Abstract
To determine the role of tumor necrosis factor (TNF ) in lipopolysaccharide (LPS)-induced inflammation, 12 healthy subjects received an intravenous injection with LPS (2 ng/kg) preceded by infusion of either a recombinant human dimeric TNF receptor type II-IgG fusion protein (TNFR:Fc; 6 mg/m2; n = 6) or vehicle (n = 6) from −30 minutes to directly before LPS injection. LPS elicited a transient increase in plasma TNF activity, peaking after 1.5 hours (219 ± 42 pg/mL; P < .05). Infusion of TNFR:Fc completely neutralized endogenous TNF activity. LPS administration was associated with an early activation of fibrinolysis (plasma concentrations of tissue-type plasminogen activator, plasminogen activator activity, and plasmin-α2 -antiplasmin complexes), followed by inhibition (plasma plasminogen activator inhibitor type I), changes that were completely prevented by TNFR:Fc. By contrast, TNFR:Fc did not influence LPS-induced activation of coagulation (plasma levels of prothrombin fragment F1 + 2 and thrombin-antithrombin III complexes). TNFR:Fc strongly inhibited endothelial cell activation (plasma levels of soluble E-selectin), modestly reduced neutrophil responses (neutrophilia and plasma concentrations of elastase-α1 -antitrypsin complexes and lactoferrin), but did not affect the release of secretory phospholipase A2 or lipopolysaccharide-binding protein (P < .05). Infusion of TNFR:Fc only (without LPS) in another 6 normal subjects did not induce any inflammatory response. These data indicate that TNF is involved in only some inflammatory responses to intravenous LPS in humans.
TUMOR NECROSIS FACTOR α (TNF ) is a pluripotent proinflammatory cytokine that plays an important role in the pathogenesis of sepsis.1 Intravenous infusion of bacteria or endotoxin is associated with early and transient release of TNF into the circulation.2-4 Because administration of recombinant TNF can reproduce the major features of sepsis,5 this cytokine has been implicated as a mediator of systemic inflammation associated with bacteremia and endotoxemia. In accord, neutralization of endogenous TNF activity in models of systemic infection confers a strong protection against lethality.6 7
Recent investigations focusing on the role of TNF in specific inflammatory responses have indicated that endogenous TNF is involved only partly in the activation of host mediator systems known to contribute to tissue injury during sepsis. Although injection of TNF into humans or baboons resulted in activation of both coagulation and fibrinolysis,8-10 treatment with a neutralizing anti-TNF monoclonal antibody (MoAb) of endotoxemic chimpanzees only inhibited activation of the fibrinolytic system,11,12 while not influencing activation of the coagulation system.11 Furthermore, despite its potent capacity to induce degranulation of neutrophilic granulocytes in vivo,13 anti-TNF only modestly attenuated this response during primate endotoxemia.11 14
Knowledge of the role of endogenous TNF in endotoxin-induced inflammation in humans is limited. Recently, infusion of a recombinant dimeric TNF receptor type II (TNFR:Fc), administered at 10 or 60 mg/m2, was reported to neutralize TNF activity and to inhibit cytokine, chemokine, and stress hormone release after injection of endotoxin into normal humans.15 A remarkable finding of that study was, that, although both TNFR:Fc doses provided a large excess of TNF neutralizing capacity, the lower dose exerted the most potent antiinflammatory effects.15 In the present study, we sought to determine the antiinflammatory effects of an even lower dose of TNFR:Fc (6 mg/m2) on activation of coagulation, endothelial cells, and leukocytes during endotoxemia in normal humans.
MATERIALS AND METHODS
Study design and subjects.The present study was performed simultaneously with an investigation on the effects of TNFR:Fc on endotoxin-induced changes in the surface expression of TNF and interleukin-1 (IL-1) receptors, the results of which are reported elsewhere.16 Eighteen healthy male subjects, 28 ± 2 (mean ± SE) years of age, were admitted to the Adult Clinical Research Center of the New York Hospital-Cornell University Medical Center (New York, NY) after documentation of good health by history, physical examination, and hematologic and biochemical screening. The study was approved by the Institutional Review Board, and written informed consent was obtained from all subjects before enrollment in the study. Twelve subjects received an intravenous injection with lipopolysaccharide (LPS; National Reference Endotoxin, Escherichia coli 0113 (lot EC-5); generously provided by Dr H.D. Hochstein, The Bureau of Biologics, Food and Drug Administration, Bethesda, MD) at a dose of 2 ng/kg body weight, at 9:00 am. These 12 subjects were randomized to also receive 30 minutes of intravenous infusion (starting at 8:30 am) with either a recombinant dimeric TNF receptor (TNFR:Fc; Immunex Corp, Seattle, WA) at a dose of 6 mg/m2 (n = 6) or vehicle (n = 6). The remaining 6 subjects received 30 minutes of intravenous infusion of TNFR:Fc (6 mg/m2) only (ie, without LPS). TNFR:Fc was manufactured by fusing two identical extracellular portions of the type II TNF receptor with the Fc domain of IgG1.17 The resulting dimeric TNF receptor binds TNF with a 50-fold greater affinity (Ki = 1010 mol/L−1) than monomeric receptor.17 TNFR:Fc was reconstituted with 1 mL of sterile water for injection from a lyophilized powder containing 10 mg of TNFR:Fc, 1.2 mg Tris (trimethamine), 10 mg sucrose, and 40 mg mannitol. The final dose of TNFR:Fc (or vehicle) was diluted in 100 mL of isotonic saline before infusion. Two hours before the administration of LPS (or saline), a radial arterial catheter was placed in all subjects to continuously monitor heart rate and blood pressure (Datascope model 2000A; Datascope Corp, Paramus, NJ) and for blood sampling.
Effects of TNFR:Fc in Normal Subjects
| Time . | TAT Complexes . | PAP Complexes . | Soluble E-Selectin . | Secretory PLA2 . | Neutrophils . | Elastase-α1-Antitrypsin . | IL-6 (pg/mL) . |
|---|---|---|---|---|---|---|---|
| (hr) . | (ng/mL) . | (nmol/L) . | (ng/mL) . | (ng/mL) . | (×109/L) . | Complexes (ng/mL) . | . |
| Pre | 6.4 ± 0.9 | 4.1 ± 0.3 | 13.3 ± 3.1 | 2.38 ± 0.54 | 1.91 ± 0.65 | <40 | <4 |
| 2 | 5.9 ± 0.8 | 4.6 ± 0.9 | 13.8 ± 2.8 | 1.97 ± 0.45 | 2.14 ± 0.39 | <40 | <4 |
| 4 | 6.4 ± 1.9 | 5.6 ± 1.4 | 13.2 ± 2.5 | 1.82 ± 0.39 | 3.25 ± 0.62 | <40 | <4 |
| 6 | 7.3 ± 2.4 | 5.5 ± 0.6 | 13.5 ± 2.7 | 1.73 ± 0.33 | 3.31 ± 0.45 | <40 | <4 |
| 12 | 2.4 ± 0.2 | 6.8 ± 0.9 | 13.7 ± 2.8 | 1.60 ± 0.32 | 2.78 ± 0.24 | <40 | <4 |
| 24 | 2.0 ± 0.2 | 4.4 ± 0.7 | 12.6 ± 2.5 | 1.83 ± 0.27 | 2.16 ± 0.30 | <40 | <4 |
| Time . | TAT Complexes . | PAP Complexes . | Soluble E-Selectin . | Secretory PLA2 . | Neutrophils . | Elastase-α1-Antitrypsin . | IL-6 (pg/mL) . |
|---|---|---|---|---|---|---|---|
| (hr) . | (ng/mL) . | (nmol/L) . | (ng/mL) . | (ng/mL) . | (×109/L) . | Complexes (ng/mL) . | . |
| Pre | 6.4 ± 0.9 | 4.1 ± 0.3 | 13.3 ± 3.1 | 2.38 ± 0.54 | 1.91 ± 0.65 | <40 | <4 |
| 2 | 5.9 ± 0.8 | 4.6 ± 0.9 | 13.8 ± 2.8 | 1.97 ± 0.45 | 2.14 ± 0.39 | <40 | <4 |
| 4 | 6.4 ± 1.9 | 5.6 ± 1.4 | 13.2 ± 2.5 | 1.82 ± 0.39 | 3.25 ± 0.62 | <40 | <4 |
| 6 | 7.3 ± 2.4 | 5.5 ± 0.6 | 13.5 ± 2.7 | 1.73 ± 0.33 | 3.31 ± 0.45 | <40 | <4 |
| 12 | 2.4 ± 0.2 | 6.8 ± 0.9 | 13.7 ± 2.8 | 1.60 ± 0.32 | 2.78 ± 0.24 | <40 | <4 |
| 24 | 2.0 ± 0.2 | 4.4 ± 0.7 | 12.6 ± 2.5 | 1.83 ± 0.27 | 2.16 ± 0.30 | <40 | <4 |
TNFR:Fc (6 mg/m2) was infused for 30 minutes. Data are the mean ± SE of 6 normal subjects obtained before the start of the infusion (Pre) and at various time points after the end of the infusion.
Sampling and assays.Arterial blood was obtained at 8:30 am (ie, directly before the start of the infusion with TNFR:Fc or vehicle, t = −0.5 hours), directly before the injection of LPS or saline (t = 0 hours), and 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 12, and 24 hours thereafter. All blood samples (except samples for determination of leukocyte counts) were centrifuged at 4°C for 20 minutes at 1,600g and stored at −70°C until assayed. Assays of coagulation and fibrinolysis were performed in citrated plasma; all other assays were performed in EDTA anticoagulated plasma. Plasma TNF activity was determined using the WEHI 164 clone 13 fibroblast cytotoxicity assay,18 exactly as described previously.19 Coagulation activation was determined by measuring the prothrombin fragment F1 + 2 and thrombin-antithrombin III (TAT) complexes (enzyme-linked immunosorbent assays [ELISAs]; Behringwerke AG, Marburg, Germany). Fibrinolytic activation was monitored by measurements of tissue-type plasminogen activator (tPA; ELISA; Innogenetics, Nijmegen, The Netherlands), plasminogen activator (PA) activity (by amidolytic assay as described),20 plasminogen activator inhibitor type I (PAI-1; ELISA; Innogenetics), and plasmin-α2 -antiplasmin (PAP) complexes (radioimmunoassay [RIA]).21 Plasma concentrations of soluble E-selectin (ELISA), IL-6 (ELISA; CLB, Amsterdam, The Netherlands), IL-8 (ELISA; CLB), and IL-10 (ELISA; Pharmingen, San Diego, CA) were measured as described.22-25 sPLA2 was measured by ELISA using a monoclonal antihuman PLA2 antibody (185.1) for coating, a rabbit polyclonal antihuman anti-PLA2 antibody, a goat antirabbit horseradish peroxidase-labeled antibody for detection, and recombinant human PLA2 as standard. Leukocyte counts were determined by flow cytometric light scatter analysis. Plasma levels of elastase-α1 -antitrypsin complexes and lactoferrin were assayed with RIAs.26 Plasma lipopolysaccharide-binding protein (LBP) was determined by ELISA.27
Statistical analysis.All values are given as means ± SEM. Changes within treatment groups were analyzed by one-way analysis of variance. Changes between treatment groups were analyzed by two-way analysis of variance (interaction treatment and time). P < .05 was considered to represent a statistically significant difference.
RESULTS
Effects of TNFR:Fc only.Infusion of TNFR:Fc only (without LPS) was not associated with any adverse effects. In addition, no significant changes were registered in the inflammatory responses under investigation (Table 1).
Plasma TNF activity and clinical responses.Details of the in vivo neutralizing capacity of TNFR:Fc at the dose administered are reported elsewhere.16 Infusion of TNFR:Fc effectively neutralized LPS-induced TNF activity. In subjects injected with LPS only, plasma TNF activity peaked after 1.5 hours (219 ± 42 pg/mL; P < .05 v time), whereas in subjects infused with LPS and TNFR:Fc, TNF activity remained undetectable (P < .05 v LPS only). Furthermore, the addition of 10 ng/mL recombinant TNF to plasma samples obtained from TNFR:Fc-treated subjects at 1.5 hours or 24 hours after injection of LPS did not result in detectable TNF activity, indicating that the amount of TNFR:Fc administered offered an excess of TNF neutralizing capacity. LPS induced a febrile response, which was significantly blunted by TNFR:Fc16; peak temperatures were 38.2°C ± 0.1°C (LPS only) and 37.7°C ± 0.1°C (LPS with TNFR:Fc; P < .05). TNFR:Fc did not significantly influence flu-like symptoms induced by LPS, including chills, headache, and nausea.
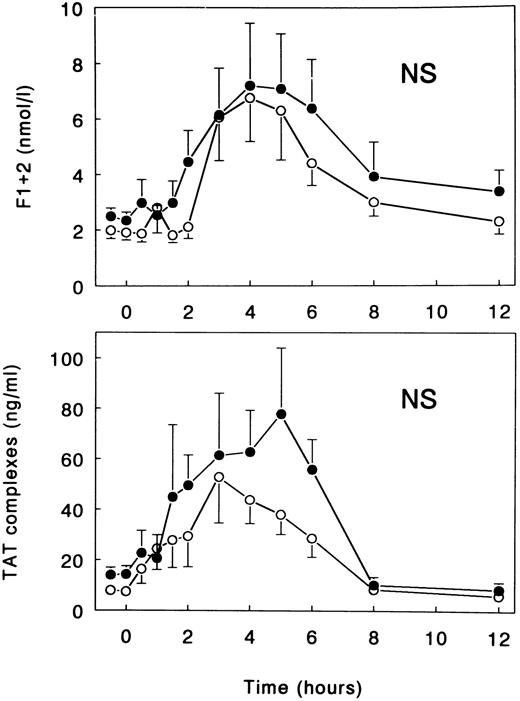
Coagulation and fibrinolysis.LPS injection was associated with a potent activation of the coagulation system, as reflected by increases in the plasma concentrations of F1 + 2 prothrombin fragment and TAT complexes, peaking after 4 hours (7.21 ± 2.24 nmol/L) and 5 hours (77.8 ± 26.1 ng/mL), respectively (both P < .05 v time; Fig 1). This LPS-induced coagulant response was not significantly influenced by treatment with TNFR:Fc. Peak levels of F1 + 2 and TAT complexes in TNFR:Fc-infused subjects were 6.77 ± 1.58 nmol/L (P = .99 v LPS only) and 52.8 ± 18.2 ng/mL (P = .87), respectively (Fig 1).
Mean (± SE) plasma concentrations of prothrombin fragment F1 + 2 and TAT complexes after intravenous injection of LPS (lot EC-5; 2 ng/kg) at t = 0 hours in subjects receiving either (○) TNFR:Fc (6 mg/m2; n = 6) starting at t = −0.5 hours or (•) vehicle (n = 6). NS, nonsignificant for the difference between treatment groups.
Mean (± SE) plasma concentrations of prothrombin fragment F1 + 2 and TAT complexes after intravenous injection of LPS (lot EC-5; 2 ng/kg) at t = 0 hours in subjects receiving either (○) TNFR:Fc (6 mg/m2; n = 6) starting at t = −0.5 hours or (•) vehicle (n = 6). NS, nonsignificant for the difference between treatment groups.
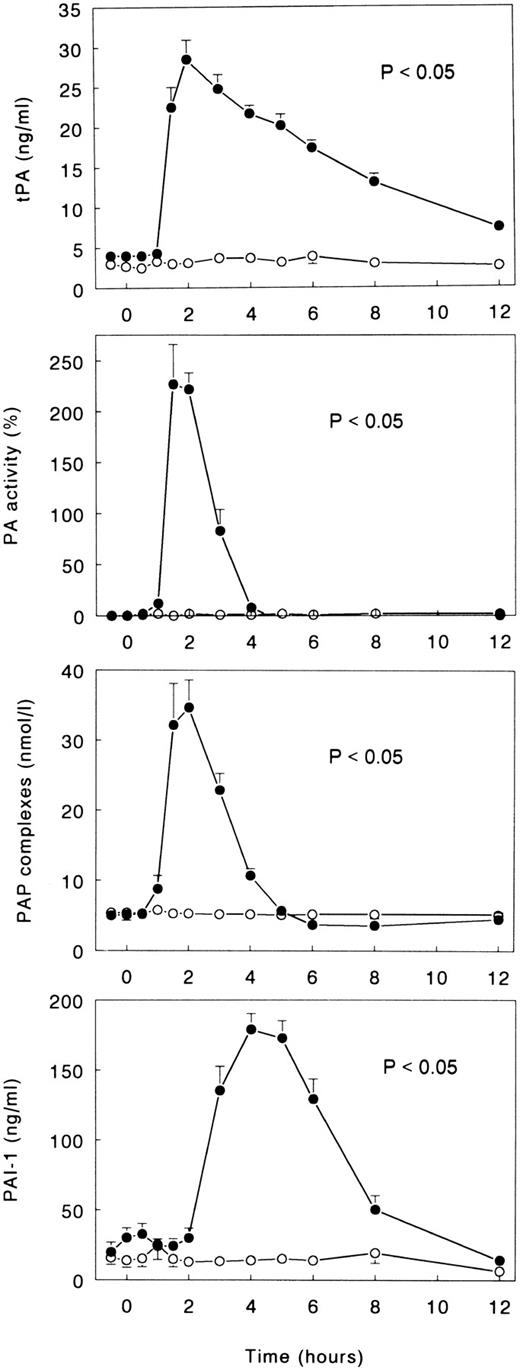
Changes in the fibrinolytic system induced by LPS were characterized by an initial activation, as reflected by transient increases in the plasma concentrations of tPA, PA activity, and PAP complexes, followed by inhibition, as indicated by a more delayed increase in the plasma levels of PAI-1 (all P < .05 v time; Fig 2). Peak concentrations of indexes of activation of fibrinolysis were measured after 1.5 to 2 hours (tPA, 28.5 ± 2.4 ng/mL; PA activity, +227% ± 39%; PAP complexes, 34.7 ± 3.9 nmol/L), and peak levels of PAI-1 were measured after 4 hours (179.2 ± 11.1 ng/mL). Infusion of TNFR:Fc completely prevented LPS-induced fibrinolytic changes. Thus, none of the parameters of fibrinolysis increased significantly from baseline (all P < .05 v LPS only; Fig 2).
Mean (± SE) plasma concentrations of tPA, PA activity, PAP complexes, and PAI-1 after intravenous injection of LPS (lot EC-5; 2 ng/kg) at t = 0 hours in subjects receiving either (○) TNFR:Fc (6 mg/m2; n = 6) starting at t = −0.5 hours or (•) vehicle (n = 6). P value indicates the difference between treatment groups.
Mean (± SE) plasma concentrations of tPA, PA activity, PAP complexes, and PAI-1 after intravenous injection of LPS (lot EC-5; 2 ng/kg) at t = 0 hours in subjects receiving either (○) TNFR:Fc (6 mg/m2; n = 6) starting at t = −0.5 hours or (•) vehicle (n = 6). P value indicates the difference between treatment groups.
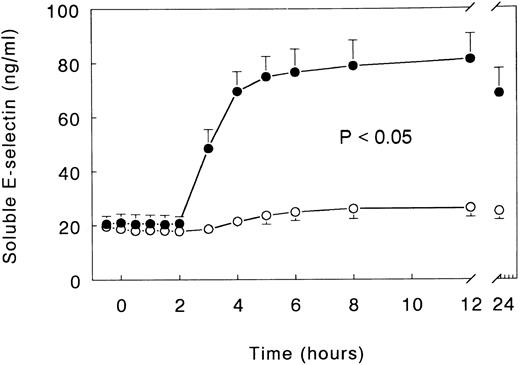
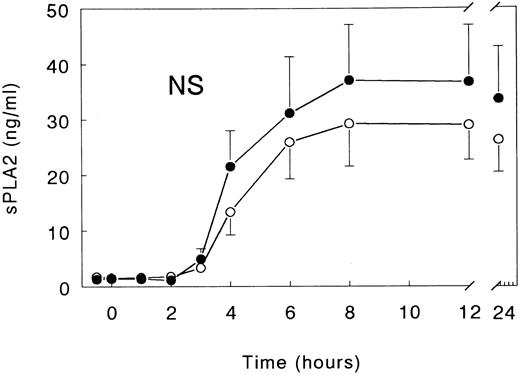
Soluble E-selectin and secretory PLA2.Injection of LPS elicited a marked increase in the plasma concentrations of soluble E-selectin, peaking after 12 hours (81.2 ± 9.4 ng/mL; P < .05 v time). Infusion with TNFR:Fc almost completely prevented this increase, with peak soluble E-selectin levels being detected of only 26.4 ± 3.4 ng/mL (P < .05 v LPS only; Fig 3). sPLA2 concentrations reached a plateau between 8 and 12 hours after LPS administration (8 hours, 37.1 ± 10.0 ng/mL; P < .05 v time), a response that was not significantly influenced by treatment with TNFR:Fc (Fig 4).
Mean (± SE) plasma concentrations of soluble E-selectin after intravenous injection of LPS (lot EC-5; 2 ng/kg) at t = 0 hours in subjects receiving either (○) TNFR:Fc (6 mg/m2; n = 6) starting at t = −0.5 hours or (•) vehicle (n = 6). P value indicates the difference between treatment groups.
Mean (± SE) plasma concentrations of soluble E-selectin after intravenous injection of LPS (lot EC-5; 2 ng/kg) at t = 0 hours in subjects receiving either (○) TNFR:Fc (6 mg/m2; n = 6) starting at t = −0.5 hours or (•) vehicle (n = 6). P value indicates the difference between treatment groups.
Mean (± SE) plasma concentrations of sPLA2 after intravenous injection of LPS (lot EC-5; 2 ng/kg) at t = 0 hours in subjects receiving either (○) TNFR:Fc (6 mg/m2; n = 6) starting at t = −0.5 hours or (•) vehicle (n = 6). P value indicates the difference between treatment groups.
Mean (± SE) plasma concentrations of sPLA2 after intravenous injection of LPS (lot EC-5; 2 ng/kg) at t = 0 hours in subjects receiving either (○) TNFR:Fc (6 mg/m2; n = 6) starting at t = −0.5 hours or (•) vehicle (n = 6). P value indicates the difference between treatment groups.
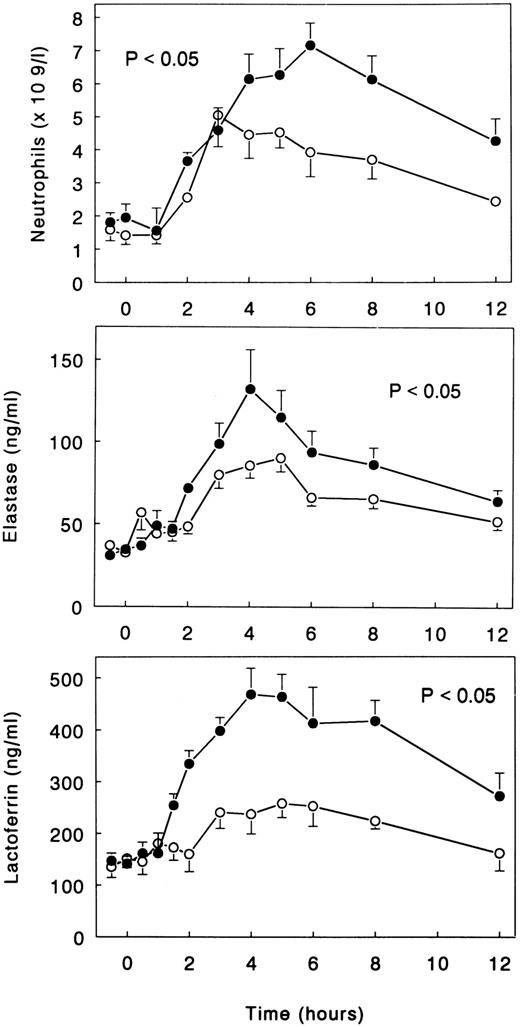
Neutrophils.LPS administration resulted in an increase in neutrophil counts, peaking after 6 hours (7.2 ± 0.7 × 109/L; P < .05 v time; Fig 5). LPS also elicited degranulation of neutrophilic granulocytes, as reflected by increases in the plasma concentrations of elastase-α1 -antitrypsin complexes and lactoferrin, peaking after 4 hours (132.2 ± 24.0 and 467.9 ± 50.4 ng/mL, respectively; both P < .05 v time; Fig 5). Treatment with TNFR:Fc attenuated these LPS-induced responses. Neutrophil counts increased to only 5.1 ± 1.0 × 109/L (P = .06 v LPS only), and the increases in elastase-α1-antitrypsin complexes and lactoferrin were reduced to 90.3 ± 8.4 and 240.4 ± 30.9 ng/mL, respectively (both P < .05 v LPS only; Fig 5).
Mean (± SE) neutrophil counts and plasma concentrations of elastase-α1 -antitrypsin complexes and lactoferrin after intravenous injection of LPS (lot EC-5; 2 ng/kg) at t = 0 hours in subjects receiving either (○) TNFR:Fc (6 mg/m2; n = 6) starting at t = −0.5 hours or (•) vehicle (n = 6). P value indicates the difference between treatment groups.
Mean (± SE) neutrophil counts and plasma concentrations of elastase-α1 -antitrypsin complexes and lactoferrin after intravenous injection of LPS (lot EC-5; 2 ng/kg) at t = 0 hours in subjects receiving either (○) TNFR:Fc (6 mg/m2; n = 6) starting at t = −0.5 hours or (•) vehicle (n = 6). P value indicates the difference between treatment groups.
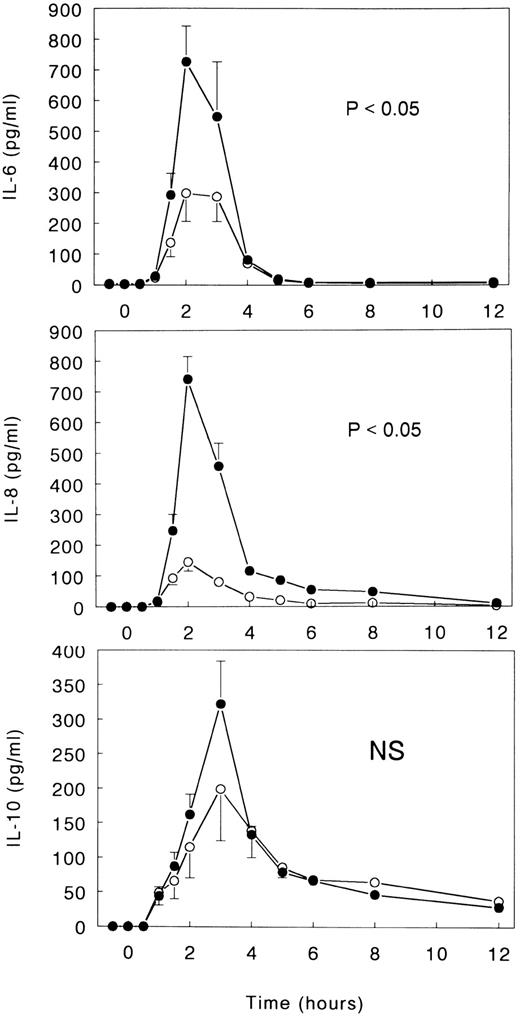
Cytokines.LPS induced transient increases in the plasma concentrations of IL-6, IL-8, and IL-10 (all P < .05 v time; Fig 6). IL-6 and IL-8 reached peak levels after 2 hours (727 ± 115 and 741 ± 74 pg/mL, respectively), and IL-10 reached peak levels after 3 hours (322 ± 62 pg/mL). TNFR:Fc inhibited LPS-induced IL-6 and IL-8 release (both P < .05 v LPS only) and tended to reduce the increase in IL-10 concentrations (nonsignificant). Peak levels of these cytokines in TNFR:Fc-infused subjects were 299 ± 92 pg/mL for IL-6, 145 ± 29 pg/mL for IL-8, and 199 ± 75 pg/mL for IL-10 (Fig 6).
Mean (± SE) plasma concentrations of IL-6, IL-8, and IL-10 after intravenous injection of LPS (lot EC-5; 2 ng/kg) at t = 0 hours in subjects receiving either (○) TNFR:Fc (6 mg/m2; n = 6) starting at t = −0.5 hours or (•) vehicle (n = 6). P value indicates the difference between treatment groups. NS, nonsignificant.
Mean (± SE) plasma concentrations of IL-6, IL-8, and IL-10 after intravenous injection of LPS (lot EC-5; 2 ng/kg) at t = 0 hours in subjects receiving either (○) TNFR:Fc (6 mg/m2; n = 6) starting at t = −0.5 hours or (•) vehicle (n = 6). P value indicates the difference between treatment groups. NS, nonsignificant.
LPS-binding protein.LPS administration resulted in a pronounced increase in plasma concentrations of LBP, peaking after 12 hours (P < .05 v time; Table 2). Infusion of TNFR:Fc tended to reduce this response (nonsignificant v LPS only).
Administration of TNFR:Fc Does Not Significantly Influence Plasma Concentrations of LBP During Endotoxemia
| Time (hr) . | LPS Only . | LPS + TNFR:Fc . |
|---|---|---|
| −0.5 | 18.9 ± 1.8 | 20.8 ± 2.5 |
| 0 | 17.5 ± 2.0 | 18.9 ± 3.0 |
| 8 | 58.0 ± 8.7 | 43.8 ± 4.3 |
| 12 | 66.7 ± 6.9 | 54.9 ± 6.1 |
| 24 | 58.9 ± 8.2 | 52.4 ± 6.8 |
| Time (hr) . | LPS Only . | LPS + TNFR:Fc . |
|---|---|---|
| −0.5 | 18.9 ± 1.8 | 20.8 ± 2.5 |
| 0 | 17.5 ± 2.0 | 18.9 ± 3.0 |
| 8 | 58.0 ± 8.7 | 43.8 ± 4.3 |
| 12 | 66.7 ± 6.9 | 54.9 ± 6.1 |
| 24 | 58.9 ± 8.2 | 52.4 ± 6.8 |
Mean ± SE plasma concentrations of LBP (in micrograms per milliliter) after intravenous injection of LPS (lot EC-5; 2 ng/kg) at t = 0 hours in subjects receiving either TNFR:Fc (6 mg/m2; n = 6) starting at t = −0.5 hours or vehicle (n = 6).
DISCUSSION
Sepsis is associated with excessive activation of a number of host mediator systems, including the cytokine network, the hemostatic mechanism, and leukocytes, each of which can contribute to the development of tissue injury.28 TNF is the first cytokine detectable in the circulation during models of systemic infection. Numerous studies have documented that neutralization of this early TNF activity inhibits the induction of the cytokine network and has a strong protective effect against lethality associated with intravenous bacterial challenges.1 However, despite its effect on lethality, investigations in animals have suggested that TNF only is involved in the activation of some inflammatory responses during sepsis. We show here for the first time in humans that neutralization of endogenous TNF activity does not influence LPS-induced coagulation activation, while completely preventing the activation of the fibrinolytic system. Further, elimination of TNF resulted in reduced neutrophil and endothelial cell responses.
Administration of TNFR:Fc at a dose of 6 mg/m2 effectively neutralized TNF activity produced in response to intravenous LPS, as determined by the WEHI cytotoxicity assay. In a recent study, TNFR:Fc administered at higher doses (10 and 60 mg/m2) exerted a paradoxical effect on cytokine and stress hormone release during human endotoxemia, ie, as the dose of TNFR:Fc increased, the degree of inhibition decreased.15 Because the lower TNFR:Fc dose used in the earlier volunteer study still provided a large excess of TNF neutralizing capacity,15 we chose to administer TNFR:Fc at 6 mg/m2. Furthermore, in light of these findings, we infused 6 healthy men with TNFR:Fc only (ie, without LPS) to exclude an inflammatory effect of TNFR:Fc per se, which could not be detected. The absence of changes in standard laboratory measurements, such as coagulation parameters, hematology, and chemistry, was previously reported after administration of TNFR:Fc to normal subjects in doses up to 60 mg/m2.29 Similar to our study, TNF activity remained neutralized up to 24 hours after LPS injection, thereby ruling out the occurrence of delayed and prolonged release of TNF activity from TNF-TNFR:Fc complexes, as has been reported in a mouse model of lethal gram-negative sepsis.30
In line with previous reports,4,31 injection of LPS was associated with an early activation of the fibrinolytic system mediated by the release of tPA, followed in time by an abrupt inhibition of fibrinolytic activity by the appearance of PAI-1. Only later, the common pathway of the coagulation system became activated. Administration of TNF to humans or baboons causes similar changes in the fibrinolytic and coagulation systems as LPS.8-10 TNF has a net procoagulant effect on endothelial cells by enhancing the synthesis and surface expression of tissue factor, the essential cofactor of the extrinsic pathway of the coagulation system; by downregulating thrombomodulin and protein S secretion; and by inhibiting the fibrinolytic response via inducing the synthesis and secretion of PAI-1.32-35 Although these data strongly suggest that TNF is an important mediator of LPS-induced stimulation of coagulation and fibrinolysis, TNFR:Fc only inhibited fibrinolytic changes. These results are in accordance with studies in endotoxemic and bacteremic primates, in which infusion of a neutralizing anti-TNF MoAb did not influence activation of the coagulation system.7,11 Thus, TNF is crucial for LPS-induced stimulation of fibrinolysis, but does not contribute to LPS-induced coagulation activation. The latter response has been found to be mediated by tissue factor,21,36 possibly in conjunction with IL-6.37,38 Hence, inhibition of TNF during sepsis may have a net procoagulant effect by selective inhibition of fibrinolysis without a corresponding effect on the coagulation system. Furthermore, this study shows that also in humans activation of coagulation and fibrinolysis in response to intravenous LPS are independent phenomena, as previously shown in chimpanzees in which inhibition of coagulation with an antitissue factor MoAb did not affect fibrinolytic changes.21
TNFR:Fc infusion not only prevented tPA release, but also strongly attenuated the increase in the plasma levels of soluble E-selectin, another molecule that is shed by the vascular endothelium upon activation.39 These data therefore suggest that TNF plays a significant role in endothelial cell activation during endotoxemia. By contrast, the release of sPLA2 was not influenced by TNFR:Fc. sPLA2 is a central mediator of inflammation controling the synthesis of eicosanoids and platelet-activating factor that can be produced by a variety of cell types, including endothelial cells and macrophages.40 The plasma concentrations of sPLA2 have been found elevated in sepsis, in which it may contribute to tissue injury.40 We previously showed that injection of TNF into baboons results in an increase in plasma sPLA2 levels.10 TNF may elicit sPLA2 release directly by an effect on endothelial cells.41 In baboons infected with live E coli, treatment with an anti-TNF MoAb significantly reduced sPLA2 release.42 The results of the present study suggest that, during low-grade endotoxemia, TNF does not play an important role in this inflammatory reaction. Thus, the production of sPLA2 during endotoxemia seems to be regulated by different (TNF-independent) mechanisms and/or different cell types than the production of the more specific endothelial cell markers tPA and soluble E-selectin. It remains to be established which factors sustain sPLA2 production. IL-6, of which the release into the circulation was inhibited but not prevented by TNFR:Fc, is also able to induce sPLA2 release in vitro.41
TNFR:Fc infusion abrogated LPS-induced neutrophil responses, which confirms and extends an earlier study in human volunteers.15 However, the reduced neutrophilia in subjects treated with TNFR:Fc contrasts with the previously reported enhancement of neutrophilia in endotoxemic subjects infused with higher doses of TNFR:Fc,15 suggesting that indeed lower doses of the compound exert more potent antiinflammatory effects. This supposition is further supported by our finding of slightly reduced LBP levels in TNFR:Fc-infused subjects, whereas in the earlier volunteer study the acute-phase protein response tended to be enhanced.15
Recently, a dose-response relation between treatment with TNFR:Fc, administered at doses of 0.15, 0.45, or 1.5 mg/kg body weight, and mortality was found in a randomized, placebo-controlled trial with patients with septic shock.43 The explanation of this unexpected finding remains uncertain. Considering the present data and data from other laboratories,15,29 a toxic effect of TNFR:Fc is unlikely. Furthermore, it seems unlikely that TNFR:Fc functioned as an intravascular carrier of TNF prolonging the activity of the cytokine, because neither in this nor in the previous human study with TNFR:Fc15 could delayed appearance of TNF activity be detected. However, it is conceivable that some patients included in clinical sepsis trials do not benefit from complete neutralization of endogenous TNF activity. Indeed, animal data suggest that anti-TNF treatment may impair host defense during localized infections, such as peritonitis or pneumonia.44 45 Hence, at early stages of an infection, the local production of TNF likely is needed to combat invading microorganisms. Therefore, the failure of anti-TNF treatment (whether administered in the form of antibodies or TNF receptor fusion proteins) in patients with clinically defined sepsis may be related to the fact that the currently used inclusion criteria do not appropriately identify patients who may benefit from elimination of excessive TNF activity.
The results presented herein indicate that TNF cannot be considered the central mediator of LPS-induced systemic inflammatory responses in humans. Neutralization of TNF in this model was associated with a shift toward a more procoagulant state due to prevention of the fibrinolytic response while leaving coagulation activation unaltered. Furthermore, TNF is not involved in sPLA2 release and only modestly contributes to neutrophil responses during human endotoxemia. Although systemic administration TNF can reproduce the majority of inflammatory effects observed in models of systemic infection, it seems likely that TNF is not absolutely necessary for many of such responses during sepsis.
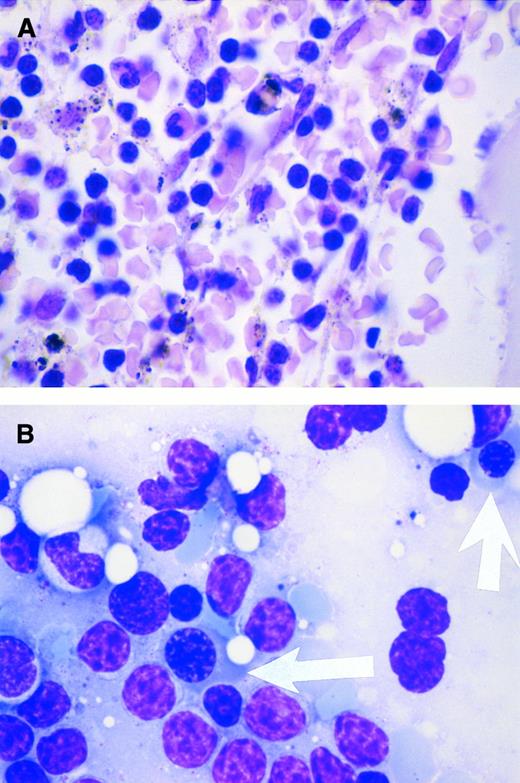
Response to cyclosporin in pure red blood cell aplasia. A 54-year-old man has had acquired pure red blood cell aplasia associated with B-cell chronic lymphocytic leukemia since 1994. At diagnosis, the Direct Antiglobulin Test was negative. In May 1996, while receiving prednisolone (70 mg/d), his reticulocyte count was 7.1 × 109/L (reference range, 20 to 80 × 109/L); his hemoglobin level varied from 5.6 to 6.2 g/dL before red blood cell transfusions that he required every 1 to 2 weeks; his platelet count was 133 × 109/L; his leukocytes were 22.7 × 109/L, with a lymphocytosis of 15.9 × 109/L; and he had a normal granulocyte count of 5.6 × 109/L. The bone marrow showed severe erythroid hypoplasia, a reduced number of megakaryocytes, a normal proportion of granulocyte precursors, and marked lymphocytic infiltration (A). Two weeks after starting cyclosporin A at 250 mg twice daily, the drug concentration in whole blood was 155 μg/L and his reticulocyte count had increased to 145 × 109/L. By 4 weeks, the hemoglobin level had increased to 11.4 g/dL without red blood cell transfusion and the prednisolone decreased to 5 mg/d. Repeat marrow biopsy 3 weeks after initiating cyclosporin therapy showed an increased number of erythroid precursor cells (arrows in B). (Courtesy of I.E. Okpala, MRCPath, FWACP, Bronglais General Hospital, Aberystwyth, UK.)
Response to cyclosporin in pure red blood cell aplasia. A 54-year-old man has had acquired pure red blood cell aplasia associated with B-cell chronic lymphocytic leukemia since 1994. At diagnosis, the Direct Antiglobulin Test was negative. In May 1996, while receiving prednisolone (70 mg/d), his reticulocyte count was 7.1 × 109/L (reference range, 20 to 80 × 109/L); his hemoglobin level varied from 5.6 to 6.2 g/dL before red blood cell transfusions that he required every 1 to 2 weeks; his platelet count was 133 × 109/L; his leukocytes were 22.7 × 109/L, with a lymphocytosis of 15.9 × 109/L; and he had a normal granulocyte count of 5.6 × 109/L. The bone marrow showed severe erythroid hypoplasia, a reduced number of megakaryocytes, a normal proportion of granulocyte precursors, and marked lymphocytic infiltration (A). Two weeks after starting cyclosporin A at 250 mg twice daily, the drug concentration in whole blood was 155 μg/L and his reticulocyte count had increased to 145 × 109/L. By 4 weeks, the hemoglobin level had increased to 11.4 g/dL without red blood cell transfusion and the prednisolone decreased to 5 mg/d. Repeat marrow biopsy 3 weeks after initiating cyclosporin therapy showed an increased number of erythroid precursor cells (arrows in B). (Courtesy of I.E. Okpala, MRCPath, FWACP, Bronglais General Hospital, Aberystwyth, UK.)
Supported by Grants No. RO1 GM 34695 and RR0047 from the US Public Health Service. T.v.d.P. is a fellow of the Royal Netherlands Academy of Arts and Sciences.
Address reprint requests to Stephen F. Lowry, MD, UMDNJ-Robert Wood Johnson Medical School, One Robert Wood Johnson Place, CN-19, New Brunswick, NJ 08903.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal