Abstract
The antineoplastic agent bryostatin-1 (bryo-1) possesses powerful immunomodulatory properties and can function as a biological response modifier in vivo. However, there is currently little information regarding the effects of bryo-1 on cells of the monocytic lineage. In this study, we demonstrate that bryo-1 can potently induce the production of proinflammatory cytokines from human peripheral blood monocytes. Stimulation of monocytes with subnanomolar concentrations of bryo-1 significantly upregulated the constitutive levels of interleukin-8 (IL-8) mRNA and induced the expression of IL-1β, tumor necrosis factor-α (TNF-α), and IL-6 mRNA in a time and dose-dependent manner. Accordingly, secretion of all four proinflammatory cytokines was induced after monocyte exposure to bryo-1. Furthermore, we showed that bryo-1 selectively synergized with IL-2 in triggering monocyte activation, and this effect seemed to be dependent, at least in part, on the ability of bryo-1 to upregulate IL-2Rγ chain expression. Finally, we demonstrated that the responses of monocytes to bryo-1 could be blocked by the protein kinase C (PKC) inhibitors staurosporine and UCN-01, indicating a role for PKC in monocyte activation by bryo-1. These results show for the first time that bryo-1 is a powerful activator of human monocytes and suggest that stimulation of monokine secretion by bryo-1 may represent at least one of the mechanisms responsible for the in vivo antitumor activity of this drug.
BRYOSTATIN 1 (bryo-1), a macrocyclic lactone isolated from the marine bryozoan Bugula neritina,1 exhibits a unique pattern of biological activities (for a review, see Kraft2 ). Bryo-1 is a potent ligand and activator of the phorbol ester receptor, protein kinase C (PKC),3-5 and it can mimic certain effects of the phorbol esters in some biological systems.6 However, several other properties of bryo-1 are distinct from the phorbol esters,7,8 and more important, unlike the phorbol esters, this compound lacks tumor-promoting capabilities, counteracting tumor promotion induced by tetradecanoylphorbol 13-acetate (TPA).9 Bryo-1 has attracted considerable interest because of its potent in vitro and in vivo antineoplastic activity. It has been shown to inhibit the in vitro growth of a variety of murine and human tumor cell lines,10-13 and significant antitumor activity has been observed in in vivo murine models for leukemia, ovarian carcinoma, B-cell lymphoma, reticulum cell sarcoma, and melanoma.2,10,11 The potential clinical use of bryo-1 as an antineoplastic agent is currently being evaluated,14,15 and promising therapeutic effects against metastatic malignant melanoma were recently observed.16 Although the exact mechanism responsible for its antitumor activity is not clear, numerous in vitro studies have demonstrated that bryo-1 can act as a cytostatic or a cytotoxic agent on several types of tumors. Application of bryo to either freshly isolated cells from patients with acute and chronic myelogenous and lymphocytic leukemias12,13 or to both myeloid and lymphoid human leukemia cell lines12,13,17,18 promotes their terminal differentiation and halts their growth. Moreover, we (Espinoza-Delgado et al, manuscript in preparation) and others19 have shown that bryo-1 directly induces growth arrest and apoptosis in human B-cell lymphomas. However, bryo-1 may also inhibit tumor growth in vivo by indirect mechanisms, related to its ability to stimulate host immune responses. It has been shown that bryo-1 enhances human polymorphonuclear leukocytes' cytotoxicity, oxidative burst, and degranulation,4,20,21 induces the proliferation and activation of human B and T cells,14,22-24 triggers cytotoxic T lymphocyte (CTL) development,23 and stimulates lymphocyte secretion of interferon-γ (IFN-γ) and interleukin-2 (IL-2).25
One of the key elements in the host defense against neoplasia is the monocyte/macrophage.26 Mononuclear phagocytes represent a main recognition system for foreign cells, including tumor cells,27 play a major role in antigen presentation to lymphocytes,26 and are an important source of a wide repertoire of secretory products endowed with antitumor and immunomodulatory properties, including the proinflammatory cytokines IL-1β, tumor necrosis factor-α (TNF-α), IL-6, and IL-8.26,29 Indeed, IL-1, TNF-α, and IL-6 were reported to exert direct cytotoxic or cytostatic effects on tumor cells and to stimulate T and B lymphocyte activity.30-34 IL-8, on the other hand, has been shown to induce chemotaxis and activation of a wide variety of immune cells, such as neutrophils, basophils, and T lymphocytes, thus contributing to leukocyte recruitment and amplifying host immune responses.35,36 Monocytes/macrophages can be induced to exert effector, secretory, and antigen-presenting functions by various physiological and environmental stimuli.27,37 Among them, IL-2 has been shown to be a powerful activator of human peripheral blood monocytes.38 IL-2–treated monocytes express tumoricidal and microbicidal activity39,40 and show enhanced synthesis and release of hydrogen peroxide, superoxide, prostaglandin E2 , and thromboxane B2 ,39,41 as well as increased production of several cytokines and growth factors.38
The present study was designed to investigate the effects of bryo-1 on the activation of human monocytes and, specifically, on their production of proinflammatory cytokines, and to analyze its possible interaction with IL-2. We demonstrate that bryo-1 induces IL-1β, TNF-α, IL-6, and IL-8 mRNA expression and protein secretion from human monocytes and acts synergistically with IL-2 in triggering monocyte activation. Finally, our findings suggest a role for PKC in mediating monocyte response to bryo-1.
MATERIALS AND METHODS
Monocyte isolation and culture conditions.Peripheral blood leukocytes were obtained from normal healthy volunteers (who provided informed consent) by leukapheresis using a Fenwal CS-3000 blood cell separator (Fenwall Laboratories, Deerfield, IL). Mononuclear cells were separated by density gradient centrifugation on lymphocyte separation medium (LSM; Organum Teknika Corp, Durham, NC), and then purified in suspension from the unfractionated mononuclear leukocyte preparation by counter-current centrifugal elutriation in a Beckman JE6 elutriation chamber and rotor system (Beckman Instruments, Inc, Palo Alto, CA) as described elsewhere.42 The purity of monocyte preparations was 94% ± 3% as assessed by morphology on Giemsa-stained cytocentrifuge slide preparations and by flow cytometry using anti-CD14 monoclonal antibodies (MoAbs). Large granular lymphocytes (LGL) represented less than 1% of the monocyte preparation, as determined by the LGL marker CD56 (Becton Dickinson, Mountain View, CA). Viability, determined by trypan blue exclusion, was greater than 99%. Monocytes were cultured in RPMI 1640 (Advanced Biotechnology, Columbia, MD), supplemented with 100 U/mL penicillin, 100 U/mL streptomycin, 2 mmol/L glutamine, 20 mmol/L HEPES (GIBCO, Grand Island, NY) and 10% heat-inactivated fetal calf serum (FCS) (HyClone Laboratories, Logan, UT), hereafter referred to as complete medium (CM).3
Reagents.Bryostatin-1 (lot. 2017-25-1), kindly provided by the BRMP Repository (NCI-FCRDC, Frederick, MD), was isolated from the marine bryozoan Bugula neritina, as previously described1 and dissolved in dimethyl sulfoxide (DMSO) at 100 μg/mL (conversion factor: 1.0 ng/mL = 0.904 nmol/L). Clinical grade bryo-1 was a gift from A. Fallavollita Jr, M.S. (Cancer Therapy Evaluation Program, Division of Cancer Treatment, Diagnosis, and Centers [DCTDC], National Cancer Institute, National Institutes of Health [NCI-NIH], Bethesda, MD). Highly purified human recombinant IL-2 from Escherichia coli (sp. act. 18 × 105 IU/mg, LPS content <0.6 pg/mL) was obtained from Cetus Corporation (Emeryville, CA).43 Human recombinant IFNγ (sp. act. 2.02 × 107 IU/mg) was kindly provided by Dr H. Michael Shepard (Genentech Labs, San Francisco, CA). Staurosporine was purchased from Sigma Chemical Co (St Louis, MO), and UCN-01 was a gift from Kamiya Biomedical Company (Thousand Oaks, CA). Both PKC inhibitors were dissolved in DMSO at 40 and 2 mmol/L, respectively, and further diluted in CM. All reagents were demonstrated to be endotoxin free by the Limulus amebocyte lysate test (M.A. Bioproducts, Walkersville, MD; sensitivity 0.06 IU/mL).
Detection of cytokine release.Monocytes were cultured in 15 cm Lux plates (Miles Scientific, Wapersville, CA) at 2 × 106 cells/mL and stimulated for 18 hours with the indicated factors. At the end of the incubation period, cell-free supernatants were harvested and assayed for IL-1β, TNF-α, IL-6, and IL-8 activity using an IL-6–specific enzyme-linked immunosorbent assay (ELISA) from Biosource (Camarillo, CA) and IL-1β–, TNF-α–, and IL-8–specific ELISAs from R&D Systems (Minneapolis, MN), following the manufacturer's instructions.
Northern blot analysis.Monocytes were cultured for the indicated time points in 15 cm Lux plates at 2 × 106 cells/mL in the presence of IL-2 or bryo-1, alone or in combination. In a few experiments, monocytes were preincubated with Staurosporine or UCN-01 for 1 hour before the addition of the stimuli. Cells were then lysed in RNAzol (Tel-Test, Inc, Friendswood, TX), and total RNA was purified according to the manufacturer's instructions. A total of 20 μg of RNA from each sample was electrophoresed under denaturing conditions on a 1.2% agarose gel containing 2.2 mol/L formaldehyde, transferred onto Nytran membranes (Schleicher & Schuell Inc, Keene, NH) and cross-linked by ultraviolet (UV) irradiation. Filters were prehybridized for 6 hours at 42°C in Hybrisol solution (Oncor Inc, Gaithersburg, MD) and hybridized overnight with 2 × 106 cpm/mL of 32P-labeled probe. Membranes were then washed three times at room temperature for 10 minutes in 2× SSC, 0.1% sodium dodecyl sulfate (SDS) and twice at 60°C for 15 minutes in 0.2× SSC, 0.1% SDS, before being autoradiographed using Kodak XAR-5 film (Rochester, NY) and intensifying screens at −80°C. Probes were labeled by random priming reaction using a commercial kit (Boehringer Mannheim Biochemicals, Indianapolis, IN) and deoxycytidine 5′-[α-32P] triphosphate ([α-32P]dCTP) (3,000 Ci/mmol; Amersham Corp, Arlington Heights, IL). The sp. act. was always higher than 109 cpm/μg. For IL-8 detection, the hIL-8 full-length cDNA from the pUC19 vector, kindly provided by Dr K. Matsushima (Kanazawa University Cancer Institute, Kanazawa, Ishikawa-ken, Japan), was used. For IL-1β detection, the hIL-1β cDNA from the PBR322 vector, obtained from Dr D. Carter (The Upjohn Co, Kalamazoo, MI) was used. For IL-6 detection, the 900-bp Pstl fragment of hIL-6 cDNA44 was used. The hTNF-α cDNA was kindly provided by Dr S.A. Nedospasov (Institute of Molecular Biology, Academy of Sciences of Russia, Moscow, Russia). For IL-2Rγ detection, the 1.5-kb full-length cDNA from pIL-2Rγ2, kindly provided by Dr Kazuo Sugamura (Tohoku University School of Medicine, Sendai, Japan), was used. The human glyceraldehyde-3 phosphate dehydrogenase (G3PDH) probe was purchased from Clontech (Palo Alto, CA). When needed, the autoradiographs were scanned using a light densitometer.
Immunofluorescence analysis.Monocytes were washed with phosphate-buffered saline (PBS) and then labeled according to the following procedure. A total of 1 × 106 cells were resuspended in 100 μL of PBS supplemented with 2% human AB serum (GIBCO) and 0.05% of sodium azide (GIBCO), here referred to as immunofluorescence buffer (IB), and incubated for 30 minutes at 4°C with the anti-IL-2Rγ MoAb 1A11,45 kindly provided by Dr J. Ritz (Dana-Farber Cancer Institute, Boston, MA), or control mouse myeloma IgG1 , obtained from Cappel (Organon Teknika Corp, West Chester, PA). After two washings with cold IB, monocytes were incubated with a 1:25 dilution of fluorescein isothiocyanate (FITC)-conjugated goat F(ab′ )2 antimouse IgG (Tago Immunologicals, Burlingame, CA) in 100 μL of IB for 30 minutes at 4°C. Thereafter, the cells were washed twice and surface fluorescence was quantitated on an EPICS Profile flow cytometer (Coulter Corp, Hialeah, FL), with gate settings specific for monocytes. The data are expressed as mean fluorescence intensity (MFI in arbitrary fluorescence units) that is an indication of the density of surface antigens presents on individual cells.
RESULTS
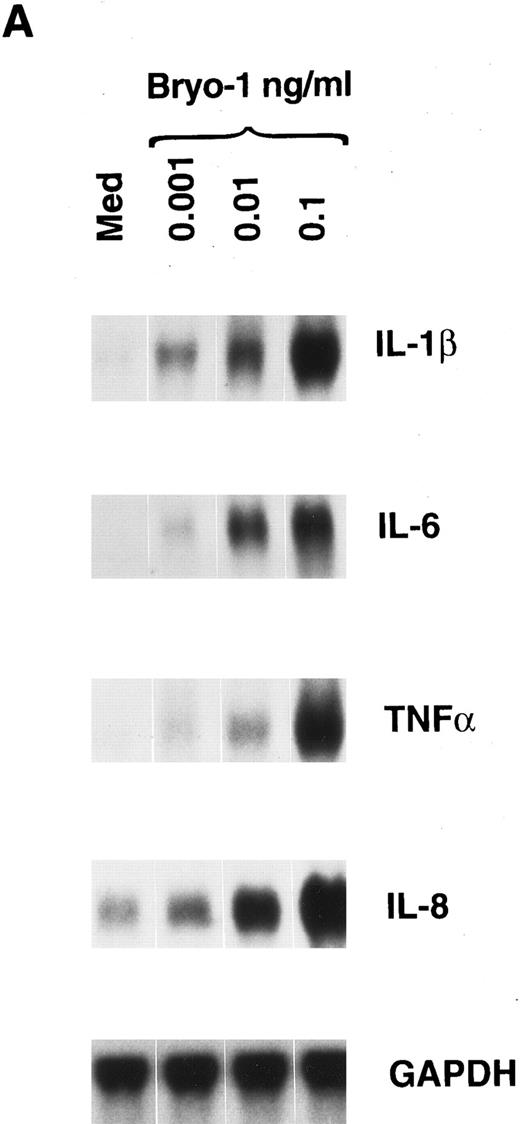
Induction of IL-1β, TNF-α, IL-6, and IL-8 mRNA expression and protein secretion by bryo-1 in human monocytes.In the first set of experiments, we analyzed the effects of bryo-1 on the expression of IL-1β, IL-6, TNF-α, and IL-8 mRNA in purified peripheral blood human monocytes. Northern blot analysis was performed on total RNA extracted from monocytes cultured with increasing concentrations of bryo-1 for 6 hours. This time point was shown to be optimal for mRNA induction by different stimuli.44,46 As shown in Fig 1A, control monocytes expressed low constitutive levels of IL-8 mRNA, whereas no basal expression of IL-6, IL-1β, and TNF-α mRNA was detectable, in agreement with data previously reported.44 46 Stimulation with bryo-1 resulted in a dose-dependent induction of all four transcripts, with as low as 0.001 ng/mL sufficient to upregulate IL-8 mRNA expression and to induce detectable levels of the mRNA for IL-1β, TNF-α, and IL-6. A major increase was observed with a dose of bryo-1 of 0.1 ng/mL, which was optimal for monocyte activation, because no further mRNA augmentation was detected at higher concentrations (data not shown). Similar results were obtained in 5 independent determinations, although different degrees of mRNA induction were evident, representing donor-to-donor variations. At the concentrations used, Bryo-1 did not affect monocyte viability, as determined by trypan blue dye exclusion test, though cytotoxic effects were seen at higher doses (data not shown).
Bryo-1 induces IL-1β, IL-6, TNF-α, and IL-8 mRNA expression in human monocytes. (A) Dose response. Monocytes were cultured for 6 hours in the presence of increasing concentrations of bryo-1. (B) Time-course. Cells were stimulated with 0.1 ng/mL of bryo-1 for the times specified. Total RNA was isolated and Northern blot analysis was performed as described in Materials and Methods. The blots were sequentially hybridized with the cDNAs for the indicated cytokines. GAPDH levels were determined to control that equal amounts of RNA were loaded in each lane.
Bryo-1 induces IL-1β, IL-6, TNF-α, and IL-8 mRNA expression in human monocytes. (A) Dose response. Monocytes were cultured for 6 hours in the presence of increasing concentrations of bryo-1. (B) Time-course. Cells were stimulated with 0.1 ng/mL of bryo-1 for the times specified. Total RNA was isolated and Northern blot analysis was performed as described in Materials and Methods. The blots were sequentially hybridized with the cDNAs for the indicated cytokines. GAPDH levels were determined to control that equal amounts of RNA were loaded in each lane.
To determine the kinetics of mRNA induction, cytokine expression was assessed at different times in monocytes optimally stimulated with bryo-1. Figure 1B shows the results of one representative experiment of three independent determinations. Maximal induction of IL-1β mRNA occurred within 3 hours of stimulation, gradually decreasing thereafter. Very low levels of IL-6 transcripts were observed after 3 hours of culture, reaching a maximum at 6 hours and becoming almost undetectable at 18 hours. TNF-α mRNA expression was significantly induced 3 hours after stimulation, and it was further increased in a time-dependent manner to reach peak levels at 18 hours. Finally, bryo-1 caused a dramatic and maximal increase in IL-8 mRNA within 3 hours, and similar levels of expression were still observed at 18 hours, starting to decline thereafter.
To establish whether mRNA induction in response to bryo-1 was paralleled by protein secretion, supernatants from bryo-1–stimulated monocytes were assayed for the presence of IL-1β, TNF-α, IL-6, and IL-8 (Table 1). A significant and dose-dependent increase in cytokine release, relative to control cells, was induced by monocyte stimulation with bryo-1 for 18 hours. Similar results were obtained in two independent determinations, although some variability was observed. This variability is a common phenomenon in freshly isolated cells and represent donor-to-donor variations. Secretion of IL-1β, TNF-α, and IL-8 from bryo-1–treated monocytes was observed as early as 3 hours after the onset of the culture in three independent experiments. Similar data were obtained with clinical grade bryo-1 (data not shown). These results provide the first evidence that bryo-1 is a potent activator of human monocytes and can induce monocyte production of several cytokines.
Dose-Dependent Induction by Bryo-1 of Cytokine Secretion From Human Monocytes
| Treatment . | Cytokines (pg/mL) . | |||
|---|---|---|---|---|
| . | IL-1β . | IL-8 . | TNF-α . | IL-6 . |
| Donor 1 | ||||
| Medium | 0 | 399 | 0 | 48 |
| Bryo 0.001 ng/mL | 117 | 1,514 | 77 | 2,925 |
| Bryo 0.01 ng/mL | 146 | 2,914 | 189 | 4,831 |
| Bryo 0.1 ng/mL | 89,400 | 16,413 | 11,700 | 6,051 |
| Donor 2 | ||||
| Medium | 0 | 1,880 | 0 | 0 |
| Bryo 0.001 ng/mL | 12 | 3,272 | 33 | 45 |
| Bryo 0.01 ng/mL | 41 | 7,483 | 62 | 191 |
| Bryo 0.1 ng/mL | 400 | 84,260 | 1,000 | 2,438 |
| Treatment . | Cytokines (pg/mL) . | |||
|---|---|---|---|---|
| . | IL-1β . | IL-8 . | TNF-α . | IL-6 . |
| Donor 1 | ||||
| Medium | 0 | 399 | 0 | 48 |
| Bryo 0.001 ng/mL | 117 | 1,514 | 77 | 2,925 |
| Bryo 0.01 ng/mL | 146 | 2,914 | 189 | 4,831 |
| Bryo 0.1 ng/mL | 89,400 | 16,413 | 11,700 | 6,051 |
| Donor 2 | ||||
| Medium | 0 | 1,880 | 0 | 0 |
| Bryo 0.001 ng/mL | 12 | 3,272 | 33 | 45 |
| Bryo 0.01 ng/mL | 41 | 7,483 | 62 | 191 |
| Bryo 0.1 ng/mL | 400 | 84,260 | 1,000 | 2,438 |
Monocytes were cultured at 2 × 106/mL in medium alone or supplemented with increasing concentrations of bryo-1. Supernatants were collected after 18 hours and assayed for the indicated cytokines by specific ELISA. Bryo-1 conversion factor: 1.0 ng/mL = 0.904 nmol/L.
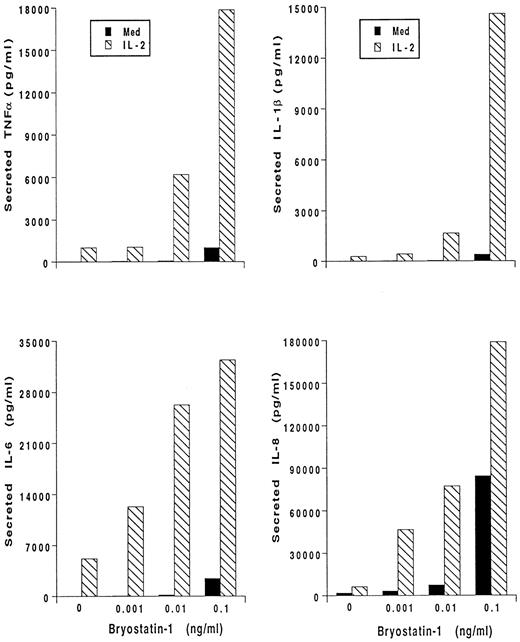
Bryo-1 and IL-2 synergistically activate human monocytes.Bryo-1 has been shown to induce the production of IL-2 from activated lymphocytes, and to potentiate several IL-2 immune modulating effects.14,22,25 Human monocytes can be stimulated by IL-2 to release proinflammatory cytokines.36 We, therefore, wished to determine whether the combination of IL-2 and bryo-1 had additive or even synergistic effects on the activation of monocyte secretory functions. Monocytes were stimulated for 18 hours with optimal doses of IL-2,44,46 alone or in the presence of increasing concentrations of bryo-1, and cytokine production was determined. A representative experiment is depicted in Fig 2. As previously shown,45 IL-2 induced secretion of IL-1β, TNF-α, IL-6, and IL-8. Addition of 0.1 ng/mL of bryo-1 to the culture synergistically enhanced cytokine release caused by IL-2. In fact, when bryo-1 and IL-2 were used together, an increase in the amounts of secreted cytokines, up to 30-fold higher than that induced by either stimulus alone, was observed in three different experiments. A high degree of synergy was detectable even when suboptimal (0.01 to 0.001 ng/mL) concentrations of bryo-1 were used.
Bryo-1 synergizes with IL-2 in triggering cytokine secretion from monocytes. Cells were stimulated for 18 hours with medium alone (▪) or supplemented with IL-2 (1,000 U/mL) (▧) in the presence or absence of increasing concentration of bryo-1. Supernatants were harvested and assayed for secreted IL-1β, IL-6, TNF-α, and IL-8 by specific ELISA. Results from one representative experiment, expressed as ng/mL per 2 × 106 cells/mL, are shown.
Bryo-1 synergizes with IL-2 in triggering cytokine secretion from monocytes. Cells were stimulated for 18 hours with medium alone (▪) or supplemented with IL-2 (1,000 U/mL) (▧) in the presence or absence of increasing concentration of bryo-1. Supernatants were harvested and assayed for secreted IL-1β, IL-6, TNF-α, and IL-8 by specific ELISA. Results from one representative experiment, expressed as ng/mL per 2 × 106 cells/mL, are shown.
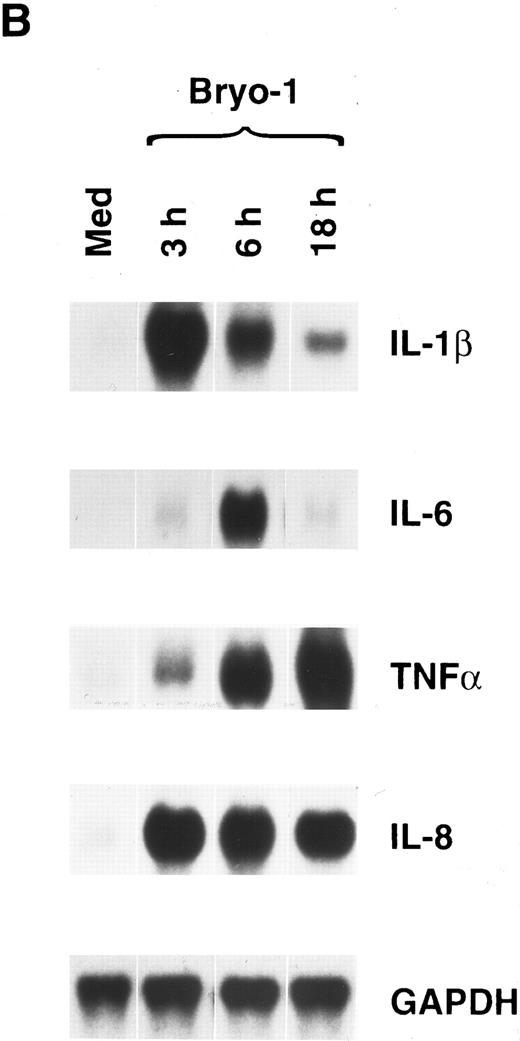
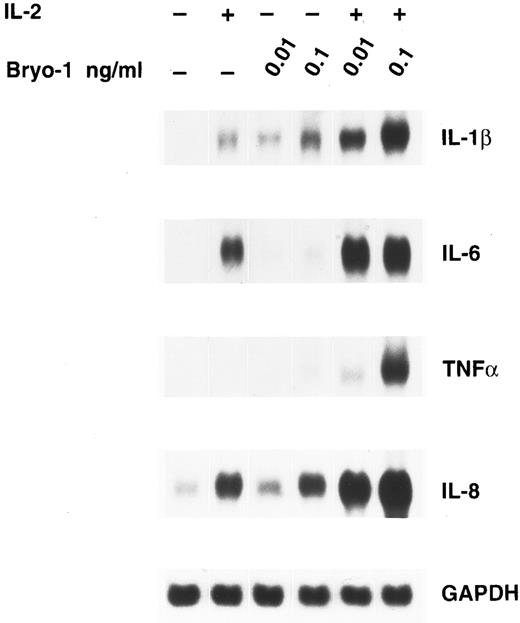
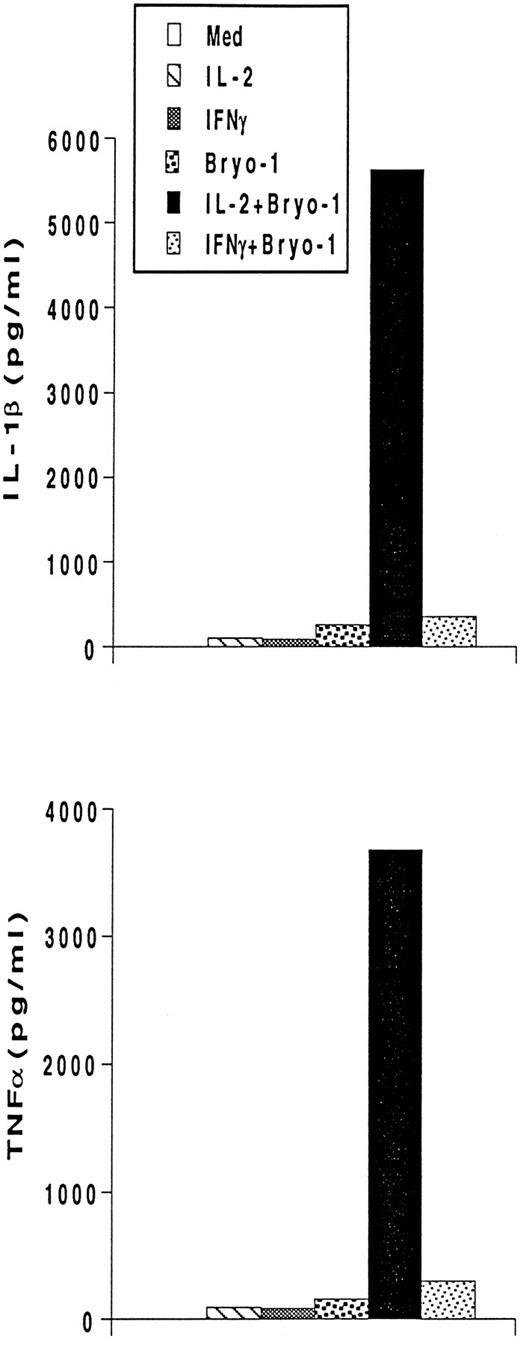
To investigate whether the synergism between IL-2 and bryo-1 was exerted at the level of gene expression, monocytes were stimulated with IL-2 in the presence or absence of bryo-1, and Northern blot analysis was performed. As shown in Fig 3, stimulation of monocytes with IL-2 in combination with optimal or suboptimal doses of bryo-1 resulted in a dramatic upregulation of IL-1β, TNF-α, IL-6, and IL-8 mRNA above the levels induced by either individual stimulus. This synergistic effect, which occurred as early as 3 hours after the onset of the culture, was further increased at 18 hours (data not shown). Finally, we wished to determine whether the synergistic effect of bryo-1 was IL-2–specific or would occur with any stimulus capable of generating the activated phenotype in monocytes. To address this issue, we tested in parallel experiments the effects of bryo-1 on monocyte activation by IL-2 or IFN-γ. IFN-γ has been previously shown to induce monocyte production of IL-1β and TNF-α.37 In agreement with these observations, treatment of monocytes with IFN-γ induced an increase in the amounts of secreted IL-1β and TNF-α relative to the basal levels, that was comparable to that observed in response to IL-2 (Fig 4). Addition of bryo-1 to the culture caused a synergistic enhancement of monocyte activation by IL-2, but not by IFN-γ. Three independent experiments yielded comparable results. We concluded that bryo-1 selectively synergizes with IL-2 in triggering cytokine secretion from monocytes, and that this effect is exerted, at least in part, at the mRNA level.
Bryo-1 enhances IL-2–induced expression of IL-1β, TNF-α, IL-6, and IL-8 mRNA. Monocytes were incubated for 3 hours with IL-2 (1,000 U/mL) alone or in combination with 0.1 or 0.01 ng/mL of bryo-1 before total RNA was isolated and sequentially probed for cytokine mRNA levels by Northern blot. The blot was then rehybridized with the GAPDH probe to check the RNA loading. These data are representative of three different experiments.
Bryo-1 enhances IL-2–induced expression of IL-1β, TNF-α, IL-6, and IL-8 mRNA. Monocytes were incubated for 3 hours with IL-2 (1,000 U/mL) alone or in combination with 0.1 or 0.01 ng/mL of bryo-1 before total RNA was isolated and sequentially probed for cytokine mRNA levels by Northern blot. The blot was then rehybridized with the GAPDH probe to check the RNA loading. These data are representative of three different experiments.
Bryo-1 differentially affects IL-2– and IFN-γ–dependent secretion of IL-1β and TNF-α. Monocytes were stimulated with IL-2 (1,000 U/mL) or IFNγ (500 U/mL) in the presence or absence of 0.1 ng/mL of bryo-1. Supernatants were collected after 18 hours of treatment and assayed for IL-1β and TNF-α by ELISA. Data from one representative experiment are shown.
Bryo-1 differentially affects IL-2– and IFN-γ–dependent secretion of IL-1β and TNF-α. Monocytes were stimulated with IL-2 (1,000 U/mL) or IFNγ (500 U/mL) in the presence or absence of 0.1 ng/mL of bryo-1. Supernatants were collected after 18 hours of treatment and assayed for IL-1β and TNF-α by ELISA. Data from one representative experiment are shown.
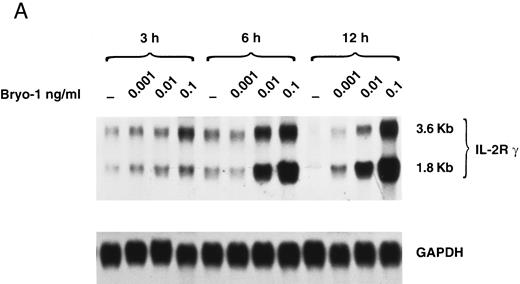
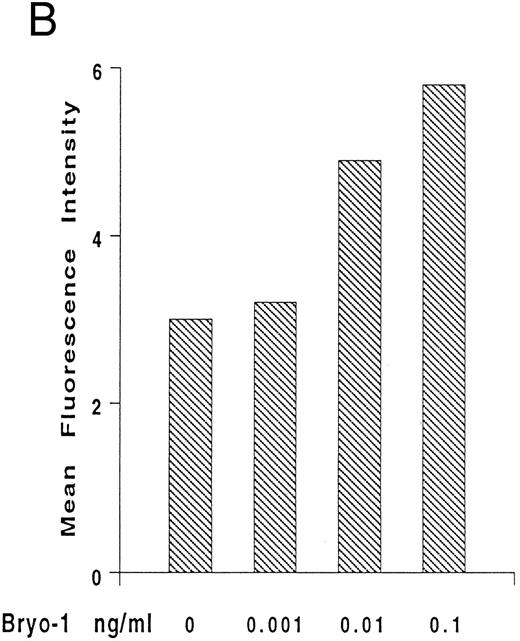
Upregulation by bryo-1 of IL-2Rγ chain expression.We have previously shown that human monocytes constitutively express the β and γ chain of the IL-2R, but not the α subunit.47-49 To gain insights into the mechanisms involved in the synergistic action of bryo-1 on monocyte response to IL-2, experiments were designed to investigate whether bryo-1 affected the expression of the IL-2R components. Monocytes were incubated for different lengths of time with increasing amounts of bryo-1, and Northern blot analysis was performed. Figure 5A shows the results of a representative experiment. Medium-treated monocytes expressed low levels of the 3.6- and 1.8-kb species of IL-2Rγ mRNA, and slight fluctuations in their basal expression were detectable over the time course, confirming earlier results.48 Interestingly, a dramatic augmentation of IL-2Rγ mRNA occurred in response to bryo-1 in a time- and dose-dependent fashion. An initial upregulation was observed within 3 hours of culture with optimal doses of bryo-1 and was further increased at 6 hours and 12 hours. Concentrations of bryo-1 suboptimal for cytokine production, but able to significantly enhance IL-2–triggered effects (0.001 to 0.01 ng/mL), were sufficient to cause a marked upregulation of IL-2Rγ mRNA expression. The increase in mRNA levels was paralleled by enhanced expression of p64 protein on the cell surface, as assessed by flow cytometry analysis (Fig 5B). Bryo-1 did not affect the constitutive expression of IL-2Rβ and did not induce IL-2Rα (data not shown). Three independent experiments yielded similar results. These data demonstrated that bryo-1 can significantly upregulate the synthesis and the expression of IL-2Rγ chain on human monocytes and suggested that this may represent at least one of the mechanisms by which this compound enhances monocyte activation by IL-2.
Upregulation by bryo-1 of IL-2Rγ chain expression. (A) Total RNA was extracted from monocytes stimulated for different time points with medium alone or supplemented with increasing concentrations of bryo-1, and analyzed by Northern blot for IL-2Rγ mRNA expression (upper panel). The blot was then rehybridized with the GAPDH probe (lower panel). The two IL-2Rγ mRNA species of 1.8 and 3.6 Kb are indicated in the figure. (B) Flow cytometric analysis was performed on medium-treated monocytes or on monocytes stimulated for 12 hours with 0.001 to 0.1 ng/mL of bryo-1. Cells were stained by indirect immunofluorescence with the anti-p64 MoAb 1A11, followed by FITC-conjugated goat-antimouse IgG, as detailed in Materials and Methods. Analysis was performed on an EPICS Profile flow cytometer with gate settings specific for monocytes. Values shown indicate the MFI, determined on a logarithmic scale, of the FITCI-1A11 MoAb staining (directly related to the density of IL-2Rγ molecules in individual cells) calculated by subtracting the MFI of isotype-matching IgG1 from the MFI of 1A11-stained cells.
Upregulation by bryo-1 of IL-2Rγ chain expression. (A) Total RNA was extracted from monocytes stimulated for different time points with medium alone or supplemented with increasing concentrations of bryo-1, and analyzed by Northern blot for IL-2Rγ mRNA expression (upper panel). The blot was then rehybridized with the GAPDH probe (lower panel). The two IL-2Rγ mRNA species of 1.8 and 3.6 Kb are indicated in the figure. (B) Flow cytometric analysis was performed on medium-treated monocytes or on monocytes stimulated for 12 hours with 0.001 to 0.1 ng/mL of bryo-1. Cells were stained by indirect immunofluorescence with the anti-p64 MoAb 1A11, followed by FITC-conjugated goat-antimouse IgG, as detailed in Materials and Methods. Analysis was performed on an EPICS Profile flow cytometer with gate settings specific for monocytes. Values shown indicate the MFI, determined on a logarithmic scale, of the FITCI-1A11 MoAb staining (directly related to the density of IL-2Rγ molecules in individual cells) calculated by subtracting the MFI of isotype-matching IgG1 from the MFI of 1A11-stained cells.
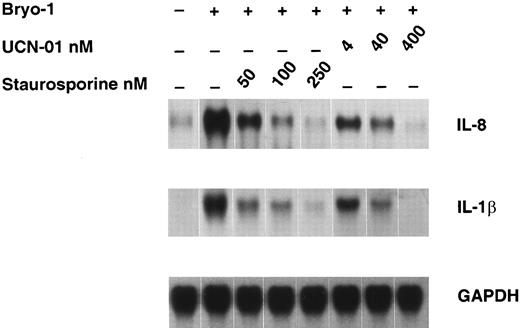
Effect of staurosporine and UCN-01 on bryo-1–induced cytokine expression.Several biological effects of bryo-1 are mediated through binding and activation of PKC.3-5 To study the requirement for PKC activation in the induction of cytokine secretion from human monocytes, we examined the effects of the PKC inhibitors staurosporine and UCN-0150 on bryo-1–induced cytokine mRNA expression in monocytes. As depicted in Fig 6, pretreatment of monocytes for 1 hour with either PKC inhibitor caused a dose-dependent inhibition of IL-8 and IL-1β mRNA expression in bryo-1–stimulated monocytes, with an IC50 of staurosporine and UCN-01 lower than 50 and 4 nmol/L, respectively. Cell viability was not affected by treatment with either PKC inhibitor, as determined by trypan blue dye exclusion test, and a similar profile of suppression was observed in two additional experiments. These results indicate a role for PKC in monocyte activation by bryo-1.
Dose-dependent downregulation of bryo-1–induced IL-8 and IL-1β mRNA by PKC inhibitors. Monocytes were preincubated with the indicated concentrations of staurosporine or UCN-01 for 1 hour and then stimulated with bryo-1 (0.1 ng/mL) for 3 hours. Northern blot analysis was performed as previously described, and the same filter was sequentially hybridized with IL-8 (upper panel), IL-1β (middle panel), and GAPDH (lower panel) probes.
Dose-dependent downregulation of bryo-1–induced IL-8 and IL-1β mRNA by PKC inhibitors. Monocytes were preincubated with the indicated concentrations of staurosporine or UCN-01 for 1 hour and then stimulated with bryo-1 (0.1 ng/mL) for 3 hours. Northern blot analysis was performed as previously described, and the same filter was sequentially hybridized with IL-8 (upper panel), IL-1β (middle panel), and GAPDH (lower panel) probes.
DISCUSSION
The PKC modulator bryo-1 has received considerable attention in the past few years as an antineoplastic agent.10-17,19 In addition to its antitumor activity, bryo-1 has been found to possess powerful immunomodulatory properties and to function as a biological response modifier in vivo by potentiating host immune mechanisms.4,14,20-25 51 However, there is currently little information regarding the effects of bryo-1 on monocyte/macrophage functions. The data presented here demonstrate for the first time that bryo-1 can potently stimulate human monocytes and induce the production of the proinflammatory cytokines IL-1β, TNF-α, IL-6, and IL-8. Moreover, we provide evidence of an interplay between the activity of IL-2 and bryo-1, which trigger increased response in a synergistic fashion.
Bryo-1 can exert pleiotropic effects on several types of immune cells.2 However, the range of effective concentrations varies depending on the cell lineage. Subnanomolar concentrations of bryo-1 are sufficient to maximally stimulate erythroid and granulocyte-macrophage colony formation,51 whereas higher amounts are required for the activation of B- and T-lymphocyte proliferation,14,22,24,25 as well as for the induction of histamine secretion from human basophils.20 Here we show that subnanomolar doses of bryo-1 are required to induce IL-1β, TNF-α, IL-6, and IL-8 mRNA and, accordingly, to activate cytokine secretion from monocytes. As little as 0.001 ng/mL of bryo-1 was sufficient to increase cytokine production above the basal levels and maximal induction was obtained with 0.1 ng/mL. Our results confirm and extend previous findings by Steube and Drexler,52 who have recently reported that bryo-1 induces TNF-α secretion from the human macrophage cell line MonoMac 6. However, concentrations of bryo-1 100- to 1,000-fold higher than those active on fresh monocytes were used in that study. The discrepancy between these results may reflect differences in the responsiveness of peripheral blood monocytes and macrophages to the drug. Consistent with this possibility are recent findings from our laboratory demonstrating that bryo-1 acts as a costimulus with IFN-γ for the production of NO2− and the induction of iNOS gene expression in a murine macrophage cell line, and that maximal effects are exerted at concentrations of 1 to 10 ng/mL (Taylor et al, manuscript submitted). It is noteworthy that the range of concentrations of bryo-1 effective on monocytes in vitro was comparable to that detectable in vivo in the plasma of cancer patients following systemic administration of tolerated doses of the drug.53
Induction of IL-1β, IL-8, and TNF-α mRNA by bryo-1 occurred very rapidly, being detectable within 3 hours of stimulation, suggesting a direct response to bryo-1. IL-6 mRNA expression was observed 6 hours after monocyte incubation with bryo-1, raising the possibility that its induction was in part caused by IL-1β autocrine stimulation.54 This possibility is currently under investigation. Bryo-1 can induce the production of IFNγ from activated lymphocytes.25 In our experimental system, we never detected IFNγ in the supernatants of bryo-1–treated monocytes (data not shown). These data argue against the possibility that the stimulatory effects of bryo-1 on monocyte secretory functions may be due to small amounts of IFN-γ released in the medium by potential contaminating lymphocytes. This conclusion is further supported by the demonstration that IFNγ does not stimulate, but rather inhibits, IL-8 and IL-6 production.44 46 Finally, we can rule out the possibility that endotoxin (lipopolysaccharide [LPS]) contamination might have contributed to monocyte activation, because bryo-1 preparations did not contain any detectable levels of LPS, as judged by the Limulus amebocyte lysate assay, and similar stimulatory effects were elicited by clinical grade preparations of bryo-1 (data not shown). Collectively, these observations indicate that bryo-1 directly stimulates monocyte secretory functions. Further studies would be required to elucidate the molecular mechanisms controlling the expression of the genes for the different cytokines.
Another interesting finding is the synergistic effect of bryo-1 on IL-2-induced monocyte activation. We have previously reported that IL-2 is a potent inducer of monokine mRNA expression and protein secretion by human monocytes.38,45 Here we show that bryo-1 significantly potentiated the ability of IL-2 to stimulate monocyte secretory functions, even when used at suboptimal concentrations. This effect was mainly exerted at the level of gene expression and was selective for IL-2 because bryo-1 did not affect the response of monocytes to IFNγ. Enhancement of IL-2–induced T lymphocyte proliferation by bryo-1 has been ascribed to the induction of IL-2Rα chain expression.14,22 However, this mechanism cannot account for our results because monocytes did not express IL-2Rα chain on stimulation with bryo-1. On the other hand, IL-2Rγ upregulation might be responsible for, or at least contribute to, the synergistic action of bryo-1. Previous work from our laboratory has shown that IL-2Rβ and IL-2Rγ chains are constitutively expressed on human monocytes and are required for the formation of functional IL-2Rs,45,47-49 and that the expression of IL-2Rγ is the limiting factor regulating IL-2 binding to monocytes.48 Thus, bryo-1, by increasing IL-2Rγ chain expression, could augment the number of functional IL-2Rs on monocytes, allowing a more efficient cell response to IL-2. However, we cannot rule out the possibility that postreceptor binding events may be involved in the early phase of the IL-2 and bryo-1 synergistic effect. Further studies would be required to address this issue. The fact that bryo-1 can synergize with IL-2 in triggering monocyte activation makes this compound an interesting candidate for clinical trials in combination with IL-2.
Bryo-1 is known to be a potent ligand for PKC, and a large number of studies suggest that most of its biological effects are mediated through the activation of PKC.3-5,20,22,24 However, bryo-1 can also antagonize a number of PKC-mediated effects,7-9 and a recent report by Szallasi et al55 demonstrated that bryo-1 antitumor activity in the B16/F10 mouse melanoma model was independent of PKC. Monocyte response to bryo-1 was dependent, at least in part, on PKC activation, as suggested by the finding that induction of cytokine expression by bryo-1 was markedly inhibited by staurosporine and UCN-01 at concentrations that are highly specific for inhibition of PKC activity.50 No effect on cell viability was observed on treatment with either drug, as assessed by trypan blue dye exclusion test, demonstrating that the decrease in mRNA levels detectable in bryo-1-stimulated monocytes could not be accounted for by toxicity of the treatment.
In conclusion, we have provided the first evidence that bryo-1 is capable of stimulating human monocytes to generate IL-1β, TNF-α, IL-6, and IL-8, adding a new function to the spectrum of immunomodulatory activities of this compound. The central role of mononuclear phagocyte-derived cytokines in the regulation of host immune responses, inflammatory processes, and hematopoiesis is well established.26,29 Moreover, these inflammatory mediators are involved in controlling the expression of monocyte/macrophage antitumor activity either by exerting a direct cytostatic or cytotoxic effect on tumor cells31,33 or indirectly through the activation of an immune response.30,34-36 Philip et al16 have recently reported that increased plasma levels of TNF-α and IL-6 were detectable in cancer patients undergoing phase I clinical trials with bryo-1.
Moreover, bryo-1–dependent inhibition of the proliferation of chronic myelomonocytic leukemia cells in culture has been found to be associated with an increase in TNF-α production, and to be reversed by neutralizing MoAbs to TNF-α,13 suggesting that bryo-1–antitumor effects in this system were in part due to TNF-α. Interestingly, we found that the production of NO−2 a major mediator of macrophage tumoricidal activity, from murine macrophages in response to bryo-1 was also partially mediated by TNF-α secretion (Taylor et al, manuscript submitted). Therefore, it is possible that stimulation of monokine secretion by bryo-1 may represent at least one of the mechanisms responsible for the in vivo antitumor activity of the drug.
In addition to immunomodulatory and antitumor properties, inflammatory cytokines, such as IL-1β and IL-6, have well-documented growth stimulatory and radioprotective capacity on hematopoietic progenitors51.29,32 Bryo-1 has been reported to stimulate the proliferation and differentiation of normal myeloid and erythroid progenitor cells both in vitro and in vivo,51,56 to exert radioprotective effects in lethally irradiated mice,57 and to potentiate the radioprotective capacity of granulocyte macrophage colony-stimulating factor (GM-CSF ) toward human hematopoietic precursors.57 It is noteworthy that the range of concentrations of bryo-1 active on hematopoietic progenitors51 is comparable to those which are effective in stimulating monocyte secretory functions, raising the possibility that the release of inflammatory mediators by monocytes might contribute to the hematopoietic effects of bryo-1.
Finally, the finding that bryo-1 upregulates IL-2Rγ chain expression on monocytes indicates that this compound may affect monocyte responsiveness to various cytokines other than IL-2, as IL-2Rγ is a functional component of the receptors for IL-4, IL-7, IL-9, and IL-15.58 Studies are currently in progress to determine whether bryo-1 can modulate the response of monocytes to these cytokines.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Address reprint requests to Igor Espinoza-Delgado, MD, Department of Medicine and Stanley S. Scott Cancer Center, Louisiana State University Medical Center, 1542 Tulane Ave, New Orleans, LA 70112.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal