In this issue of Blood, Eckmann et al use preclinical models of multiple myeloma (MM) to investigate the mode of action of forimtamig, a novel GPRC5D-targeting T-cell–bispecific antibody (TCB) with a 2+1 format and bivalent binding to GPRC5D.1
In recent years, the G protein–coupled receptor, class C, group 5, member D (GPRC5D), has gained increased attention as a novel immunotherapeutic target for the treatment of patients with MM. GPRC5D is a 7-pass transmembrane receptor protein with a short extracellular N-terminal domain, primarily expressed in plasma cells and hard keratinized tissues, and highly enriched in malignant plasma cells.2 Therefore, several anti-GPRC5D therapeutic strategies, including chimeric antigen receptor T-cell and TCB approaches, have been developed.3
Talquetamab is the first GPRC5D-targeted TCB approved for the treatment of MM. It has a symmetrical antibody design with monovalent binders for CD3 and GPRC5D. In the phase 1/2 MonumenTAL-1 trial it has demonstrated promising results, with an overall response rate of 71% in patients with triple-class-exposed relapsed/refractory multiple myeloma (RRMM) and manageable safety profiles, including oral toxicity and skin/nails changes related to on-target off-tumor effects, and lower infection rates when compared with B-cell maturation antigen (BCMA)-targeted TCB.4,5 On the basis of these encouraging results, talquetamab is currently under evaluation in combination with other anti-MM drugs such as daratumumab, pomalidomide, or the BCMA-targeted TCB teclistamab.
Forimtamig is a novel TCB targeting GPRC5D that features a unique 2:1 asymmetrical configuration with bivalent GPRC5D binding sites. It is currently under clinical evaluation and the initial results coming from an ongoing phase 1 clinical study have indicated its high activity in patients with heavily pretreated RRMM when administered as an intravenous or subcutaneous formulation.6 Dermal and oral toxicity was also reported but managed with dose delays and/or reductions.6
The mechanism of TCB action against tumor cells is based on the ability of simultaneous binding to target antigen on the tumor and CD3 on the T cells. This leads to the formation of immunological synapses between them, ultimately resulting in lysis of the tumor cell.7 The TCB design and structure, including antibody geometry, size, flexibility, target affinity, and avidity, together with targeted tumor antigen copy number, size, and distance of epitope to membrane, have been described to influence their efficacy.8 In terms of affinity, although it is important to be low for the fragment targeting CD3 to avoid T-cell activation in the absence of target cells and to decrease the level of CRS, the affinity (as well as avidity) to the target antigen is essential and constitutes an important factor mediating TCB activity. The CrossMAb platform, which ensures correct pairing of antibody light and heavy chains to improve the stability and functionality of bispecific antibodies, has allowed the development of bivalent TCB structures and multivalent TCB with high affinity to the target antigen.9 This has been further improved by using avidity in multivalent antibody formats, such as with foramtamig, which has a novel 2:1 format, bivalent to GPRC5D and monovalent to CD3.8
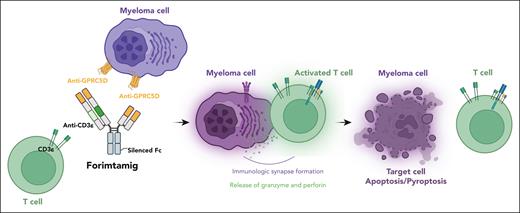
In their study, Eckmann et al performed binding studies and show how forimtamig binds with high avidity to the N-terminus of human GPRC5D, inducing formation of stable synapses between T and tumor cells, irrespective of target expression levels (see figure). As a result of its unique configuration and bivalent binding to GPRC5D, forimtamig has higher in vitro and in vivo potency to induce tumor cell killing and T-cell activation as compared with the 1+1 format GPRC5D TCB and anti-BCMA TCB. In addition, the authors demonstrate that the combination of forimtamig with novel anti-MM agents, including BCMA TCB and cereblon E3 ligase modulatory drugs, was potent and prevented the emergence of GPRC5D-negative tumor relapse.
A schematic representation of the forimtamig structure and mode of antimyeloma action. Figure generated using BioRender.com.
A schematic representation of the forimtamig structure and mode of antimyeloma action. Figure generated using BioRender.com.
This finding has great clinical significance because it is now evident that the gene aberration of GPRC5D correlates with the efficacy of MM immunotherapy and antigenic loss due to biallelic loss or more often convergent evolution leading to biallelic antigenic exclusion are often seen in patients progressing on talquetamab.10 Therefore, the combination of anti-GPRC5D TCB with other anti-MM agents with known direct antitumor activity and immunomodulatory effects such as daratumumab or the cereblon E3 ligase modulatory drugs, should be explored to increase the depth and durability of response seen with these therapies.
Similarly, T-cell–redirecting therapies targeting simultaneously or sequentially 2 or more antigens on tumor cells could also enhance antitumor activities and improve long-term efficacy, as well as potentially reduce the incidence of antigen-negative escape. In this setting, a careful evaluation of the risks and adverse events related to on-target off-tumor effects is also critical to avoid unnecessary toxicity. Dynamic surveillance for antigen escape and functional assessment of T-cell fitness will also be necessary to elucidate the optimal treatment regimen and sequence to use when treating patients with MM.
In conclusion, Eckmann et al have elegantly described the novel and unique mode of action of forimtamig (see figure). They support a “doubling-down” design with the bivalent targeting of tumoral antigen. Importantly, they highlight, as also shown with anti-BCMA TCBs,10 that not all TCBs targeting the same antigen are created equal. Target valency, affinity, and avidity, as well as antibody geometry and flexibility, render unique properties to these TCBs that translate into differential cross-resistance to targeted antigen mutants.
In future clinical studies, TCBs’ properties, along with the surveillance profiling for the emergence of targeted antigen mutant clones, will need to be accounted for in selecting, sequencing, and possible retreating with these molecules, even when targeting the same antigen.
Conflict-of-interest disclosure: P.N. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal