Abstract
Anti-CD3 monoclonal antibodies (MoAbs) and glucocorticoid hormones induce apoptosis in immature thymocytes and peripheral T lymphocytes. This process is inhibited by a number of growth factors, including interleukin-2 (IL-2), IL-3, and IL-4, as well as by triggering of the adhesion molecule CD44, which would indicate that signals generated by membrane receptors can modulate the survival of lymphoid cells. To investigate whether triggering of CD2 may also affect apoptosis in lymphoid cells, we analyzed the effect of stimu-lation with anti-CD2 MoAbs on T-cell apoptosis induced by two stimuli, anti-CD3 MoAbs and dexamethasone (DEX), using a hybridoma T-cell line and a T-helper cell clone. The results show that CD2 engagement decreased anti-CD3 MoAb-induced apoptosis, but did not influence DEX-induced cell death. Furthermore, the decrease appeared to be related to the expression of Fas/APO-1 (CD95) and Fas-ligand (Fas-L). In fact, we show that CD2 stimulation inhibits apoptosis by preventing the CD3-induced upregulation of Fas and Fas-L in a Fas-dependent experimental system. These data suggest that a costimulatory molecule may control a deletion pathway and may therefore contribute to the regulation of peripheral tolerance.
APOPTOSIS (PROGRAMMED cell death) is a common mechanism often triggered by environmental stimuli.1-4 It is operative in tissue remodeling and involution during embryogenesis, control of cell growth in adult life, various degenerative diseases of the central nervous system, and regulation of neoplastic cell growth.5-8 Apoptosis involves a cascade of specific biochemical and morphologic events. The most significant of these is activation of endogenous endonucleases, which are responsible for internucleosomal DNA fragmentation.9 10
Apoptosis is also important in T-cell repertoire development.11-13 These cells are controlled by a complex process that includes both positive and negative selection. Negative selection is the result of apoptosis activated through Ag–T-cell receptor (TCR) interaction.14 It has been suggested that glucocorticoid hormones (GCH) and cytokines are also critical regulators of T-cell death.10,15-18 Mutual exclusion between two apoptosis inducers, namely anti-CD3 monoclonal antibodies (MoAbs) and dexamethasone (DEX), has also been described in a T-cell hybridoma.19
Recent evidence suggests that susceptibility to apoptosis is not restricted to immature or transformed T cells because it can be triggered in vivo and in vitro in mature peripheral T cells through Ag engagement of TCR/CD3.20,21 In particular, engagement of the TCR/CD3 complex of primary T cells, either by antigen-presenting cells (APCs) presenting antigenic peptide or antibody to TCR/CD3, triggers a series of activation events, whereas CD3-stimulation of previously activated T cells or hybridoma cell lines induces apoptosis. This process has been termed activation-induced cell death (AICD).22 AICD is also mediated by the Fas/Fas-ligand (Fas-L) system.23-25 Fas/APO-1 (CD95) is a type I membrane protein belonging to the tumor necrosis factor (TNF )/nerve growth factor (NGF ) receptor family.26,27 It is expressed on the surface of a variety of transformed cell lines and chronically stimulated T cells and transduces a direct apoptotic death signal after ligation with Fas-L.28-30 Fas-L is present constitutively in the spleen and at low levels in the thymus as a type II transmembrane protein that may be proteolytically released from the cell membrane and it is physiologically active in soluble form.31-33 Anti-CD3 MoAbs cross-linking induces Fas-L and upregulates Fas. The engagement of Fas by Fas-L activates the cell death program involved in the regulation of peripheral lymphocyte tolerance.23-25
CD2 is an adhesion molecule involved, through its interaction with LFA-3 (CD58), in facilitating CD3/TCR recognition of the antigens presented via the major histocompatibility complex (MHC) and in T-cell activation.34-37 Moreover, CD2 activation regulates expression of interleukin-2 (IL-2), IL-2 receptor-α, and transcription of HLA-DR and IL-6 genes and induces transcription factors that bind to the NF-AT, AP-1, and NF-κB sites.38-40 Recently, it has been reported that in vivo administration of anti-CD2 or anti-CD48 MoAbs inhibits the T-cell response in mice and rats.41-43
Recent evidence that AICD could be a peripheral negative selection mechanism28 and that adhesion molecules play a role in controlling programmed cell death44,45 prompted us to study the role of CD2 in AICD. We used three models: (1) a mouse hybridoma T-cell line (3DO) that undergoes apoptosis by TCR cross-linking and in which Fas/Fas-L interaction plays a dominant role in mediating AICD,46 (2) a T-helper cell clone, and (3) primary polyclonal lymphocytes.
Our results clearly show that CD2 stimulation by MoAbs protects T cells from TCR/CD3-induced apoptosis, but does not influence DEX-induced apoptosis. This protection was associated with a decrease in Fas and Fas-L expression. Therefore, CD2 may be involved in the control of T-cell activation and survival by modulating TCR-induced Fas and Fas-L expression.
MATERIALS AND METHODS
Animals
C3H/HeN mice purchased from Charles River (Chalco, Milan, Italy) were used at the age of 8 weeks as donors of T cells.
Cell Suspensions
A CD3+, CD4+, CD2+, CD44+ subline of the OVA-specific hybridoma T-cell line 3DO47 obtained in our laboratory was used in this study. Cells maintained in suspension in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS) and 10 μmol/L HEPES buffer were centrifuged at pre-established times at 200g for 10 minutes, washed, and adjusted to the desired concentrations (see below). An antigen-specific, nontransformed T-cell clone was also used, the keyhole limpet hemocyanin (KLH)-specific CD3+, CD4+ CD2+ T-helper clone HDK-148 (kindly provided by Dr F. Colotta, Pharmacia, Milan, Italy), and was maintained in complete medium supplemented with IL-2 and stimulated with KLH (Calbiochem, San Diego, CA) plus irradiated (2 Gy) spleen cells as feeder every 4 weeks, as previously described.48
Lymph nodes were ground between frosted glass slides to produce a cell suspension and then passed twice through nylon columns to obtain a 95% pure T-cell population, as established by CD4, CD8 expression.
Antibody Cross-Linking and Cell Treatment
Hamster antimouse CD3ε (clone 145-2C11; Pharmingen, San Diego, CA) MoAbs at 1 μg/well and/or rat antimouse CD2 (clone RM2-5; Pharmingen) MoAbs, at a concentration of 5 or 1 μg/well, were allowed to adhere in flat-bottomed, high-binding 96-well plates (Costar, Cambridge, MA) at 4°C in 100 μL phosphate-buffered saline (PBS). After 20 hours, plates coated with MoAbs were washed, incubated at 37°C for 2 hours with PBS supplemented with 10% FCS, and washed again. The hybridoma T cells or untransformed HDK-1 cells were then plated at 1 × 105 cells/well and incubated at 37°C for 20 hours. Isotype-matched rat antimouse IgG2b MoAbs (clone R35-38; Pharmingen) were used as control. Some cultures were established in the presence of 100 nmol/L DEX (Sigma, St Louis, MO). Cells recovered after culturing were used to measure cell death.
To evaluate Fas-mediated killing, 3DO cells (1 × 106) were incubated at room temperature for 30 minutes with 10 μg/mL of the antibody to Fas (hamster antimouse, clone Jo2; Pharmingen) and then washed and plated in wells coated with an antibody to hamster IgG (5 μg/well; Pharmingen) for the cross-linking of the anti-Fas MoAb. The CD2 MoAbs (5 μg/well) were either adhered to the plates at 0°C or to the cells at room temperature with or without anti-Fas MoAbs.
To block anti-CD3–induced apoptosis, in selected experiments anti-Fas MoAb (clone Jo2) or isotype-matched hamster antimouse IgG MoAb (clone UC8-4B3; Pharmingen) was used in soluble non–cross-linked form. For this purpose, the MoAbs were added after the incubation of anti-CD3 pretreated plates with 100 μL/well FCS.
Lymph node T cells at the concentration of 2 × 105 cells/well were cultured for 5 days in the presence of 100 U/mL IL-249 (Genzyme, Cambridge, MA).
IL-2 and Proliferation Assays
Supernatants from cells stimulated with anti-CD3 and/or anti-CD2 for 20 hours were tested for their concentration of IL-2 by two-site enzyme-linked immunosorbent assay (ELISA) using MoAb JES6-1A12 as the primary reagent and biotinylated monoclonal S4B6 as the secondary reagent. Both antibodies were purchased from Pharmingen. The IL-2 titer (mean ± SD of replicate samples) was expressed as picograms per milliliter, calculated by reference to standard curves constructed with known amounts of IL-2. The sensitivity limit was approximately 20 pg/mL.
In some wells, [3H]-thimidine (1 μCi/well) was added 12 hours before harvesting and proliferation assayed as described elsewhere.42
Flow Cytometry Analysis
A single-cell suspension (1 × 106 cells/sample) was incubated for 30 minutes on ice in 50 μL staining buffer (PBS plus 5% FCS) containing 10 μg/mL hamster antimouse Fas MoAb directly conjugated to R-phycoerytrin (PE) or PE-hamster IgG (isotype control). Both MoAbs were purchased from Pharmingen. Cells were also stained with rabbit polyclonal antibody raised against a peptide, corresponding to the amino acids 260 to 279 mapping at the carboxy terminus of human FAS-L (Santa Cruz Biotechnology, Inc, Santa Cruz, CA) or with isotype-matched antibody and anti-rabbit IgG fluorescein isothiocyanate (FITC) conjugate, F(ab′)2 fragment (Sigma), as the second-step reagent.
For intracellular Fas-L staining, cells were fixed and stained by the paraformaldehyde-saponin procedure. Cells were harvested, washed twice in Hanks' balanced salt solution (HBSS) with 1% HEPES buffer solution, and transferred to a FACS tube (1 × 106/tube). Then, 50 μL of ice-cold 4% phosphate-buffered paraformaldehyde, pH 7.4 (40 g/L paraformaldehyde, 16.88 g/L NaH2PO4⋅H2O, 3.85 g/L NaOH, and 5.4 g/L glucose), was added for 5 minutes. The tubes were washed once in HBSS-HEPES and then in HBSS-HEPES containing 0.1% saponin (HBSS-saponin) to render cells permeable. To stain cells, 50 μL of the predetermined dilution of Fas-L or isotype-matched antibodies in HBSS-saponin was added to each reaction field and left to incubate for 30 minutes. After one washing in HBSS-saponin, 50 μL of FITC-labeled antirabbit IgG F(ab′)2 fragment diluted in HBSS-saponin was added. An aliquot of cells was stained with the secondary antibody alone (background). After 30 minutes, the samples were washed and analyzed on a FACScan flow cytometer. The Fas-L antibody, an affinity-purified rabbit polyclonal antibody raised against a peptide corresponding to amino acids 2 to 19 mapping at the amino terminus of rat origin Fas-L, was purchased from Santa Cruz Biotechnology. Isotype-matched antibodies, rabbit antirat and rabbit antihuman, were purchased from Jackson Immuno Research Laboratories, Inc (West Grove, PA). In selected experiments, a portion of T cells was stained with anti–IL-2 receptor MoAb conjugated to R-phycoerythrin (IL-2R, P55; Pharmingen). The median or percentage values of Fas and Fas-L histograms were calculated using Lysis II research software (Becton Dickinson, Mountain View, CA).
Apoptosis Evaluation
Apoptosis evaluation by propidium iodide solution.Apoptosis was measured by flow cytometry as described elsewhere.50 After culturing, cells were centrifuged and the pellets were gently resuspended in 1.5 mL hypotonic propidium iodide solution (PI; 50 μg/mL in 0.1% sodium citrate plus 0.1% Triton X-100; Sigma). The tubes were kept overnight at 4°C in the dark. The PI fluorescence of individual nuclei was measured by flow cytometry using standard FACScan equipment (Becton Dickinson). The nuclei traversed a 488-nm Argon laser light beam. A 560-nm dichroid mirror (DM 570) and a 600-nm band pass filter (band width, 35 nm) were used to collect the red fluorescence due to PI DNA staining, and the data were recorded in logarithmic scale in a Hewlett Packard (HP 9000, model 310; Palo Alto, CA) computer. The percentage of apoptotic cell nuclei (subdiploid DNA peak in the DNA fluorescence histogram) was calculated with specific FACScan research software (Lysis II).
Apoptosis evaluation by fluorescein (terminal deoxynucleotidyl transferase [TdT] assay).A kit based on labeling of DNA strand breaks (Boehringer Mannheim, Milan, Italy) was used to detect and quantify apoptosis.51
Cells were washed twice in PBS containing 1% bovine serum albumin at 4°C, adjusted to 1 × 107 cells/mL, and then transferred into a U-bottomed 96-well plate. A paraformaldehyde solution (4% in PBS) was added to the cells, which were fixed by incubating for 30 minutes at room temperature. After washing, cells were permeabilized with a solution of 0.1% sodium citrate for 2 minutes on ice. After further washing, DNA of fixed cells was labeled by adding fluorescein dUTP at strand breaks by means of terminal transferase enzyme (TdT). Fixed and permeabilized cells labeled with fluorescein dUTP without terminal transferase were used as negative controls. After incubation for 60 minutes at 37°C and washing in PBS, the cells were analyzed by flow cytometry.
Immunoprecipitation
The cell-surface proteins were biotinylated using biotin NHS ester (Amersham Life Science, Buckinghamshire, UK). The cells (5 × 106/sample) were lysed in an NP-40–containing buffer and preadsorbed with normal hamster IgG. After removing the protein A-Sepharose by centrifugation, 10 μg of purified antimouse Fas MoAb, lines 2 through 5, or isotype-matched antibody, line 1, was added to the supernatant. After incubation for 60 minutes on ice, protein A-Sepharose was added and the mixture was incubated at 4°C overnight. The immunoprecipitates were then washed three times with cold lysis buffer, boiled for 3 minutes, and then analyzed by electrophoresis in 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels followed by transfer to nitrocellulose (Bioblot-NC; Costar) for 5 hours at 250 mA, 4°C in 25 mmol/L Tris/glycine, pH 8.3, and 20% vol/vol methanol. The proteins were detected using the enhanced chemiluminescence system (Amersham) after staining with streptavidin-conjugated horseradish peroxidase (Amersham).
Cytotoxicity Assay
The lysis of P815 Fas+ tumor cell line within 16 hours was used as an indicator of Fas-L expression. This tumor cell line was grown in RPMI 1640 and 10% FCS and subcultured 2 to 3 times per week. Different concentrations of 3DO cells were cultured for 20 hours on plates coated with anti-CD3 (1 μg/well) and/or anti-CD2 (5 μg/well) or control medium. The 51Cr labeling and assay were as previously described.24 Spontaneous release or release in the presence of anti-CD3 and/or anti-CD2 with no effector cells was ≤15% of the total release. The percentage of specific lysis at various E:T ratios was calculated as follows: % Cytotoxicity = (Test cpm − Spontaneous Release cpm)/(Total Release cpm) × 100, where test cpm is the mean cpm released in the presence of effector cells, spontaneous release is the mean of cpm released from targets cultured in medium alone, and total release cpm is the mean of cpm obtained by lysing target with 0.5% Triton X-100.
Statistical Analysis
Each experiment was performed at least three times. Representative experiments are shown, unless otherwise indicated in the figure legends. The means ± SD of three different experiments are included in the text. Because of the non-normal distribution of the data, nonparametric tests (Kruskall-Wallis' analysis of variance) were adopted for statistical evaluation.
RESULTS
Stimulation of CD2 Inhibits CD3-Driven Apoptosis
Treatment with anti-CD3 MoAbs mimics the effect of Ag-TCR/CD3 interaction. Previous reports indicate that anti-CD2 antibodies increase in vitro or decrease in vivo CD3-induced activation.35,36 41-43 We used a CD3+ CD2+ T-cell hybridoma subline, 3DO, and a nontransformed T-cell clone, HDK-1, to analyze the effect of CD2 on apoptosis induced by anti-CD3 MoAb treatment.
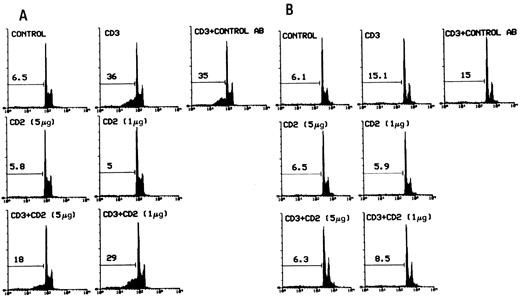
The cells were cultured for 20 hours in 96-well plates coated with activating anti-CD3 MoAbs in the presence or absence of anti-CD2 MoAbs. Figure 1 shows the results of a representative experiment obtained by flow cytometry analysis of PI-labeled nuclei. It is apparent that treatment with two doses of anti-CD2 MoAbs inhibited, in a dose-dependent manner, CD3-induced apoptosis in 3DO cells (Fig 1A) as well as in HDK-1 cells (Fig 1B). The mean ± SD of three experiments with 3DO gave the following results: untreated control, 6.0 ± 1; anti-CD3–treated (1 μg/well), 37 ± 4; anti-CD2 (5 μg/well), 4.7 ± 2; and anti-CD3 (1 μg/well) plus anti-CD2–treated (5 μg/mL), 17 ± 1. The difference between anti-CD3–treated and anti-CD3 plus anti-CD2–treated groups was statistically significant (P < .01). This effect was dose-dependent and specific, because control MoAbs, with the same isotype as anti-CD2 MoAbs and at the higher dose used for anti-CD2, had no effect (Fig 1).
Flow cytometric analysis of PI-stained 3DO (A) and HDK-1 (B) nuclei after 20 hours of in vitro culture in 96-well plates with medium alone, coated with anti-CD3 (1 μg/well) and/or anti-CD2 MoAbs (5 or 1 μg/well; adhered as described in the Materials and Methods). Numbers above histograms indicate the percentage of apoptotic nuclei (broad hypodiploid peak) in a representative experiment. Control AB MoAbs, with the same isotype as anti-CD2 MoAbs, were used as control. PI fluorescence versus the number of nuclei is shown.
Flow cytometric analysis of PI-stained 3DO (A) and HDK-1 (B) nuclei after 20 hours of in vitro culture in 96-well plates with medium alone, coated with anti-CD3 (1 μg/well) and/or anti-CD2 MoAbs (5 or 1 μg/well; adhered as described in the Materials and Methods). Numbers above histograms indicate the percentage of apoptotic nuclei (broad hypodiploid peak) in a representative experiment. Control AB MoAbs, with the same isotype as anti-CD2 MoAbs, were used as control. PI fluorescence versus the number of nuclei is shown.
Similar results were obtained when apoptosis was evaluated by the TdT assay. Treatment with two doses of anti-CD2 MoAbs countered 3DO cell death induced by anti-CD3 MoAbs in a dose-dependent manner (Fig 2).
TdT assay of 3DO-fixed cells after 20 hours of in vitro culture in 96-well plates with medium alone, coated with anti-CD3 (1 μg/well) and/or anti-CD2 MoAbs (5 or 1 μg/well). The numbers above the histograms indicate the percentage of apoptosis in a representative experiment. Also shown is the relative fluorescence intensity versus cell number.
TdT assay of 3DO-fixed cells after 20 hours of in vitro culture in 96-well plates with medium alone, coated with anti-CD3 (1 μg/well) and/or anti-CD2 MoAbs (5 or 1 μg/well). The numbers above the histograms indicate the percentage of apoptosis in a representative experiment. Also shown is the relative fluorescence intensity versus cell number.
Cell count analysis, by trypan blue exclusion assay and thymidine uptake, confirmed the protective effect of CD2 triggering. The data in Table 1 show that treatment with anti-CD2 MoAbs countered the decrease in cell number and thymidine uptake due to anti-CD3–induced apoptosis. Furthermore, Table 1 shows that T-cell activation markers, such as IL2R (CD25) and IL-2, decreased with anti-CD2 treatment, suggesting, as previously reported,41-43 that ligation of CD2 may result in inhibition of anti-CD3–induced activation.
Effect of CD2 Ligation on Some T-Cell Activation Markers
| . | Cell No. (×105/mL) . | CPM (×10−3) . | IL-2R+ (%) . | IL-2 (pg/mL) . |
|---|---|---|---|---|
| Medium | 19 ± 0.7 | 32 ± 3 | 2.7 ± 0.5 | 13 ± 0.3 |
| Anti-CD3 (1 μg) | 10 ± 0.5 | 19 ± 2 | 16.2 ± 3 | 489 ± 4 |
| Anti-CD2 (5 μg) | 20 ± 0.8 | 33 ± 1 | 1.8 ± 1 | 15 ± 0.5 |
| Anti-CD3 + anti-CD2 | 16.8 ± 0.4* | 26 ± 0.8* | 5.8 ± 1.7* | 410 ± 5* |
| . | Cell No. (×105/mL) . | CPM (×10−3) . | IL-2R+ (%) . | IL-2 (pg/mL) . |
|---|---|---|---|---|
| Medium | 19 ± 0.7 | 32 ± 3 | 2.7 ± 0.5 | 13 ± 0.3 |
| Anti-CD3 (1 μg) | 10 ± 0.5 | 19 ± 2 | 16.2 ± 3 | 489 ± 4 |
| Anti-CD2 (5 μg) | 20 ± 0.8 | 33 ± 1 | 1.8 ± 1 | 15 ± 0.5 |
| Anti-CD3 + anti-CD2 | 16.8 ± 0.4* | 26 ± 0.8* | 5.8 ± 1.7* | 410 ± 5* |
Data are means ± SD of three experiments. Cell count using trypan blue exclusion, [3H]thymidine uptake, IL-2 detection by ELISA, and IL-2R staining were performed after incubating 3DO cells for 20 hours.
P < .01 comparing line 4 with line 2.
The results suggest that CD2-triggering may result in survival of lymphocytes exposed to anti-CD3 apoptotic stimuli.
CD2 Stimulation Does Not Inhibit DEX-Induced Apoptosis
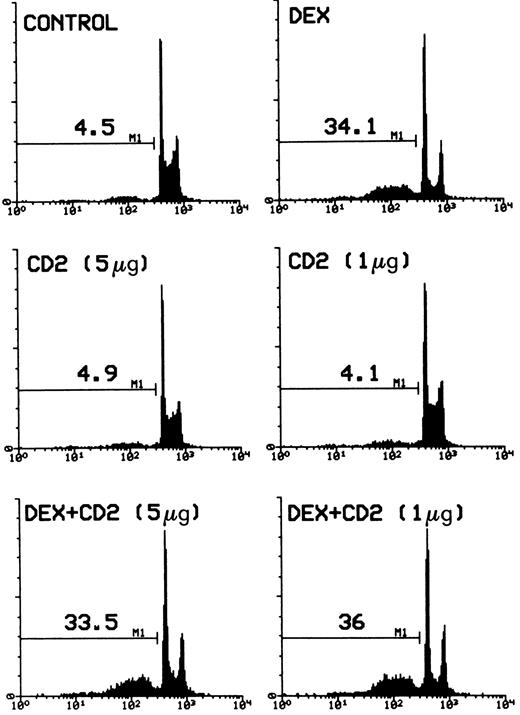
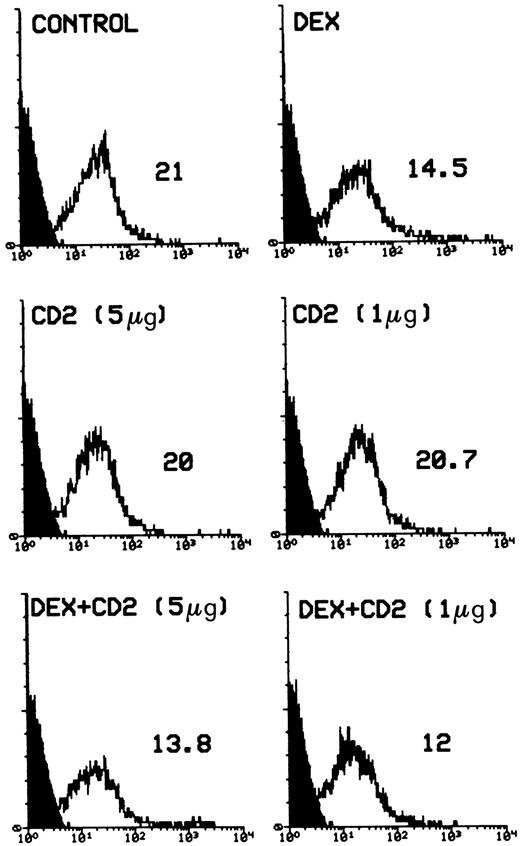
It has been shown that GCH induce apoptosis in both undifferentiated thymocytes and more mature T lymphocytes.10,15,16 19 To test the effect of CD2 triggering on this induction, a series of experiments was performed. Figure 3 gives the flow cytometry profile of PI-labeled nuclei from cells treated with DEX (100 nmol/L) in the presence or absence of anti-CD2 MoAb treatment. The results clearly indicate that stimulation of CD2 did not counter the DEX-induced apoptotic stimuli.
Percentage of apoptotic nuclei of cells treated with DEX alone (100 nmol/L) or DEX plus different concentrations (number in parentheses) of anti-CD2 MoAbs adhered on 96-well plates. Analysis of apoptosis by flow cytometry was performed after incubation for 20 hours. The percentage of apoptotic nuclei (broad hypodiploid peak) in a representative experiment is indicated in each histogram. PI fluorescence (DNA content) versus number of nuclei is shown.
Percentage of apoptotic nuclei of cells treated with DEX alone (100 nmol/L) or DEX plus different concentrations (number in parentheses) of anti-CD2 MoAbs adhered on 96-well plates. Analysis of apoptosis by flow cytometry was performed after incubation for 20 hours. The percentage of apoptotic nuclei (broad hypodiploid peak) in a representative experiment is indicated in each histogram. PI fluorescence (DNA content) versus number of nuclei is shown.
Taken together, these results suggest that CD2 stimulation specifically rescues T lymphocytes from AICD but does not protect them from DEX-induced apoptosis.
CD2-Induced Inhibition of CD3-Activated Apoptosis Correlates With Modulation of Fas and Fas-L
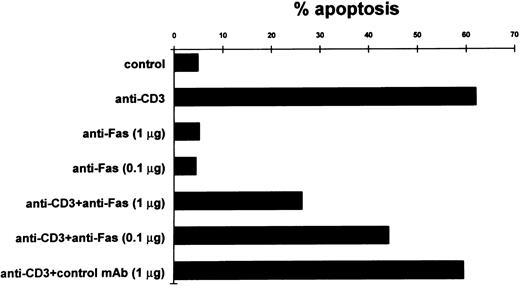
It has been suggested that T-cell AICD is also dependent on Fas/Fas-L interaction. Our results show that this system is involved in AICD of 3DO cells. In fact, in experiments with soluble (non–cross-linked) anti-Fas MoAbs, there was a substantial dose-dependent inhibition of anti-CD3–induced apoptosis, whereas no effect was observed with control antibody (Fig 4). Figure 4 also shows that anti-Fas MoAb in soluble form did not induce apoptosis.
Inhibition of apoptosis in anti-CD3–stimulated 3DO cells by soluble anti-Fas MoAb. In one experiment representative of three, anti-CD3–stimulated cells (10 μg/well) were cultured in the presence of soluble anti-Fas or control MoAbs. Apoptosis was evaluated by flow cytometric analysis of PI-stained nuclei after 20 hours of culture.
Inhibition of apoptosis in anti-CD3–stimulated 3DO cells by soluble anti-Fas MoAb. In one experiment representative of three, anti-CD3–stimulated cells (10 μg/well) were cultured in the presence of soluble anti-Fas or control MoAbs. Apoptosis was evaluated by flow cytometric analysis of PI-stained nuclei after 20 hours of culture.
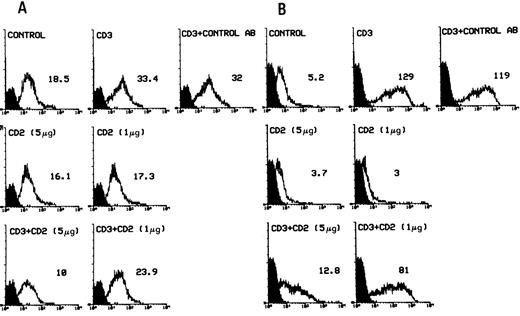
We examined the expression of Fas/Fas-L in anti-CD3– and/or anti-CD2–treated cells to determine if they were involved in the effects mediated by anti-CD2 stimulation. Results (Fig 5) show that anti-CD2 MoAb treatment of 3DO or HDK-1 cells inhibited the anti-CD3–induced upregulation (Fig 5A and B), but did not change the DEX-induced downmodulation of Fas expression in 3DO cells (Fig 6). Treatment with anti-CD2 alone did not modulate Fas expression (Figs 5 and 6).
Fas expression (flow cytometry with anti-Fas MoAbs directly conjugated to R-PE) in 3DO (A) and HDK-1 (B) cells after 20 hours of in vitro culture in 96-well plates with medium alone (control) or coated with anti-CD3 MoAbs (1 μg/well) and /or anti-CD2 MoAbs (5 or 1 μg/well). The number represents the value of the histogram median calculated by Lysis II. Fluorescence intensity versus cell number is shown.
Fas expression (flow cytometry with anti-Fas MoAbs directly conjugated to R-PE) in 3DO (A) and HDK-1 (B) cells after 20 hours of in vitro culture in 96-well plates with medium alone (control) or coated with anti-CD3 MoAbs (1 μg/well) and /or anti-CD2 MoAbs (5 or 1 μg/well). The number represents the value of the histogram median calculated by Lysis II. Fluorescence intensity versus cell number is shown.
Surface expression of Fas on DEX-stimulated 3DO cells. Cells were cultured in the presence or absence of DEX (100 nmol/L) and/or anti-CD2 MoAbs (5 or 1 μg/well). After 20 hours, cells were stained with Fas-PE MoAb and FACS analysis was performed as described. Shown is the relative fluorescence intensity versus cell number. The number represents the value of the histogram median.
Surface expression of Fas on DEX-stimulated 3DO cells. Cells were cultured in the presence or absence of DEX (100 nmol/L) and/or anti-CD2 MoAbs (5 or 1 μg/well). After 20 hours, cells were stained with Fas-PE MoAb and FACS analysis was performed as described. Shown is the relative fluorescence intensity versus cell number. The number represents the value of the histogram median.
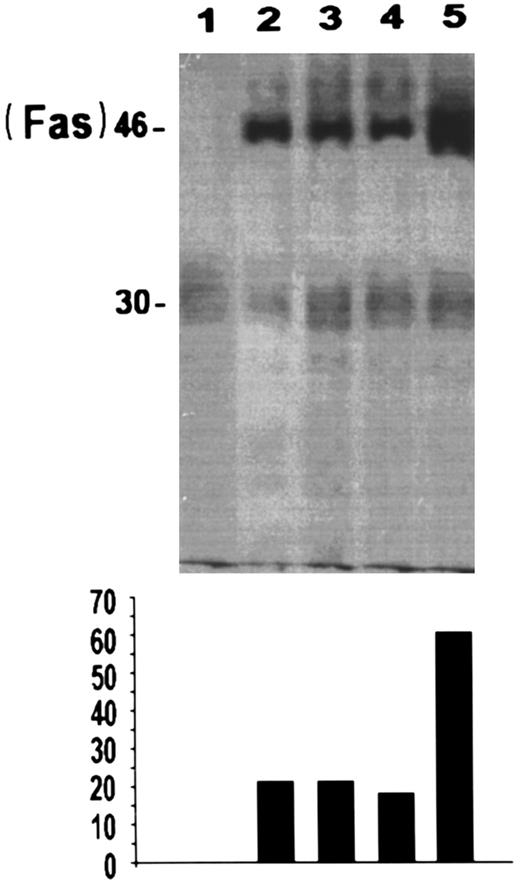
To further analyze the effects of CD2 on apoptosis induced by CD3 MoAbs, we evaluated the levels of Fas by immunoprecipitation experiments. Results (Fig 7) show that CD3 triggering increases Fas expression in 3DO cell line and that this increase is countered by CD2 (compare lane 5 with lane 3).
Immunoprecipitation of Fas protein in 3DO cells: untreated (lane 2), treated with anti-CD2 (5 μg/well) plus anti-CD3 (1 μg/well; lane 3), anti-CD2 (5 μg/well) alone (lane 4), or anti-CD3 (1 μg/well) alone (lane 5) for 20 hours. Cells were also immunoprecipitated with isotype-matched antibody (lane 1). Densitometric analysis (relative units) of the film is also reported at the bottom of the figure.
Immunoprecipitation of Fas protein in 3DO cells: untreated (lane 2), treated with anti-CD2 (5 μg/well) plus anti-CD3 (1 μg/well; lane 3), anti-CD2 (5 μg/well) alone (lane 4), or anti-CD3 (1 μg/well) alone (lane 5) for 20 hours. Cells were also immunoprecipitated with isotype-matched antibody (lane 1). Densitometric analysis (relative units) of the film is also reported at the bottom of the figure.
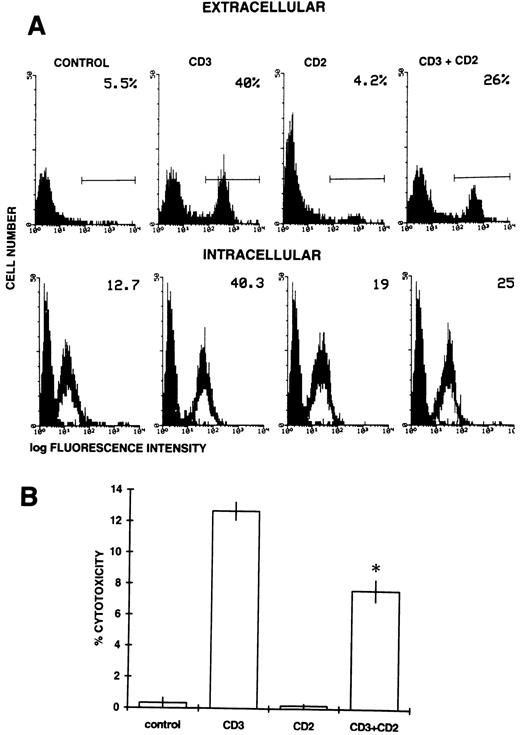
We also evaluated the effects of treatment with anti-CD2 MoAbs on Fas-L expression in untreated and anti-CD3–treated 3DO cells. Expression of Fas-L was assessed by FACScan and functional analysis using 51Cr-labeled P815 as a target population.24 An antibody that recognizes an epitope located on the extracellular portion of the Fas-L molecule was used in flow cytometry analysis. In agreement with previous reports,23 24 the results show that anti-CD3 activation induced surface expression of Fas-L. CD2 stimulation decreased this anti-CD3–induced expression of Fas-L (Fig 8A, top panel).
(A) Extracellular (top panel) and intracellular (bottom panel) expression of Fas-L. Anti-CD3 (1 μg/well) and/or anti-CD2 (5 μg/well) MoAbs were allowed to adhere in 96-well flat-bottomed plates. 3DO cells were cultured for 20 hours on coated plates and then stained with either anti–Fas-L Ab against the extracellular portion or anti–Fas-L Ab against the intracellular portion of the molecule, as described in the Materials and Methods. The number in the upper right corner represents the percentage of cells expressing Fas-L (top panel) or the value of the histogram median (bottom panel). (B) Fas-L expression as evaluated by the cytotoxicity assay (see the Materials and Methods) with 3DO cells untreated or treated with anti-CD3 (1 μg/well) and/or anti-CD2 (5 μg/well) for 20 hours. The results represent the average of three experiments (each in triplicate culture). *P < .01 comparing anti-CD3 + anti-CD2–treated versus anti-CD3–treated group. E:T ratio, 25:1.
(A) Extracellular (top panel) and intracellular (bottom panel) expression of Fas-L. Anti-CD3 (1 μg/well) and/or anti-CD2 (5 μg/well) MoAbs were allowed to adhere in 96-well flat-bottomed plates. 3DO cells were cultured for 20 hours on coated plates and then stained with either anti–Fas-L Ab against the extracellular portion or anti–Fas-L Ab against the intracellular portion of the molecule, as described in the Materials and Methods. The number in the upper right corner represents the percentage of cells expressing Fas-L (top panel) or the value of the histogram median (bottom panel). (B) Fas-L expression as evaluated by the cytotoxicity assay (see the Materials and Methods) with 3DO cells untreated or treated with anti-CD3 (1 μg/well) and/or anti-CD2 (5 μg/well) for 20 hours. The results represent the average of three experiments (each in triplicate culture). *P < .01 comparing anti-CD3 + anti-CD2–treated versus anti-CD3–treated group. E:T ratio, 25:1.
To exclude that CD2 stimulation could simply affect cell surface Fas-L expression by increasing its shedding,33 an aliquot of anti-CD3– and/or anti-CD2–triggered 3DO cells was permeabilized with saponin and stained with an antibody that recognizes the intracellular portion of the molecule. The results of intracellular staining (Fig 8A, bottom panel) confirmed those of extracellular staining. We assume that the control group was negative for intracellular Fas-L, although there was a difference between the group stained with the secondary antibody alone (background) and the group stained with Fas-L plus antirabbit antibody. This false-positive may represent the nonspecific staining inherent in the system.52
The effect of CD2 engagement was also evident when Fas-L expression was evaluated as the ability to induce cytotoxicity in a Fas-L-sensitive target, P815, in a 51Cr-release assay. Lysis of the target cells was observed after 16 hours of incubation, when 3DO cells were activated with anti-CD3 antibody. Killing was not detected in the control group or in the group treated with anti-CD2 alone. Significant inhibition of the lysis was observed when the cells were treated with both antibodies (Fig 8B).
Similar results were obtained with normal lymphocytes. As previously reported, chronic stimulation is required for the Fas-L expression and apoptosis induction in primary lymphocytes.49 53 In fact, as shown in Fig 9, anti-CD3 stimulation induced cell death only after 5 days of culture, when Fas was upregulated and Fas-L was induced. This effect was partially reverted by CD2 ligation (Fig 9).
Effect of anti-CD2 MoAb on primary lymphocytes activated with anti-CD3 MoAb. Anti-CD3 (1 μg/well) and/or anti-CD2 MoAb (1 μg/well) were allowed to adhere in 96-well flat-bottomed plates as described above. T lymphocytes (2 × 105/well) were cultured for different times on coated plates in the presence of 100 U/mL IL-2. Results are expressed as (A) the percentage of apoptosis (broad hypodiploid peak), (B) the median of Fas expression, or (C) the percentage of Fas-L+ cells and are the mean of three separate experiments. The standard errors (<10%) are omitted for clarity. *P < .01, significant inhibition comparing anti-CD3 + anti-CD2–treated with anti-CD3–treated group.
Effect of anti-CD2 MoAb on primary lymphocytes activated with anti-CD3 MoAb. Anti-CD3 (1 μg/well) and/or anti-CD2 MoAb (1 μg/well) were allowed to adhere in 96-well flat-bottomed plates as described above. T lymphocytes (2 × 105/well) were cultured for different times on coated plates in the presence of 100 U/mL IL-2. Results are expressed as (A) the percentage of apoptosis (broad hypodiploid peak), (B) the median of Fas expression, or (C) the percentage of Fas-L+ cells and are the mean of three separate experiments. The standard errors (<10%) are omitted for clarity. *P < .01, significant inhibition comparing anti-CD3 + anti-CD2–treated with anti-CD3–treated group.
Taken together, these results indicate that anti-CD2–induced protection correlates with downmodulation of Fas and Fas-L expression induced by TCR-triggering.
CD2-Stimulation Does Not Rescue 3DO Cells From Anti-Fas–Induced Apoptosis
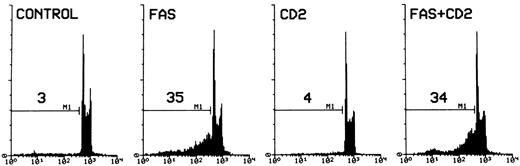
To determine whether CD2 could inhibit apoptosis induced by ligation of Fas, 3DO cells were treated with agonist anti-Fas MoAbs and then plated on wells coated with an antibody to IgG in the presence or absence of CD2 MoAbs. According to previous data, Fas MoAb induces apoptosis in 3DO cells.46 CD2 stimulation failed to inhibit the Fas-induced death of hybridoma T cells (Fig 10), suggesting that CD2 delivers signals that could affect the expression of TCR-mediated Fas and Fas-L, but did not interfere directly with the Fas cell death program.
Fas-induced apoptosis of 3DO cells. Ninety-six–well plates were coated with an antibody to hamster IgG (5 μg/well) with or without an antibody to CD2 (5 μg/well). Cells were incubated for 30 minutes with medium alone or medium containing anti-Fas MoAb (10 μg/mL). Apoptosis was evaluated by flow cytometric analysis of PI-stained nuclei after 20 hours of culture. The percentage of apoptotic nuclei is indicated in each histogram. PI fluorescence versus number of nuclei is shown.
Fas-induced apoptosis of 3DO cells. Ninety-six–well plates were coated with an antibody to hamster IgG (5 μg/well) with or without an antibody to CD2 (5 μg/well). Cells were incubated for 30 minutes with medium alone or medium containing anti-Fas MoAb (10 μg/mL). Apoptosis was evaluated by flow cytometric analysis of PI-stained nuclei after 20 hours of culture. The percentage of apoptotic nuclei is indicated in each histogram. PI fluorescence versus number of nuclei is shown.
DISCUSSION
Ag-induced extrathymic tolerance is reached by elimination of mature T cells in peripheral lymphoid organs.20,21,54 Clonal activation and/or expansion is due to a balance between apoptotic and activating signals and it has been proposed that AICD serves to limit the expansion of an immune response by eliminating lymphocytes that are no longer needed.28 Several studies have shown that one of the key events in AICD is the expression and interaction of Fas/Fas-L and that interference with the Fas/Fas-L system inhibits TCR-mediated apoptosis.23-25,27 30 Therefore, Fas/Fas-L is one of the systems by which specific clonal expansion is self-controlled.
Because costimulatory signals delivered by the interaction of T-cell surface receptors with their ligands on antigen presenting cells are required for antigen-specific T-cell activation55 56 and because overlapping signal transduction pathways are involved in both apoptotic and proliferative events, it is reasonable to suggest that costimulatory molecules play a role in controlling TCR-mediated apoptosis.
It is now well established that CD2/CD58 interaction can enhance the MHC class I- and class II-restricted T-cell antigen recognition and also have a signalling function, triggering T-cell activation or controlling the levels of Ag TCR-driven activation.34-37,40,43 Furthermore, it has been proposed that ligation of CD2 inhibits apoptosis induced by the interaction of the human immunodeficiency virus (HIV)-envelope glycoprotein gp120 with the CD4 molecule.57
Our aim was to determine whether CD2 could control the levels of AICD. We used a T-cell hybridoma in which AICD is mediated by Fas/Fas-L interaction46 (Fig 4) and compared the results with those obtained using a nontransformed CD3+ CD2+ T-cell clone. Murine T-cell hybridomas are considered to be a useful experimental model for studying apoptotic mechanisms because they undergo apoptosis after TCR triggering so mimicking the physiologic process of clonal deletion. Hybridomas, as well as previously activated T cells, respond to signals delivered by TCR with cell suicide and/or fratricide cell death.19,58 59
Our results indicate that CD2 stimulation by anti-CD2 MoAbs leads to a substantial dose-dependent inhibition of TCR-induced apoptosis, as evaluated by the flow cytometry assay with PI-labeled cells and TdT assay (Figs 1 and 2). CD2-induced rescue from AICD correlated with a decrease of Fas and Fas-L expression induced by CD3 triggering (Figs 5, 7, and 8). Similar results were obtained using primary lymphocytes after 5 days of chronic stimulation, when they upregulate Fas and express Fas-L (Fig 9). Furthermore, in agreement with previous results,42 our data show that CD2 decreases 3DO activation evaluated by IL-2 production and IL-2R expression (Table 1). These results suggest that activation and deletion pathways may share common mediators.
However, CD2 did not rescue 3DO cells from Fas-induced cell death (Fig 10), suggesting that CD2 could interfere with the CD3-, but not with the Fas-signal transduction pathway. The inhibition of CD3-induced expression of Fas/Fas-L could be the mechanism responsible for the CD2-mediated effect. Based on studies performed on cell models in which T-cell activation is induced by CD3 and/or CD2 triggering,36-38,40 it can be hypothesized that an interaction at the membrane level is responsible for the inhibition of apoptosis.60,61 However, this effect could also be due to modulation of the kinases activated by TCR triggering62 or to a direct control of Fas /Fas-L expression at the transcription or posttranscription level. To assess if the phenomenon studied above was specific for TCR-mediated apoptosis, we performed experiments using a different apoptotic signal such as DEX that induces cell death by a mechanism distinct from that activated by the TCR/CD3 complex.10,15,16,19 Triggering of CD2 failed to inhibit DEX-induced apoptosis (Fig 3), further suggesting that CD2 protection is specific for TCR/CD3-induced cell death. As shown in previous reports, DEX treatment downmodulated Fas expression24 and CD2 ligation did not affect the DEX-induced decrease of Fas expression (Fig 6).
Even though CD2 countered CD3-induced upregulation of Fas to the levels of untreated controls (Fig 4), its effect on apoptosis was only partial (35% in anti-CD3–treated, 18% in anti-CD3 and anti-CD2–treated, and 6.5% in untreated controls). This may be due to the fact that AICD is also controlled by the expression of Fas-L.24,25 CD2 stimulation only in part counteracted CD3-induced expression of Fas-L (Fig 8). Furthermore, triggering of CD2 may not influence other concomitant apoptotic signals, such as those delivered by TNF, which also mediates TCR/CD3-induced apoptosis.63
Although the in vivo importance of our results remains to be established, these data suggest that CD2 may be involved not only in the physiologic control of T-cell activation, but also in modulating lymphocyte death. This protective activity could be important in terms of immunologic memory by contributing to the survival of CD2+ immune T cells. In fact, although previously activated T cells are susceptible to Fas-mediated apoptosis, only a portion of these cells undergo AICD.22 This would suggest that APC provide a critical costimulatory signal(s) that can modulate Fas/Fas-L and therefore control the level of AICD. We suggest that one of these APC-derived signals could be delivered by CD2 triggering.
This CD2-mediated effect may also have some clinical relevance. Results obtained with lpr and gld mice suggest that autoimmune diseases could be mediated by a defect of Fas-induced apoptosis,64 and the involvement of the Fas/Fas-L system in human diseases is worthy of investigation. In particular, Fas antigen is found on various lymphoma and leukemia cells as well as on lymphoblastoid cells transformed with human T-lymphotropic virus type 1 (HTLV-1) or Epstein-Barr virus.65 Moreover, recent findings suggest that an abnormal Fas-mediated cell death could be involved in the pathogenesis of acquired immunodeficiency syndrome.66 Therefore, the modulation of the Fas/Fas L system may represent a target for new therapeutic strategies.
Supported by the Italian Association for Cancer Research (AIRC), by Progetto Strategico ‘Ciclo Cellulare e Apoptosi’ CNR Italy, and by PFACRO.
Address reprint requests to Carlo Riccardi, PhD, MD, Section of Pharmacology Department of Clinical Medicine, Pathology, and Pharmacology, Via del Giochetto, 06100 Perugia, Italy.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal