Abstract
Interferon-γ (IFN-γ) is recommended as prophylaxis against infections in patients with chronic granulomatous disease (CGD). However, since the optimal dose, the dosing interval, and the mechanisms of action are not well-defined, we studied the effects on CGD neutrophil (PMN) functions ex vivo of interferon-γ (IFN-γ). Evaluations were made on oxidative capacity, measured by superoxide anion production and chemiluminescence after stimulation with f-met-leu-phe (f-MLP) or phorbol-myristate-acetate, the killing of Aspergillus fumigatus hyphae (assessed as conversion of the tetrazolium salt MTT to formazan), and on the expression of FcγRI receptor (CD64). After randomization, 9 CGD patients (4 with gp91phox, 3 with p47phox, 1 with p67phox deficiency and 1 with unspecified CGD) were given IFN-γ, either 50 or 100 μg/m2 subcutaneously on 2 consecutive days after double blinded randomization. Furthermore, one female hyperlyonized X-linked carrier with a CGD phenotype was also studied separately after IFN-γ treatment. Evaluations were made the day before and on days 1, 3, 8, and 18 after IFN-γ administration. The killing of A fumigatus hyphae, being close to zero before IFN-γ, was enhanced on day 3, being 36% higher than pretreatment values in the high-dose CGD group and 17% in the low-dose group. The expression of FcγRI on PMN increased 3.7-fold in the high-dose and 2.3-fold in the low-dose CGD group, being maximal on day 1. Oxidative functions were raised in only selected patients represented by different subtypes of CGD. The hyperlyonized carrier of X-linked CGD responded to IFN-γ with more enhanced oxidative responses and Aspergillus killing of her PMNs than the other patients. This study suggests that a higher dose of IFN-γ than currently recommended confers transient enhancements of certain PMN functions in CGD patients.
CHRONIC granulomatous disease (CGD) is a rare X-linked or autosomal recessive (AR) disease, characterized by recurrent life threatening infections affecting the skin, lung, lymph glands, liver, bones, and intestinal tract.1 Typical infectious agents are catalase-positive micro-organisms, eg, Staphylococcus aureus and Gram-negative species, for instance Escherichia coli and Pseudomonas cepacia.2 Fungal infections, and especially Aspergillus fumigatus, also poses a threat.1 The basic defect is an inability of phagocytic cells, mainly polymorphonuclear leukocytes (PMN), to produce superoxide anions and hydrogen peroxide, as a result of a lack of, or a malfunction in one of the four discrete proteins that constitute the major part of the NADPH-oxidase in these cells, the membrane bound gp91phox and p22phox and the two cytosolic factors, p47phox and p67phox.3
Based on the results of a multicenter study, recombinant human interferon-γ (r-hu-IFN-γ), administered subcutaneously (s.c.) three times a week, has been recommended as infection prophylaxis in CGD since 1991.4 IFN-γ reduced the number of serious infections and the number of days for hospitalization was lower in the IFN-γ group. Furthermore, it was noted that the number of Aspergillus infections was lower in the IFN-γ group of this study. A follow-up study concluded that this treatment is safe and the rate of infections does not change over time with treatment.5 Some case reports also point out IFN-γ as treatment in conjunction with antibiotics for active infection,6-9 but no controlled study on this issue has been published.
The mechanisms of action of IFN-γ in CGD are, however, poorly understood. Although some early reports on “variant” forms of CGD (defined as CGD with some rest activity in all their phagocytes) showed an increase of the oxidative capacity in these CGD cells after IFN-γ treatment,10-12 studies of more common CGD phenotypes did not reveal a significant increase of this function.4,5,13,14 Also, the optimal dose and the dosing interval has not been well-defined. The dose chosen for the multicenter study, 50 μg/m2 given three times a week, was based on studies of other disease.15
The aim of our study was to assess the duration and responses of two different IFN-γ doses to CGD patients on a number of ex vivo PMN functional responses. These included an aspergillicidal assay, superoxide production, chemiluminescence (CL), and the expression of FcγRI (CD64). The aspergillicidal assay was chosen because it is related to a difficult clinical problem1,3,8 and a previously recognized effect of IFN-γ administration.16 The superoxide and CL assays were chosen because previous results on oxidative capacity have been contradictory,4,5,10-14 and the expression of CD64 was chosen because it might characterize one explanation to the effect of IFN-γ in CGD.17-19
MATERIALS AND METHODS
Study design.After informed consent was obtained, patients were randomized to receive either 50 or 100 μg/m2 of IFN-γ (Imukin; Boehringer-Ingelheim) on 2 consecutive days, assuming that this regimen would induce a rapid and transient increase of the cell functions studied and yet have tolerable side effects. IFN-γ was administered subcutaneously by a nurse in a double blinded manner after preparation of the drug by a pharmacist. Venous blood samples were obtained previous to IFN-γ and on days 1, 3, 8, and 18 after the last dose, at 8 to 9 A.M. Normal controls (healthy members of the laboratory staff ), were consistently run for comparison; however, they did not receive IFN-γ. The randomization code was broken after all results were registered. Different subgroups of CGD were encountered in the study group to determine whether there were any differences in response of the PMNs. A protocol including adverse events was filled in for each visit.
The study was approved by the local ethical committee and the Swedish Board for Pharmaceutical Trials.
Patients.The nine CGD patients selected for this study (see Table 1) suffered from recurrent infections since childhood. They were free from infections at the time of receiving IFN-γ and at blood sampling for the present investigation. They had no history of previous use of IFN-γ. Their neutrophils produced no (or minute amounts of ) superoxide anions in response to the phorbol ester phorbol-myristate-acetate (PMA) and had chemiluminescence (CL) reactions of <1% compared with controls. The nitroblue tetrazolium reduction test (NBT) was negative for the 9 CGD patients. A female hyperlyonized carrier of X-linked CGD (CE) was also studied. She had 4% NBT+ cells and responded to the tripeptide N-formyl-Met-Leu-Phe (fMLP) with a minute burst of CL, ∼8% of controls. This carrier is expressing a CGD phenotype (explained by “extreme Lyonization” or unfavourable X-inactivation), and has suffered from repeated lymphadenitis, skin abscesses, Aspergillus pneumonia, and also an inflammatory bowel disease mimicking Crohn's disease of the colon.20
CGD Patients Receiving IFN-γ, Subgroups, Oxidative Function Previous to IFN-γ, and Randomization Dose. The Patient CE Is a Hyperlyonized Carrier of X-Linked CGD Yet Expressing a CGD Phenotype
| Patient/Age/Gender . | CGD Type . | fMLP-Induced CL . | NBT % Pos Cells . | Randomization Dose . |
|---|---|---|---|---|
| AA | A470 | <1% | 0 | High |
| 26/F | ||||
| MA 33/M | A470 | <1% | 0 | High |
| EF | A?0, not specified | <1% | 0 | High |
| 6/F | ||||
| HA | X910 | <1% | 0 | High |
| 17/M | ||||
| CE | X91+ | Minute ∼8% | 4 | High |
| 43/F | ||||
| Hyperlyonized female carrier | ||||
| LG | A670 | <1% | 0 | Low |
| 16/F | ||||
| TW | X910 | <1% | 0 | Low |
| 9/M | ||||
| AW | X910 | <1% | 0 | Low |
| 17/M | ||||
| PA | A470 | <1% | 0 | Low |
| 22/M | ||||
| PE | X910 | <1% | 0 | Low |
| 7/M |
| Patient/Age/Gender . | CGD Type . | fMLP-Induced CL . | NBT % Pos Cells . | Randomization Dose . |
|---|---|---|---|---|
| AA | A470 | <1% | 0 | High |
| 26/F | ||||
| MA 33/M | A470 | <1% | 0 | High |
| EF | A?0, not specified | <1% | 0 | High |
| 6/F | ||||
| HA | X910 | <1% | 0 | High |
| 17/M | ||||
| CE | X91+ | Minute ∼8% | 4 | High |
| 43/F | ||||
| Hyperlyonized female carrier | ||||
| LG | A670 | <1% | 0 | Low |
| 16/F | ||||
| TW | X910 | <1% | 0 | Low |
| 9/M | ||||
| AW | X910 | <1% | 0 | Low |
| 17/M | ||||
| PA | A470 | <1% | 0 | Low |
| 22/M | ||||
| PE | X910 | <1% | 0 | Low |
| 7/M |
Abbreviations: F, female; M, male; X indicates X-linked and A is autosomal recessive inheritance; 91, 47, and 67 indicate the defective “phox” protein; superscript symbols indicate 0, undetectable “phox” protein; +, normal expression of “phox” protein on Western blot (in this case only the expression of her defect gene).
The patients could be divided into these discrete subgroups according to immunoblotting and/or genetic sequence analysis of the CYBB gene and family pedigrees (see Table 1)20: four had X-linked CGD with defect gp91phox, and five had autosomal recessive (AR) CGD. Of the patients with AR disease, three had a p47phox deficiency and 1 had a p67phox deficiency. One girl (EF ) showed all the bands of the oxidase subcomponents on the Western blot assay and had presumed AR inheritance because oxidative functions of her parents' PMN and family pedigree suggested this. The female carrier of X-linked CGD (CE) also showed all the bands on the immunoblot, but was previously genetically defined by sequence analysis as having a missense mutation caused by a T-1117 → G substitution in one allele of the CYBB gene, leading to a Cys-369 → Gly substitution.20 Even though she has a CGD phenotype, she is excluded from the calculations in this study because her neutrophils comprise 4% normal (NBT+) cells.
Materials and chemicals.Hanks' balanced salt solution (HBSS) and RPMI 1640 with L-glutamine were obtained from GIBCO (Paisley, Scotland, UK). fMLP, PMA, luminol (dissolved in 0.2 mol/L NaOH supplemented with Trizma buffer 6 mg/mL and further diluted to pH 7.4 with HBSS), nitroblue tetrazolium (NBT), ferricytochrome C (type III, bovine heart) and the tetrazolium salt (3[4,5-dimethylthiazol-2-yl]-2,5-diphenyl-tetrazolium bromide (MTT) were from Sigma Chemical Co (St Louis, MO). Percoll, superoxide dismutase (SOD), and human serum albumin (HSA) were from Pharmacia Fine Chemicals (Uppsala, Sweden). Monoclonal mouse antibody against the gp91phox and p22phox and polyclonal goat antibodies against the p47phox and the p67phox protein were kind gifts from Dr D Roos and coworkers (Amsterdam, The Netherlands). The fluorescein isothiocyanate-labeled mouse, monoclonal anti-CD64 (anti FcγRI) was obtained from Medarex (West Lebanon, NH). The Aspergillus fumigatus was an ATCC strain, #13073 (Rockville, MD). As opsonin served pooled AB serum. Yeast nitrogen base was from Difco Laboratories (Detroit, MI). Recombinant human IFN-γ (rH-IFN-γ, Imukin) 0.2 mg/mL was kindly supplied by Boehringer-Ingelheim AB (Stockholm, Sweden).
Neutrophils were obtained by a one-step discontinuous Percoll gradient centrifugation.21 The neutrophils were washed twice before lysis of contaminating erythrocytes with ice cold ammonium chloride 0.155 mol/L. The purified neutrophils (>95% neutrophils with >95% viability) were resuspended in HBSS.
CL augmented by luminol and nitroblue tetrazolium reduction (NBT) test were assessed as previously described.21 22
Superoxide anion production was assessed essentially as described previously.21 In brief, 1.25 × 106 neutrophils/mL were suspended in HBSS and after an equilibration period of 5 minutes, PMA (1 μmol/L) was added. Samples with and without SOD were always assessed simultaneously in a spectrophotometer at 37°C. The samples were assessed for 60 minutes with 2 minute intervals and the amount of SOD-inhibitable cytochrome C reduction was used as a measure of superoxide anion formed. This prolonged assay period was used since we, and other investigators (Malech H.L., personal communication, September 1996) have observed different kinetics expressed as a later burst of oxidative activity (up to 60 minutes) in some of the CGD cells compared with controls. The production of superoxide anions is expressed as the rate nanomoles O2−/10 min/106 PMN. The intraindividual day by day variation for controls in the production of superoxide anion production was ±23% standard deviation (SD) as measured with this method.
Expression of FcγRI (CD64), flow cytometry was performed on a Becton Dickinson FacsScan (Mountain View, CA) as previously described,19 using the FITC-labeled anti-CD64 antibody. Results are expressed as mean fluorescence intensity units (MFI).
A fumigatus hyphae killing assay.We used a miniaturized version of the method previously described,16 allowing smaller quantities of blood to be drawn from the children. Briefly, 0.5 mL of Aspergillus conidia at 5 × 104/mL were incubated with yeast nitrogen base, supplemented with 1% glucose in 4-well plates at 30°C for 16 hours. Almost all hypae were germinated and were about 80 to 100 μm long at this time and the bottom of the well was covered with a lawn of hyphae. After aspiration of wells (hyphae are sticky and stay at the bottom of the well), 0.5 mL PMN (at 5 × 106 cells/mL with 20 mmol/L HEPES and 10% opsonin, pooled AB-serum) was added. PMN were gently centrifuged at 400 rpm to the bottom of the well and then incubated for 2 hours at 37°C, allowing them to kill or damage hyphae. Wells were then aspirated and phagocytes lysed with Triton, followed by three washes with sterile distilled water. To each well, 0.5 mL of RPMI 1640 with L-glutamine, containing MTT at 0.5 mg/mL was added and incubated for another 2 hours at 37°C. MTT is converted to formazan by live Aspergillus hyphae and therefore serves as an indirect indicator of the amount of live hyphae. Wells were once again aspirated dry and diluted with 200 μL of isopropanol and the amount of formazan dissolved was measured by spectrophotometry at 570 nm. Calculations were made as follows: ([abs of test wells] − [abs of blank wells])/([abs of Aspergillus wells only] − [abs of blank wells]). The intraindividual day by day variation for controls in the killing of Aspergillus hyphae is ±21% SD as measured with this method.
Statistical analysis.Student's t-test was used to compare statistical data of the different dose groups when appropriate.
RESULTS
Randomization.Four patients received the high dose, 100 μg/m2, one with X-linked CGD, two with p47phox deficiency, and one with unspecified autosomal CGD (Table 1). The remaining five patients received the low dose 50 μg/m2, three patients with X-linked disease, one with p47phox deficiency and one with p67phox deficiency. The female hyperlyonized carrier of X-linked CGD received the high dose.
Adverse effects.Two patients receiving the high dose reported headache, muscle pain, and fever of severe grade with a duration of 2 to 7 hours. Four patients receiving the low dose and one receiving the high dose reported headache and/or fever of minor/moderate grade with maximal duration of 2 hours. Two patients receiving a high dose and one receiving a low dose did not report any adverse reactions.
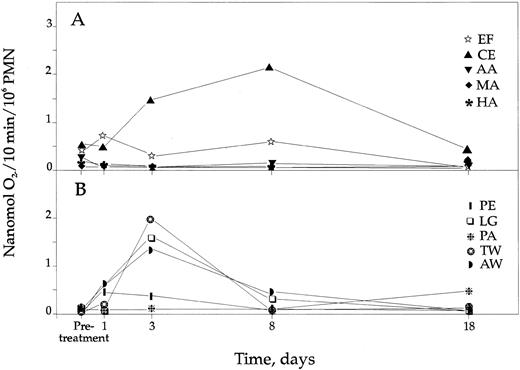
Superoxide anion production.Before IFN-γ, cells from 7 of the 9 patients had no or minute capacity to produce superoxide anions after PMN stimulation, whereas cells from two patients (AA, EF ) and the female hyperlyonized carrier of X-linked CGD (CE) produced ∼1% to 2% of controls. Control PMN responded with a maximal O2− production ∼20 minutes after stimulation, whereas some CGD PMN had a later response, up to ∼60 minutes. In the low-dose group, the response of PMNs from patient LG increased 18.7-fold on day 3 and 4.7-fold on day 8, reaching maximally 3.9% of controls (Fig 1B). Also, PMNs from two patients with X-linked disease who had almost no production of superoxide anions previous to IFN-γ (TW, AW), receiving the low dose, displayed an increased production with a maximum on day 3, being 3.6% and 5.2% of controls, respectively. Four patients in the high-dose group (AA, MA, EF, HA) and two in the low-dose group had no obvious rise. Consequently, three patients and the hyperlyonized carrier of X-linked CGD responded with increased O2− production in their PMNs after IFN-γ administration, but there was no dose dependent or any statistically proven effect of the responsiveness with this assay.
CGD PMN production of superoxide anions following IFN-γ treatment. (A) Production of superoxide anions in the 4 CGD patients and the female carrier of X-linked CGD (CE) receiving high-dose IFN-γ. (B) Production of superoxide anions in the 5 patients receiving low-dose IFN-γ. Controls not indicated but produced in mean 36.8 nmol O2−/10 min/106 PMN (±6.0 SD).
CGD PMN production of superoxide anions following IFN-γ treatment. (A) Production of superoxide anions in the 4 CGD patients and the female carrier of X-linked CGD (CE) receiving high-dose IFN-γ. (B) Production of superoxide anions in the 5 patients receiving low-dose IFN-γ. Controls not indicated but produced in mean 36.8 nmol O2−/10 min/106 PMN (±6.0 SD).
CL.The fMLP and PMA induced CL response before IFN-γ was <1% compared to controls except for cells from the female hyperlyonized carrier (CE), that exhibited ∼8% of CL response following fMLP stimulation (Table 1). When fMLP was used as the agonist, CL was raised in two patients of the high-dose group and in three patients in the low-dose group, on either day 1 and/or day 3, compared with pretreatment values. These responding patients had in mean a 3.4-fold increase of the fMLP induced CL response in the high-dose group and a 4.2-fold increase in the low-dose group to the peak value on day 1 or 3. The female hyperlyonized carrier doubled her CL response on day 3. In four of the patients (two in each dose group), there was no fMLP induced increase of the CL of the PMNs, thus, there was no statistical improvement of this function for any group. On days 8 and 18, CL values to fMLP were back to pretreatment levels in all the patients.
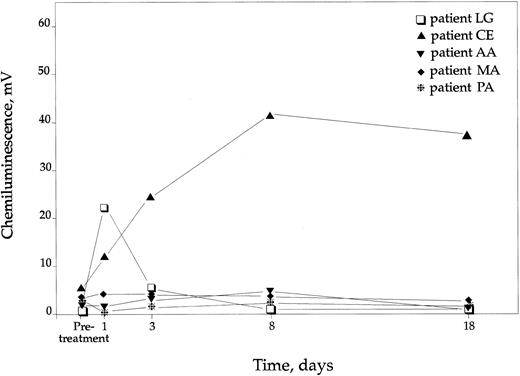
When PMA was used as the agonist, there was a pronounced increase in the CL response of only one of the nine patients, ie, the p67phox deficient patient LG, receiving the low dose (Fig 2). Cells from this patient showed a short-lived enhancement, lasting only on days 1 and 3. The female hyperlyonized carrier of X-linked CGD, receiving the high dose, showed an impressive prolonged improvement, lasting to day 18 (Fig 2). These two responding individuals also responded with increased superoxide anion production.
CL response in 5 CGD patients after PMA stimulation. The remaining patients had no rise in CL response. Controls not indicated but had in mean 570 mV (±65 SD).
CL response in 5 CGD patients after PMA stimulation. The remaining patients had no rise in CL response. Controls not indicated but had in mean 570 mV (±65 SD).
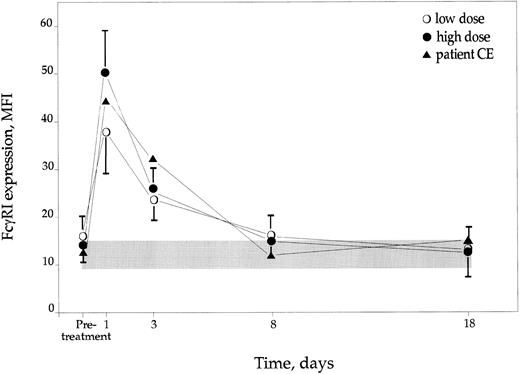
FcγRI (CD64) expression.The mean pretreatment values for CGD neutrophils were similar in the high- and the low-dose groups and did not differ from those of the controls (Fig 3). Following the administration of IFN-γ there was a clear and dose-dependent increase (P < .01) of FcγRI expression on CGD PMN, being maximal on day 1 after the two doses of IFN-γ. The low-dose group showed a 2.3-fold increase of the MFI values and the high-dose group a 3.7-fold rise (Fig 3). All patients at least doubled their expression of CD64 on day 1 after IFN-γ administration. On days 8 and 18 the expression of CD64 was back to pretreatment values. The female hyperlyonized carrier of X-linked CGD showed similar rise of the CD64 expression following IFN-γ treatment as the rest of the patients receiving high dose. Control PMN (not receiving IFN-γ) showed very consistent values over time, being similar to pretreatment CGD values.
Expression of FcγRI (CD64) on CGD PMN after IFN-γ treatment, expressed as MFI ± SD. Controls are represented by shaded area ± SD.
Expression of FcγRI (CD64) on CGD PMN after IFN-γ treatment, expressed as MFI ± SD. Controls are represented by shaded area ± SD.
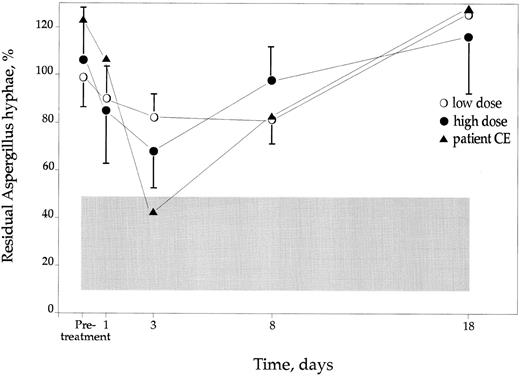
The A fumigatus hyphae killing assay.Before IFN-γ treatment the CGD PMN failed to kill or damage the hyphae totally (Fig 4), whereas controls killed or caused damage to in mean 71% (±22, mean and SD values) of the hyphae. On day 1, a slight increase in this capacity was noted in the two CGD dose groups. However, the improvement in aspergillicidal capacity of the PMNs was highest on day 3 in the high-dose group, being in mean 36% (±15) compared to pretreatment values. This increase was only 17% (±9) in the low-dose group (Fig 4) on the same day. All the patients responded with an improvement of this function after IFN-γ treatment, being at least 11% compared with pretreatment values. The female hyperlyonized carrier of X-linked CGD responded with an aspergillicidal capacity on day 3 that was similar to control cells (Fig 4). The beneficial effect of IFN-γ treatment returned to pretreatment values on day 18.
The Aspergillicidal capacity of CGD PMN after IFN-γ treatment. Values are expressed as residual % of Aspergillus hyphae compared with wells without PMN added ±SD. Controls are indicated as shaded area ±SD. In the text the effect of the IFN-γ is described as increase in % killing capacity. This is calculated as the percentage of pretreatment residual % hyphae − residual % for each separate day.
The Aspergillicidal capacity of CGD PMN after IFN-γ treatment. Values are expressed as residual % of Aspergillus hyphae compared with wells without PMN added ±SD. Controls are indicated as shaded area ±SD. In the text the effect of the IFN-γ is described as increase in % killing capacity. This is calculated as the percentage of pretreatment residual % hyphae − residual % for each separate day.
DISCUSSION
In this study we have evaluated the effects of two different doses of IFN-γ, ie, 50 and 100 μg/m2 given s.c. on 2 consecutive days, on a number of functional responses in CGD PMN to determine if host defense variables were affected and, if so, the duration of the effect. We have assessed factors that are directly dependent on the pathogenesis of CGD, ie, oxidative functions, measured as CL and superoxide anion production. We have also assessed one variable relating to a clinical problem for CGD patients, the aspergillicidal capacity. Furthermore, we followed the expression of FcγRI, the high affinity receptor for Ig on neutrophils, which is important for PMN phagocytosis.
Initial reports on oxidative functions on CGD patients after IFN-γ treatment suggested enhanced superoxide production, and increased gene expression for the oxidase components, ie, cytochrome b558 .10-12 Some of these studies were performed on a “variant” form of CGD (defined as CGD with some NADPH rest-activity in their PMNs), revealing, for some patients, a normalization of superoxide anion production.11 However, in the placebo-controlled multicenter study of IFN-γ to CGD patients, neither superoxide anion production nor bactericidal capacity was statistically improved in the IFN-γ group,4 but some patients with p47phox and p67phox deficiency responded with slight improvements in superoxide anion production. In our study, there was an increase of the oxidative capacity in some of the patients. Two of the patients with “classic” X-linked CGD displaying no oxidative capacity before IFN-γ treatment (TW, AW, being brothers), as well as one p67phox deficient patient (LG), improved their superoxide production. This might partly be due to the prolonged assay (60 minutes) used here, compared to a normal observation time of approximately 20 to 30 minutes. In addition, the female hyperlyonized carrier of X-linked CGD (CE) showed clearly increased oxidative functions, both measured as CL and superoxide production. Because she is a carrier with extreme Lyonization,20 this improvement may consist of an enhancement of the function of her 4% normal cells. However, in the early reports on the “variant” forms of CGD, these reports presented an increase of the superoxide anion production equivalent to controls.11 12 This suggests that some patients, regardless of CGD type, can improve the production of superoxide anions temporarily following IFN-γ treatment. Three p47phox patients, as well as 2 “classic” X-linked and one patient with unidentified AR CGD had no significant rise in this function.
FcγRI (CD64) is the high affinity receptor for IgG on neutrophils and monocytes and serves as a receptor for Ig opsonins on neutrophils. It has previously been claimed to be a marker for biological activity of IFN-γ.17-19 However, no studies had previously been published on the effect of various IFN-γ doses in CGD PMN. The present study suggests a dose-dependency for this function. Although the FcγRI receptor is not involved directly in the defect of CGD, its enhanced expression might serve as one explanation for the beneficial effect of IFN-γ in this setting.
The ex vivo effect on PMN aspergillicidal capacity of CGD patients after administration of IFN-γ has previously been described using the 50 μg/m2 three times weekly dose regimen.16 Our study on the short-term effect of IFN-γ shows similar results, and furthermore, suggests a dose-dependent effect, with a maximal effect on day 3 after the higher dose. However, our group of patients was too small to reach statistical significance on the results of this assay. This effect may be related to a clinical issue because A fumigatus infections have been reported to be one of the major causes of death for CGD patients.1 Some case reports have also pointed out that IFN-γ administration has a clinical effect on A fumigatus and Candida albicans infections.6 8
This study suggests that two different doses of IFN-γ induce dose-dependent responses of certain PMN functions in CGD patients. The effect on the aspergillicidal capacity was impressive, coming close to control values after treatment in some of the patients, whereas the effect on the oxidative capacity was modest and only noted in selected patients.
The duration of the different PMN responses of IFN-γ seems to be the same for the different doses used in this study. The aspergillicidal capacity as well as the superoxide production were maximal on day 3 (although two patients had a peak on the superoxide function on day 8). FcγRI receptor expression was highest on day 1, but was still enhanced on day 3. These data support the currently recommended dosing schedule, ie, three times per week.
IFN-γ has a multitude of effects on the immune system.23 Other functions than oxidative capacity could also be responsible for the beneficial effect of this drug in CGD patients. The expression of p47phox protein has also been suggested as a marker for IFN-γ effect.18,24 Investigations on CGD PMN levels of antimicrobial proteins following IFN-γ administration noted no improvement.13 Thus, the exact mechanism of the reduction of infections in CGD patients after IFN-γ treatment remains unknown.
We conclude that IFN-γ confers dose dependent and transient enhancements of certain PMN functions in CGD patients. Studies to use IFN-γ as treatment of infections in CGD patients are warranted and might include different doses of IFN-γ, especially for Aspergillus infections. Also, additional future studies to use prophylactic IFN-γ treatment in CGD patients may also include different doses.
Supported by grants from the Swedish Children's Cancer Association, the Swedish Medical Research Council (19P-9851, 19X-5991), Stiftelsen Samariten, the Funds of Karolinska Institute, Stockholm Söder Hospital and a grant from Boehringer Ingelheim AB, Stockholm, Sweden.
Address reprint requests to Anders Åhlin, MD, The Department of Pediatrics, Sachs' Children's Hospital, Box 17912, S-118 95 Stockholm, Sweden.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal