In this issue of Blood, Purvis et al1 have demonstrated that most children with B-lineage acute lymphoblastic leukemia (B-ALL) within the good-risk category can be successfully treated with low-intensity therapy, thus reducing the side effects from therapy-related toxicity. Childhood B-ALL is a success story of modern medicine, with survival rates exceeding 90%.2 Thus, there has been ongoing debate about the merits of de-escalation of therapy for patients with B-ALL, without impacting on outcomes. Purvis et al discuss this dilemma in relation to more than 500 patients treated on the St. Jude XV and XVI protocols over a 17-year period.
In this article, the authors focus on the good-risk genetic subtypes, ETV6::RUNX1 and high hyperdiploidy, which account for more than 50% of patients with B-ALL. Essentially, the refined risk stratification algorithm applied to these St. Jude protocols accurately identified which subgroups of this good-risk cohort will truly benefit from reduced-intensity therapy, which they labeled as St. Jude low-risk patients. The risk classification was further refined by minimal (measurable) residual disease (MRD) response, as indicated in the figure, which shows the relationship of this specific classification to the widely used National Cancer Institute (NCI) criteria. As previously reported,3,4 in these St. Jude trials, both ETV6::RUNX1 and high hyperdiploid-positive patients had excellent outcomes, when compared with the other B-ALL subtypes. Specifically for ETV6::RUNX1+ patients, their outcomes were excellent regardless of NCI or St. Jude risk group, utilizing MRD-directed therapy. A likely explanation is the rapid MRD clearance of these patients during the induction phase of treatment.5 That certain studies have reported good response with reduced intensity therapy specifically for ETV6::RUNX1 patients,6,7 including the results from Purvis et al, provides strong evidence that ETV6::RUNX1+ patients are ideal candidates for therapy de-escalation.
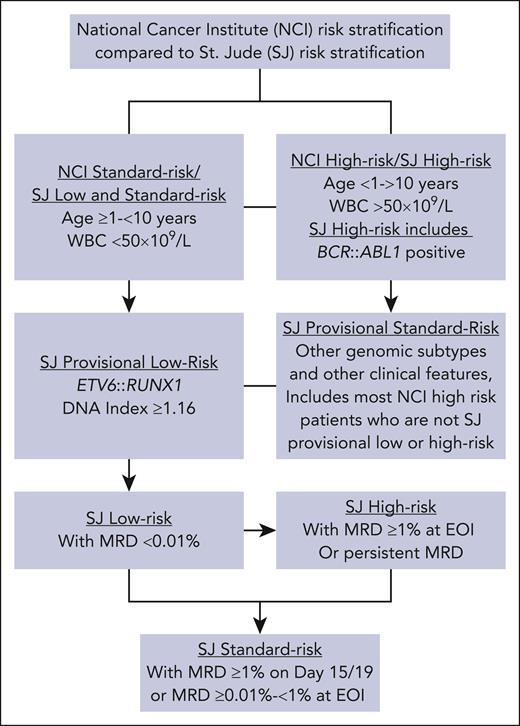
This figure explains the St. Jude (SJ) risk stratification used in this study and shows how it compares with NCI risk stratification. SJ classifies patients as low risk, standard risk, or high risk. Patients with B-ALL who were ≥1 year but <10 years of age with a white blood cell (WBC) count of <50 × 109/L at presentation (equivalent to NCI standard risk), in addition to those who had the ETV6::RUNX1 fusion or a DNA index ≥1.16, were classified as provisional low risk. Patients with BCR::ABL1 were considered high risk. The remaining patients, including those with TCF3::PBX1, hypodiploidy (<44 chromosomes), central nervous system-3 status (≥5 leukocytes/μL of cerebrospinal fluid with blasts or cranial palsy), or testicular leukemia at diagnosis, were considered provisional standard risk, which category includes most NCI high-risk patients who are not classified as provisional SJ low risk or high risk. In SJ trials, MRD levels were evaluated by flow cytometry in bone marrow samples collected on day 19 (in Total XV) or day 15 (in Total XVI) and at the end of remission induction and were used for final risk classification. Provisional low-risk patients with MRD ≥ 1% on day 15 or day 19 of induction or MRD of 0.01% to <1% at the end of induction were classified as standard risk. Patients with MRD ≥1% at the end of induction or persistent MRD during the consolidation phase were classified as high risk. EOI, end of induction.
This figure explains the St. Jude (SJ) risk stratification used in this study and shows how it compares with NCI risk stratification. SJ classifies patients as low risk, standard risk, or high risk. Patients with B-ALL who were ≥1 year but <10 years of age with a white blood cell (WBC) count of <50 × 109/L at presentation (equivalent to NCI standard risk), in addition to those who had the ETV6::RUNX1 fusion or a DNA index ≥1.16, were classified as provisional low risk. Patients with BCR::ABL1 were considered high risk. The remaining patients, including those with TCF3::PBX1, hypodiploidy (<44 chromosomes), central nervous system-3 status (≥5 leukocytes/μL of cerebrospinal fluid with blasts or cranial palsy), or testicular leukemia at diagnosis, were considered provisional standard risk, which category includes most NCI high-risk patients who are not classified as provisional SJ low risk or high risk. In SJ trials, MRD levels were evaluated by flow cytometry in bone marrow samples collected on day 19 (in Total XV) or day 15 (in Total XVI) and at the end of remission induction and were used for final risk classification. Provisional low-risk patients with MRD ≥ 1% on day 15 or day 19 of induction or MRD of 0.01% to <1% at the end of induction were classified as standard risk. Patients with MRD ≥1% at the end of induction or persistent MRD during the consolidation phase were classified as high risk. EOI, end of induction.
Although MRD and not individual chromosome gains are the subject of this study, in addition to cytogenetic analysis, fluorescence in situ hybridization to identify double and triple trisomies were used to define high hyperdiploidy. In the St. Jude trials, DNA index ≥1.16 by flow cytometry was also performed. Notably, specific subgroups of high hyperdiploid ALL have been shown to have inferior outcomes. For example, as reported by Purvis et al, high hyperdiploid cases with DNA index <1.16 had an overall worse outcome than patients with DNA index ≥1.16. In UK Childhood ALL clinical trials (UKALL), it was reported that high hyperdiploid patients with gains of chromosomes 5 and 20 had inferior outcome compared with high hyperdiploid cases without these chromosomal gains.8 As the individual chromosomes are unidentified by DNA index, the findings between the UKALL data and these from DNA index cannot be compared.
Although, generally speaking, high hyperdiploid patients have excellent outcomes, among high hyperdiploid patients in the St. Jude trials, the subgroup classified as NCI high risk and St. Jude standard/high risk was shown to have an inferior outcome. Associated with the fact that the St. Jude standard/high-risk category is defined by high MRD, this observation is likely related to the slower blast clearance during induction in these patients. If cytogenetic data were available, it would be of interest to know whether patients in this group harbored chromosomes 5 and 20 among the chromosomal gains to correlate the St. Jude standard/high-risk category with the UKALL inferior-risk high hyperdiploid group.
This article indicates that alternative therapies should be considered for the NCI high-risk/St. Jude standard or high-risk high hyperdiploid patients as their outcomes were inferior even with intensified conventional therapy. For example, sensitivity of high hyperdiploid cells to venetoclax has been demonstrated and St. Jude, based on the findings of this study,1 now treat St. Jude standard or high-risk high hyperdiploid patients with the immunotherapy blinatumomab. This approach will be introduced into the next new St. Jude high-risk B-ALL protocol. Those high hyperdiploid patients classified as low risk will continue to be treated with low-intensity chemotherapy only, with the added advantage of lower rates of adverse therapy-related effects.
This article concludes that although the majority of NCI high-risk patients with ETV6::RUNX1 and high hyperdiploidy can be successfully treated with low-intensity therapy, all patients benefit from the incorporation of MRD measurement into risk stratification algorithms. It is important to follow the refined risk stratification procedure presented here to successfully identify the NCI high-risk/St. Jude standard or high-risk high hyperdiploid group with inferior outcome to have the opportunity to offer alternative therapies to these patients. Although MRD measurement is now routinely applied in many ALL protocols,9 it is important to validate that the outcomes observed in the St. Jude high hyperdiploid risk groups are comparable between different clinical trials.
Conflict-of-interest disclosure: C.J.H. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal