required to inhibit adhesion and the complete effectiveness of relatively protease-resistant D-isomers suggest that peptides might be useful pharmacologic agents for sickle cell patients.
Supported by Grant No. DK16095 from the National Institutes of Health, Bethesda, MD, and the United Nations Development Program/World Bank/World Health Organization Special Programme for Research and Training in Tropical Diseases, and by a generous donation from the W. Duncan MacMillan Trust.
This work is dedicated to the memory of Sarah Stevens MacMillan (1931-1995).
Presented in part at the 108th Annual Meeting of the American Association of Physicians, May 5-8, 1995, San Diego, CA, and published in abstract form J Invest Med 43:A341, 1995 (suppl 2).
Address reprint requests to Bernard J.-M. Thevenin, PhD, University of California, San Francisco, Box 0134, San Francisco, CA 94143.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal