In this issue of Blood, Voso et al1 present an analysis of long-term outcomes for 1438 patients with acute promyelocytic leukemia (APL). Their data, accumulated in the European HARMONY Platform, originate from 2 international, multicenter clinical trials (GIMEMA/AMLSG/SAL-APL0406 and NCRI-AML17) and 4 European national registries (HOVON, AMLSG, Swedish AML Registry, and SAL). This valuable resource represents the largest published APL cohort, and has been exploited to examine factors that influence both short-term outcomes, primarily early death (ED) and differentiation syndrome (DS), and long-term outcomes, including cumulative incidence of relapse, event-free survival (EFS), and overall survival (OS).
APL is distinguished from other members of the broader family of acute myeloid leukemia (AML) by its unique molecular lesion involving a t(15;17) reciprocal translocation, rearrangement of the PML and RARA genes, and formation of a PML::RARα fusion protein.2 PML is a multifunctional tumor suppressor that is essential for the formation of membrane-less macromolecular nuclear organelles, known as PML nuclear bodies (NBs), involved in genome maintenance, self-renewal, apoptosis, senescence, and antiviral responses.3 RARα is a nuclear hormone receptor transcription factor that binds to retinoic acid response elements in target genes and suppresses their transcription; conformational change induced by retinoic acid binding reactivates gene expression required for myeloid differentiation.4
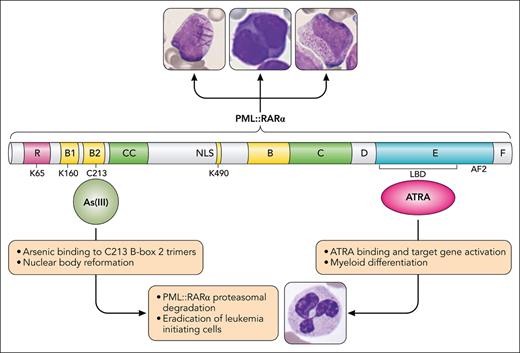
The chimeric PML::RARα fusion protein acts via both dominant negative and gain-of-function mechanisms to deregulate self-renewal and interfere with normal cell differentiation programs by disrupting NBs and by binding promiscuously to noncanonical retinoic acid response elements. The 2 most effective agents in the treatment of APL, all-trans retinoic acid (ATRA) and arsenic trioxide (ATO), counteract the consequences of the fusion protein by distinctive mechanisms (see figure). PML::RARα is insensitive to physiological amounts of retinoic acid, but pharmacological doses of ATRA restore transcriptional activation of target gene expression and cell differentiation. ATRA also activates AF2-dependent proteasomal degradation of PML::RARα.5 In contrast, As(III), the active form of arsenic trioxide, targets the PML moiety of the fusion protein. An arsenic binding pocket formed by a triad of C213 residues within PML B-box 2 trimers facilitates gel-like transition of PML and PML::RARα to induce NB reformation, and subsequent sumoylation, polyubiquitination, and 11S proteasomal degradation.6 PML::RARα degradation promotes eradication of leukemia-initiating cells, a prerequisite for cure.7
The PML::RARα fusion protein drives all the morphological variants of t(15;17) APL by disrupting nuclear bodies and blocking myeloid cell differentiation at the promyelocyte stage. Arsenic and ATRA target the PML and RARα components of the fusion protein, respectively. ATRA binds to the ligand binding domain (LBD) of RARα and induces conformational change, releasing corepressors and activating transcription of target genes involved in myeloid differentiation. As(III), the active component of arsenic trioxide, binds a pocket formed by C213 residues in PML B-box 2 trimers and induces a cascade of events resulting in nuclear body reformation, PML and PML::RARα sumoylation, polyubiquitination, and proteasomal degradation. Cellular images courtesy of the ASH Image Bank (image IDs #00005911, #00063635, and #00003608). Professional illustration by Patrick Lane, ScEYEnce Studios.
The PML::RARα fusion protein drives all the morphological variants of t(15;17) APL by disrupting nuclear bodies and blocking myeloid cell differentiation at the promyelocyte stage. Arsenic and ATRA target the PML and RARα components of the fusion protein, respectively. ATRA binds to the ligand binding domain (LBD) of RARα and induces conformational change, releasing corepressors and activating transcription of target genes involved in myeloid differentiation. As(III), the active component of arsenic trioxide, binds a pocket formed by C213 residues in PML B-box 2 trimers and induces a cascade of events resulting in nuclear body reformation, PML and PML::RARα sumoylation, polyubiquitination, and proteasomal degradation. Cellular images courtesy of the ASH Image Bank (image IDs #00005911, #00063635, and #00003608). Professional illustration by Patrick Lane, ScEYEnce Studios.
The efficacy of ATRA was initially reported by Chinese investigators,8 and ATRA was rapidly incorporated into treatment algorithms with chemotherapy. ATRA reversed the coagulopathy of APL and had a profound effect on reducing relapses. The white blood cell (WBC) count at presentation emerged as the most important determinant of ED and failure due to relapse, with high-risk (HR) disease defined as a WBC count >10 × 109/L.9 Intensification of consolidation chemotherapy for HR patients went some way toward reducing relapses, but at the cost of increased deaths in remission and long-term complications, including cardiac toxicity and therapy-related myeloid neoplasms.
The remarkable activity of ATO in APL was also a Chinese discovery,10 but concerns about toxicity and the potential for antagonism with ATRA initially slowed its adoption. However, the superiority of ATRA+ATO over ATRA+chemotherapy for standard-risk (SR) patients (WBC count ≤10 × 109/L) was clearly established by the GIMEMA/AMLSG/SAL-APL0406 and NCRI-AML17 trials. The value of ATO in HR disease has been more controversial, but several studies indicated the combination of ATRA+ATO+chemotherapy (or ATRA+ATO+gemtuzumab ozogamicin) is likely to be at least as effective (if not better) than ATRA+chemotherapy (eg, NCRI-AMLM17, ALLG-APML4, North American Leukemia Intergroup-C9710), and that impression is reinforced by preliminary data from the European Intergroup APOLLO trial.
The patients in the study by Voso and colleagues ranged widely in age (from 16 to 94 years), were treated in a mixture of clinical trial and real-world clinical practice scenarios, and >6% had therapy-related APL. Independent risk factors for ED (5.9% overall) were restricted to increasing age and HR disease. DS occurred more frequently in ATRA+ATO-treated patients, but no other significant associations were identified. This large data set provides convincing evidence that a dual-targeted attack against PML::RARα (employing ATRA+ATO) is superior to a single targeted approach (ATRA) with chemotherapy, because treatment without ATO was an independent risk factor for a higher cumulative incidence of relapse and for inferior OS and EFS. Increasing age and HR disease were also independent risk factors for OS and EFS, presumably due to their correlation with ED, but were not independent risk factors for relapse, emphasizing the importance of including ATO in the management of APL, regardless of age and risk category.
Although the size and diversity of the HARMONY data set are impressive, it has limitations attributable to differences in the spectrum of data collected by the contributing trials and registries. In particular, insufficient data regarding corticosteroid prophylaxis for DS, hemostatic support, and the extent and type of chemotherapy included for patients treated with ATRA+ATO limited the scope of the analyses. Data on late complications, particularly therapy-related myeloid neoplasms, were also unavailable. Nevertheless, the HARMONY study has convincingly corroborated the improvements in relapse rate and survival that are achievable with ATO-based therapy for patients with both SR and HR disease. Unfortunately, it has also reaffirmed the continuing problem of ED for elderly and HR patients.
Conflict-of-interest disclosure: H.J.I. served on an advisory committee for Syros.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal