Coffey et al have conducted a study demonstrating the significant impact of γ-secretase inhibition (GSI) on the bone marrow microenvironment of patients with multiple myeloma (MM) undergoing B-cell maturation antigen (BCMA)-targeted chimeric antigen receptor (CAR) T-cell therapy, which they describe in this issue of Blood.1 Their γ-secretase investigation provides another piece of evidence in solving the case of resistance to BCMA-directed therapies, especially for patients without genomic BCMA alterations.
Targeting BCMA with CAR T cells, bispecific T-cell engagers (TCEs), or antibody drug conjugates (ADCs) has significantly advanced the treatment of relapsed or refractory multiple myeloma (RRMM). However, resistance to BCMA-directed therapies is an increasing, urgent, unmet medical need. Previous studies have reported mutational and epigenetic processes that lead to expression of structurally altered BCMA or the complete loss of BCMA on myeloma cells.2-5 However, clinical experience demonstrated that genetic alterations to the BCMA locus on chromosome 16 are not the universal mode of resistance. This might be especially the case for CAR T-cell therapies, since the selective pressure on malignant plasma cells imposed by the anti-BCMA agent is limited to the few months in which CAR T cells are circulating within a patient’s body, in contrast to the continuous exposure with TCE or ADC, which can continue possibly for years. The reported incidence of BCMA-negative relapse after CAR T-cell therapy is less than 10% and almost 50% after bispecific TCE.6
Shedding of intact BCMA mediated by γ secretases decreases surface expression of BCMA on plasma cells,7 providing a possible mode of resistance for patients without genomic BCMA alterations. In their study, Coffey et al explore how GSI, when combined with CAR T-cell therapy, modulates the MM tumor and immune microenvironment. In a phase 1 clinical trial, the GSI crenigacestat was given after leukapheresis and before lymphodepletion (LDP), as well as on day 1 following infusion of FCARH143 anti-BCMA CAR T cells for patients with RRMM. Crenigacestat increased plasma cell BCMA surface density and lowered soluble BCMA (sBCMA) levels. Progression-free survival (PFS) was 11 months in the entire cohort and 29 months for BCMA-naive patients.8
To analyze the effect of GSI, bone marrow samples were collected at screening before leukapheresis and after 5 days of crenigacestat before LDP. Samples were subjected to single-nuclei RNA sequencing coupled with a single-nuclei assay for transposase-accessible chromatin sequencing. Analyses of cell-type frequencies before and after GSI revealed that nonclassical monocytes were significantly reduced after therapy. Importantly, quantification of cell types with single-cell sequencing techniques correlated with results from flow cytometry. Next, the authors demonstrated that GSI primarily impacted expression of genes targeted by NF-κB family members in tumor cells. Cell-cell interactions with nonclassical monocytes were predominantly altered after GSI.
Given the previously mentioned importance of mono- and biallelic alterations of the BCMA locus on treatment outcomes of CAR T-cell therapies, the authors performed a detailed analysis of BCMA expression and the impact of GSI. Malignant plasma cells showed a higher expression of BCMA compared with healthy plasma cells. A preexisting deletion of the TNFRSF17 locus was identified in 2 patients, leading to reduced TNFRSF17 expression in their tumor cells. Importantly, TNFRSF17 copy numbers and BCMA protein expression correlated with PFS. Although GSI exposure resulted in reduced BCMA shedding from plasma cells, it did not alter TNFRSF17 gene expression.
One of the most remarkable findings of the study by Coffey et al was the immediate reduction of sBCMA in serum with GSI (see figure). Through a comprehensive, close monitoring pharmacokinetic analysis of sBCMA levels in the serum during the first few hours following exposure to the GSI, researchers demonstrated that a significant reduction was achieved as early as 2 hours after treatment with crenigacestat. This reduction increased hourly during the first day of GSI, reaching its maximum reduction after 3 doses and 96 hours. This is a very important finding and supports results from a recent study demonstrating that sBCMA levels correlate with primary refractoriness to BCMA-directed therapies. In the study by Lee and colleagues,9 higher levels of baseline sBCMA were associated with adverse outcome after teclistamab therapy. Using whole-genome sequencing and in vitro assays, the researchers were able to show that sBCMA, tumor burden, surface BCMA expression, and TCE dosage, and the interplay between them, were major determinants of response and resistance to BCMA-directed TCE therapies. In agreement, Coffey et al found that GSI overcame higher baseline levels of sBCMA.
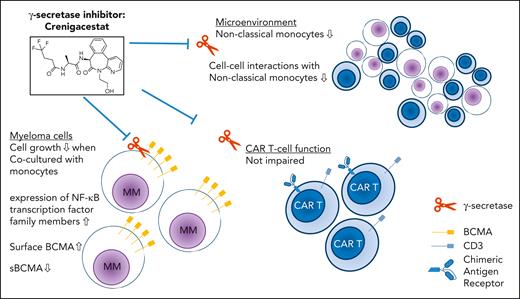
Effects of GSI inhibition with crenigacestat in the context of anti-BCMA CAR T cells. Although crenigacestat increased surface expression of BCMA on myeloma cells and decreased levels of soluble BCMA, expression of genes targeted by NF-κB family members was altered. Coculture of myeloma cells with monocytes in the presence of GSI inhibited myeloma cell growth. Changes in the microenvironment included decreased numbers and cell-cell interaction of nonclassical monocytes. Importantly, CAR T-cell function was not impaired by GSI.
Effects of GSI inhibition with crenigacestat in the context of anti-BCMA CAR T cells. Although crenigacestat increased surface expression of BCMA on myeloma cells and decreased levels of soluble BCMA, expression of genes targeted by NF-κB family members was altered. Coculture of myeloma cells with monocytes in the presence of GSI inhibited myeloma cell growth. Changes in the microenvironment included decreased numbers and cell-cell interaction of nonclassical monocytes. Importantly, CAR T-cell function was not impaired by GSI.
Coffey et al demonstrate that GSI has positive effects on surface antigen expression on target cells and sBCMA serum levels, which are important for outcomes after BCMA-directed TCE therapies. Beyond effects on myeloma cells, GSI have multiple effects on the surrounding tumor microenvironment. Importantly, these effects do not hamper T-cell function.10 This study presents compelling evidence that GSI has potential as a therapeutic adjunct to enhance the efficacy of BCMA-targeted therapies by mitigating resistance mechanisms. This study also underscores the importance of understanding the broader effects of GSI on the immune microenvironment, particularly on monocyte populations and key signaling pathways. These findings pave the way for future research into optimizing GSI dosing and combination strategies to improve patient outcomes in MM.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal