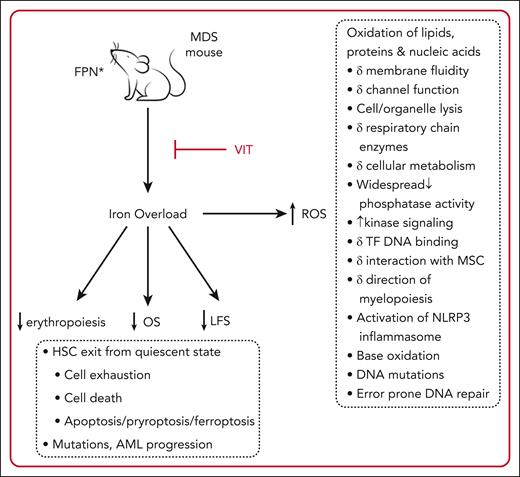
In this issue of Blood, Antypiuk et al, utilizing a murine model, demonstrate that iron overload (IOL), accumulated via a mutation in the iron importer ferroportin, accelerates the myelodysplastic syndrome (MDS) phenotype, leading to exacerbation of marrow failure, inferior leukemia-free survival, and inferior overall survival (OS).1 Conversely, blocking iron uptake with vamifeport (VIT) reverses these effects, resulting in improved erythropoiesis, superior leukemia-free survival, and superior OS (see figure). Finally, the addition of luspatercept to VIT had an additive beneficial effect on erythropoiesis.
Effects of IOL in a murine model of MDS. FPN mutation leads to increased GI iron absorption and IOL, in turn leading to inferior erythropoiesis, LFS, and OS. IOL results in formation of ROS, an indicator of oxidative stress. Cellular processes impacted by oxidative stress in preclinical studies are listed in the dashed boxes and lead to HSC death or mutation with MDS progression.2 These effects are reversed by blocking FPN with VIT. Luspatercept plus VIT has an additive beneficial effect on erythropoiesis. ∗, C326S mutation; AML, acute myeloid leukemia; FPN, ferroportin; HSC, hematopoietic stem cell; LFS, leukemia-free survival, MSC, mesenchymal stromal cells; ROS, reactive oxygen species; TF, transcription factor.
Effects of IOL in a murine model of MDS. FPN mutation leads to increased GI iron absorption and IOL, in turn leading to inferior erythropoiesis, LFS, and OS. IOL results in formation of ROS, an indicator of oxidative stress. Cellular processes impacted by oxidative stress in preclinical studies are listed in the dashed boxes and lead to HSC death or mutation with MDS progression.2 These effects are reversed by blocking FPN with VIT. Luspatercept plus VIT has an additive beneficial effect on erythropoiesis. ∗, C326S mutation; AML, acute myeloid leukemia; FPN, ferroportin; HSC, hematopoietic stem cell; LFS, leukemia-free survival, MSC, mesenchymal stromal cells; ROS, reactive oxygen species; TF, transcription factor.
IOL in β thalassemia major has long been recognized to adversely impact organ function and OS. Off-loading excess iron in these patients using iron chelation therapy (ICT) leads to dramatically improved clinical outcomes including a significant survival benefit.3 The impact of IOL and ICT in patients with MDS, however, has been less clear. Patients with MDS are older at diagnosis and have a shorter life expectancy during which to accumulate IOL. Although there are multiple studies indicating an improvement in some clinical end points such as cardiac events, infections, bone marrow function, and survival with ICT, the only randomized placebo-controlled trial of ICT in MDS, the TELESTO trial, was limited by slow enrollment, a younger median age compared with the usual MDS population, and a significant dropout rate from the placebo arm with patients subsequently receiving ICT.4 Despite these limitations, the results did show a significantly superior event-free survival, defined as time from date of randomization to first documented nonfatal event (related to cardiac or liver dysfunction and transformation to acute myeloid leukemia) or death, whichever occurred first, in the ICT arm. Unfortunately, the size of the study population had been reduced to the point where it was no longer powered to show an OS benefit, if present.4 Multiple preclinical studies indicate an adverse impact of IOL and resultant oxidative stress on cellular function and a mitigating effect of IOL reversal. Although these give some degree of comfort to undertaking control of IOL in patients with MDS, more definitive data are needed. Though in mice and not humans, the current report provides some proof of concept that iron status is clinically relevant.
In MDS, the usual clinical challenge is anemia. Because there are a limited number of medications that improve anemia in MDS, many patients are transfusion dependent. VIT is a twice daily oral medication currently in clinical trials in thalassemia and sickle cell anemia. Since VIT blocks iron uptake in the gastrointestinal (GI) tract and release from the reticuloendothelial system, the utility of VIT in transfusion-dependent patients with MDS may be limited. A significant subset of patients with MDS, however, have ring sideroblast subtypes or somatic mutation in the SF3B1 gene. Because these patients have expanded erythropoiesis at the erythroblast stage, erythroferrone is increased, hepcidin is suppressed, ferroportin is upregulated, and GI iron absorption proceeds.5 These patients may present with significantly elevated ferritin levels in the absence of transfusions and are prone to early parenchymal iron loading.6 This is an MDS subset, then, that might derive significant benefit from treatment with VIT, and in whom clinical trials could be undertaken. Clinical studies combining VIT with luspatercept treatment, as informed by the murine model, would also be of interest. Moreover, the comprehensive experiments done using this model create a context in which to study the effects of IOL on organ function and to test the effects of iron blockade with other medications used for MDS treatment, including ICT agents. It would be of interest in addition to investigate a possible interaction of the particular MDS-associated somatic mutations present with iron physiology.7
The data reported by Antypiuk et al add to those of Chan and colleagues, who used a radiation-induced AML mouse model to demonstrate that IOL promotes AML development.8 Results of gene expression profiling led the investigators to conclude that combined prodeath and promutagenic effects result in a biphasic dose-response relationship between AML rate and iron burden. Briefly, iron promotes leukemogenesis in vivo up to a point, with a further increase in iron concentration decreasing AML risk by increasing cell death.8
The data from these models increase confidence that IOL in patients with MDS is a significant clinical problem that aggravates anemia and the MDS phenotype. Development of MDS treatments has recently focused on targeted therapies. Whether therapies that reduce IOL specifically should also be considered targeted remains to be determined; there are numerous potential cellular and physiologic targets of iron that may be relevant (see figure). Regardless, the finding that IOL accelerates MDS progression and iron restriction improves erythropoiesis and survival outcomes are in keeping with clinical data suggesting a significance of IOL in patients with MDS. It supports the idea that management of IOL in MDS may confer clinical benefit and should be considered, when appropriate, in the standard care of MDS patients.9,10
Conflict-of-interest disclosure: H.A.L. reports honoraria from Bristol Myers Squibb (BMS) and Taiho and research funding from BMS.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal