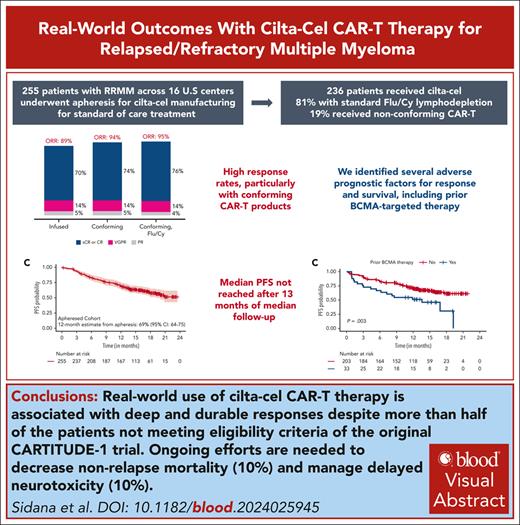
Key Points
SOC cilta-cel in RRMM results in deep and durable response despite over half the patients not meeting the CARTITUDE-1 eligibility criteria.
Close surveillance for late complications like second primary malignancies and efforts to mitigate delayed neurotoxicity and nonrelapse mortality are crucial.
Visual Abstract
Ciltacabtagene autoleucel (cilta-cel) was approved in 2022 for patients with relapsed/refractory multiple myeloma (RRMM). We report outcomes with cilta-cel in the standard-of-care setting. Patients with RRMM who underwent leukapheresis for cilta-cel manufacturing between 1 March 2022 and 31 December 2022 at 16 US academic medical centers were included. Overall, 255 patients underwent leukapheresis and 236 (92.5%) received cilta-cel, of which 54% would not have met CARTITUDE-1 eligibility criteria. In treated patients (N = 236), cytokine release syndrome was seen in 75% (grade ≥3, 5%), immune effector cell–associated neurotoxicity syndrome in 14% (grade ≥3, 4%), and delayed neurotoxicity in 10%. Overall and complete response rates were as follows: all patients who received cilta-cel (N = 236), 89% and 70%; patients receiving conforming cilta-cel (n = 191), 94% and 74%; and conforming cilta-cel with fludarabine/cyclophosphamide lymphodepletion (n = 152), 95% and 76%, respectively. Nonrelapse mortality was 10%, most commonly from infection. After a median follow-up of 13 months from cilta-cel, the median progression-free survival (PFS) was not reached, with 12-month estimate being 68% (95% confidence interval, 62-74). High ferritin levels, high-risk cytogenetics, and extramedullary disease were independently associated with inferior PFS, with a signal for prior B-cell maturation antigen–targeted therapy (P = .08). Second primary malignancies excluding nonmelanoma skin cancers were seen in 5.5% and myeloid malignancies/acute leukemia in 1.7%. We observed a favorable efficacy profile of standard-of-care cilta-cel in RRMM, despite more than half the patients not meeting the CARTITUDE-1 eligibility criteria.
Introduction
Patients with multiple myeloma (MM) commonly relapse and become resistant to multidrug regimens that often include a proteasome inhibitor, an immunomodulatory drug, and anti-CD38 antibodies, and such patients have historically had poor outcomes.1-3 Ciltacabtagene autoleucel (cilta-cel) is a camelid-derived B-cell maturation antigen (BCMA)–targeting chimeric antigen receptor (CAR) T-cell therapy with 2 high-affinity BCMA-binding single-domain antibodies on the CAR construct. It was approved by the US Food and Drug Administration (FDA) in February 2022 for the treatment of patients with relapsed or refractory MM (RRMM) after exposure to ≥4 prior lines of therapy (LOTs),4-6 based on the phase 1b/2 CARTITUDE-1 trial, which demonstrated an overall response rate (ORR) of 98%, a stringent complete response (CR) of 83%, a median progression-free survival (PFS) of 34.9 months, and a median overall survival (OS) that was not reached after a follow-up of 35 months.4,7,8
Patients treated in clinical trials often differ from those treated with standard-of-care (SOC) therapy.9,10 The CARTITUDE-1 clinical trial eligibility criteria required adequate organ function, limited comorbidities, and no prior exposure to BCMA-targeted therapy (BCMA-TT). In this multicenter retrospective study, we investigated the safety and efficacy of SOC cilta-cel for the treatment of RRMM in a real-world population.
Methods
This is a multicenter retrospective study from 16 US academic medical centers in the United States. Multiple Myeloma Immunotherapy Consortium. Each center obtained independent institutional review board approval.
Patients
All patients with RRMM who underwent leukapheresis from 1 March 2022 to 31 December 2022 with an intent to manufacture commercial cilta-cel were included. If the CAR T-cell product did not meet the FDA release criteria for an in-specification/conforming product, patients were treated under an expanded access protocol with a nonconforming/out-of-specification (OOS) CAR T-cell product. Baseline clinical and disease characteristics were abstracted retrospectively using a uniform case reporting form, and the eligibility for CARTITUDE-1 was determined at leukapheresis.
Treatment and clinical assessment
Bridging therapy was given at the discretion of the treating physician. Lymphodepleting chemotherapy was also determined by the treating physician, and a fludarabine shortage during the study period led to some patients receiving alternative lymphodepletion regimens instead of the standard regimen per package insert (fludarabine and cyclophosphamide [FluCy]). For patients with renal insufficiency, fludarabine dose was adjusted based on creatinine clearance per institutional protocols. Cytokine release syndrome (CRS) and immune effector cell associated neurotoxicity syndrome (ICANS) were graded according to American Society for Transplantation and Cellular Therapy criteria,11,12 whereas hematologic toxicities were graded by National Cancer Center Institute–Common Terminology Criteria for Adverse Events version 5.0.11,12 Toxicity management was per institutional guidelines. Response was assessed by treating investigators based on the International Myeloma Working Group criteria13 (see the supplemental Methods, available on the Blood website). High-risk cytogenetics were defined by the presence of del 17p/monosomy 17, t(4;14), and t(14;16) at any time point before CAR T-cell infusion. Extramedullary disease (EMD) was defined as myeloma involvement in soft tissues or organs, noncontiguous with bone disease. Paramedullary plasmacytomas contiguous with bone were excluded from this group. Minimal residual disease (MRD) was determined by either clonoSEQ or flow cytometry at a sensitivity of at least 10–5 nucleated cells, per institutional protocol.
Statistical analysis
The distribution of patient characteristics was examined overall and by grade ≥2 CRS, any-grade ICANS, any delayed neurotoxicity (DNT), best response of CR or better, and best ORR (partial response [PR] or better) using the χ2 or Fisher exact tests for categorical variables or the Wilcoxon rank-sum tests for continuous variables. We further investigated baseline patient characteristics (excluded Revised International Staging System [R-ISS] stage and high marrow burden due to >15% missingness) predictive of grade ≥2 CRS, any ICANS, best response of CR or better, and best ORR in multivariable logistic regression models using a stepwise variable selection approach. Due to the small number of patients with any DNT, multivariable models were not performed for this outcome.
OS was calculated as the time between the date of CAR T-cell infusion and the date of death from any cause or last contact, and PFS was calculated as the time between the date of CAR T-cell infusion and the date of progression, death, or last contact, whichever occured first. Kaplan-Meier survival curves were used to estimate overall PFS and OS, and log-rank tests were used to compare PFS of patients based on selected characteristics including prior BCMA-TT, high-risk cytogenetics, EMD, plasma cell leukemia (PCL), Eastern Cooperative Oncology Group (ECOG) performance status at lymphodepletion, age at infusion (≥70 vs <70 years), type of lymphodepleting chemotherapy, bridging therapy, response to bridging therapy, and meeting the CARTITUDE-1 eligibility criteria. Using Cox proportional hazard regression, we used a similar stepwise selection approach to investigate baseline patient characteristics associated with PFS and OS. The proportional hazard assumption was tested using covariate x time interaction terms individually and collectively. No violations of proportional hazards were observed. All analyses were conducted using R (Version 4.1.2.) or SAS (Version 9.4).
Results
Patient characteristics and disposition
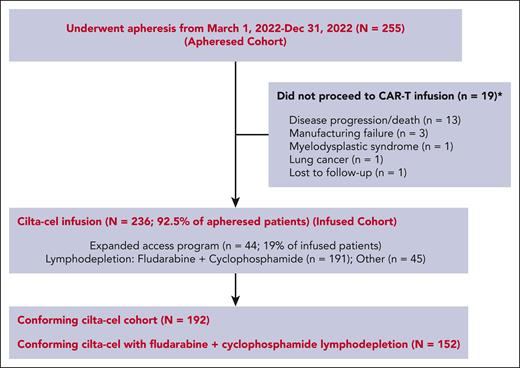
Between 1 March 2022 and 31 December 2022, a total of 255 patients with RRMM underwent apheresis with the intent to manufacture SOC cilta-cel. Of these, 236 patients (92.5%) received cilta-cel infusion as shown in Figure 1 (infused cohort). Nineteen patients did not receive cilta-cel due to complications from disease progression/death before infusion (n = 13), manufacturing failure (n = 3), interval development of myelodysplastic syndrome (n = 1), development of lung cancer (n = 1), and being lost to follow-up (n = 1). The manufacturing failure rate on first attempt was 6% (n = 15). Second manufacturing was attempted in all 15 patients and was successful in 12 patients, with an overall manufacturing failure rate of 1% (n = 3). Among patients who received infusion, 44 (19%) received an OOS CAR T-cell product. The median time from apheresis to infusion was 70 days (range, 36-275) for all patients and 65 days (36-275) for patients with a conforming product. The median CAR T-cell dose was 0.6 × 106 CAR T cells per kg of body weight (range, 0.1 × 106 to 1.0 × 106). Seven patients received CAR T-cell dose of <0.4 × 106 per kg. The median follow-up from apheresis was 15.6 months (range, 2.3-23.5) and from infusion was 13.0 months (range, 0.3-21.8).
Table 1 describes the baseline characteristics and treatment history of 255 patients who underwent apheresis and 236 patients who underwent CAR T-cell infusion. Among patients who underwent apheresis, 56% of patients would not have met the eligibility criteria for CARTITUDE-1 clinical trial, mainly due to comorbidities or treatment history, including cytopenias (18%), organ dysfunction (13%; including 22 [9%] with renal impairment), prior BCMA-TT (15%), poor performance status (11%), history of PCL, POEMS (polyneuropathy, organomegaly, endocrinopathy, monoclonal gammopathy, and skin changes) syndrome, light chain amyloidosis, or Waldenstrom macroglobulinemia (11%), central nervous system pathology (5%), or oligo/nonsecretory disease (12%). Thirty-three percent of patients undergoing apheresis were ineligible for >1 reason.
Baseline characteristics and treatment of patients who underwent apheresis for SOC cilta-cel manufacturing and those receiving cilta-cel infusion
| Characteristic . | N = 255 (apheresis) . | N = 236 (infused) . |
|---|---|---|
| n (%) or median (range) . | n (%) or median (range) . | |
| Age, y | 64 (30-84) | 64 (30-84) |
| Age ≥70 y | 65 (25%) | 62 (26%) |
| Male sex | 145 (57%) | 134 (57%) |
| Race and ethnicity | ||
| Non-Hispanic White | 190 (75%) | 177 (76%) |
| Hispanic (any race) | 20 (8%) | 19 (8%) |
| Black | 30 (12%) | 26 (11%) |
| Asian/Pacific Islander | 8 (3%) | 7 (3%) |
| American Indian/Alaskan Native | 1 (0.4%) | 1 (0.4%) |
| Other | 4 (1.6%) | 4 (1.7%) |
| Unknown | 2 | 2 |
| ECOG PS | At CAR T-cell evaluation | At lymphodepletion |
| 0-1 | 215 (92%) | 183 (89%) |
| 2-4 | 19 (8%) | 23 (11%) |
| Unknown | 21 | 30 |
| Myeloma subtype | ||
| Intact immunoglobulin | 184 (72%) | 170 (72%) |
| Light chain | 40 (16%) | 39 (17%) |
| Oligo/nonsecretory | 31 (12%) | 27 (11%) |
| R-ISS disease stage | ||
| I | 44 (27%) | 43 (28%) |
| II | 87 (53%) | 81 (53%) |
| III | 34 (21%) | 30 (19%) |
| Unknown | 90 | 82 |
| EMD | 66 (26%) | 60 (26%) |
| Unknown | 2 | 1 |
| High marrow burden, BMPCs ≥50% | 39 (19%) | 35 (18%) |
| Unknown | 46 | 39 |
| Cytogenetic abnormality | ||
| Any high-risk cytogenetics | 90 (40%) | 81 (39%) |
| Unknown | 32 | 30 |
| del(17p) | 57 (25%) | 52 (25%) |
| Unknown | 27 | 25 |
| t(4;14) | 31 (14%) | 28 (13%) |
| Unknown | 30 | 28 |
| t(14;16) | 10 (5%) | 8 (4%) |
| Unknown | 39 | 37 |
| PCL (active or history) | 17 (7%) | 13 (6%) |
| AL amyloidosis (active or history) | 8 (3%) | 8 (3%) |
| Bridging therapy | 195 (77%) | 184 (78%) |
| Unknown | 3 | 1 |
| Response to bridging therapy | ||
| PR or better | 44 (26%) | 44 (27%) |
| Unknown response | 27 | 23 |
| Prior therapies | ||
| Median prior antimyeloma therapies | 6 (2-18) | 6 (2-18) |
| Unknown | 1 | 0 |
| Prior autologous SCT | 216 (85%) | 200 (85%) |
| Unknown | 1 | 0 |
| Prior allogeneic SCT | 0 (0%) | 0 (0%) |
| Prior anti-BCMA therapy | 38 (15%) | 33 (14%) |
| Prior bispecific antibody (any target) | 12 (5%) | 10 (4%) |
| Refractory status | ||
| Immunomodulatory agent | 201 (79%) | 188 (80%) |
| Proteasome inhibitor | 197 (77%) | 184 (78%) |
| Anti-CD38 antibody | 209 (82%) | 196 (83%) |
| Triple-refractory | 176 (69%) | 163 (69%) |
| Penta-refractory | 75 (29%) | 70 (30%) |
| Was patient ineligible for the CARTITUDE-1 trial criteria at the time of leukapheresis | 144 (56%) | 128 (54%) |
| Ineligible for 1 criterion | 61 (24%) | 58 (25%) |
| Ineligible for ≥2 criteria | 83 (33%) | 70 (30%) |
| Organ dysfunction∗ (renal, cardiac, and hepatic) | 31 (13%) | 27 (12%) |
| Unknown | 9 | 8 |
| Creatinine clearance <40 mL/minute | 22 (9%) | 18 (8%) |
| Unknown | 1 | 0 |
| Prior anti-BCMA therapy | 38 (15%) | 33 (14%) |
| Cytopenias | 45 (18%) | 37 (16%) |
| Unknown | 1 | 1 |
| ECOG PS ≥2 | 28 (11%) | 25 (11%) |
| Unknown | 7 | 7 |
| History or presence of PCL, amyloidosis or POEMS | 28 (11%) | 24 (10%) |
| History of CNS myeloma and other CNS pathology | 12 (5%) | 12 (5%) |
| Lymphodepletion chemotherapy | ||
| Flu/Cy | 191 (81%) | |
| Bendamustine | 31 (13%) | |
| Cladribine + cyclophosphamide | 6 (3%) | |
| Cyclophosphamide only | 7 (3%) | |
| Unknown | 1 | |
| CAR T-cell dose (million cells per kg) | 0.6 (0.1-1.0) | |
| CAR T-cell dose ≥0.7 million cells per kg | 74 (33%) | |
| Unknown | 9 | |
| OOS/nonconforming product | 44 (19%) | |
| Time from apheresis to infusion | 70 d (36-275) |
| Characteristic . | N = 255 (apheresis) . | N = 236 (infused) . |
|---|---|---|
| n (%) or median (range) . | n (%) or median (range) . | |
| Age, y | 64 (30-84) | 64 (30-84) |
| Age ≥70 y | 65 (25%) | 62 (26%) |
| Male sex | 145 (57%) | 134 (57%) |
| Race and ethnicity | ||
| Non-Hispanic White | 190 (75%) | 177 (76%) |
| Hispanic (any race) | 20 (8%) | 19 (8%) |
| Black | 30 (12%) | 26 (11%) |
| Asian/Pacific Islander | 8 (3%) | 7 (3%) |
| American Indian/Alaskan Native | 1 (0.4%) | 1 (0.4%) |
| Other | 4 (1.6%) | 4 (1.7%) |
| Unknown | 2 | 2 |
| ECOG PS | At CAR T-cell evaluation | At lymphodepletion |
| 0-1 | 215 (92%) | 183 (89%) |
| 2-4 | 19 (8%) | 23 (11%) |
| Unknown | 21 | 30 |
| Myeloma subtype | ||
| Intact immunoglobulin | 184 (72%) | 170 (72%) |
| Light chain | 40 (16%) | 39 (17%) |
| Oligo/nonsecretory | 31 (12%) | 27 (11%) |
| R-ISS disease stage | ||
| I | 44 (27%) | 43 (28%) |
| II | 87 (53%) | 81 (53%) |
| III | 34 (21%) | 30 (19%) |
| Unknown | 90 | 82 |
| EMD | 66 (26%) | 60 (26%) |
| Unknown | 2 | 1 |
| High marrow burden, BMPCs ≥50% | 39 (19%) | 35 (18%) |
| Unknown | 46 | 39 |
| Cytogenetic abnormality | ||
| Any high-risk cytogenetics | 90 (40%) | 81 (39%) |
| Unknown | 32 | 30 |
| del(17p) | 57 (25%) | 52 (25%) |
| Unknown | 27 | 25 |
| t(4;14) | 31 (14%) | 28 (13%) |
| Unknown | 30 | 28 |
| t(14;16) | 10 (5%) | 8 (4%) |
| Unknown | 39 | 37 |
| PCL (active or history) | 17 (7%) | 13 (6%) |
| AL amyloidosis (active or history) | 8 (3%) | 8 (3%) |
| Bridging therapy | 195 (77%) | 184 (78%) |
| Unknown | 3 | 1 |
| Response to bridging therapy | ||
| PR or better | 44 (26%) | 44 (27%) |
| Unknown response | 27 | 23 |
| Prior therapies | ||
| Median prior antimyeloma therapies | 6 (2-18) | 6 (2-18) |
| Unknown | 1 | 0 |
| Prior autologous SCT | 216 (85%) | 200 (85%) |
| Unknown | 1 | 0 |
| Prior allogeneic SCT | 0 (0%) | 0 (0%) |
| Prior anti-BCMA therapy | 38 (15%) | 33 (14%) |
| Prior bispecific antibody (any target) | 12 (5%) | 10 (4%) |
| Refractory status | ||
| Immunomodulatory agent | 201 (79%) | 188 (80%) |
| Proteasome inhibitor | 197 (77%) | 184 (78%) |
| Anti-CD38 antibody | 209 (82%) | 196 (83%) |
| Triple-refractory | 176 (69%) | 163 (69%) |
| Penta-refractory | 75 (29%) | 70 (30%) |
| Was patient ineligible for the CARTITUDE-1 trial criteria at the time of leukapheresis | 144 (56%) | 128 (54%) |
| Ineligible for 1 criterion | 61 (24%) | 58 (25%) |
| Ineligible for ≥2 criteria | 83 (33%) | 70 (30%) |
| Organ dysfunction∗ (renal, cardiac, and hepatic) | 31 (13%) | 27 (12%) |
| Unknown | 9 | 8 |
| Creatinine clearance <40 mL/minute | 22 (9%) | 18 (8%) |
| Unknown | 1 | 0 |
| Prior anti-BCMA therapy | 38 (15%) | 33 (14%) |
| Cytopenias | 45 (18%) | 37 (16%) |
| Unknown | 1 | 1 |
| ECOG PS ≥2 | 28 (11%) | 25 (11%) |
| Unknown | 7 | 7 |
| History or presence of PCL, amyloidosis or POEMS | 28 (11%) | 24 (10%) |
| History of CNS myeloma and other CNS pathology | 12 (5%) | 12 (5%) |
| Lymphodepletion chemotherapy | ||
| Flu/Cy | 191 (81%) | |
| Bendamustine | 31 (13%) | |
| Cladribine + cyclophosphamide | 6 (3%) | |
| Cyclophosphamide only | 7 (3%) | |
| Unknown | 1 | |
| CAR T-cell dose (million cells per kg) | 0.6 (0.1-1.0) | |
| CAR T-cell dose ≥0.7 million cells per kg | 74 (33%) | |
| Unknown | 9 | |
| OOS/nonconforming product | 44 (19%) | |
| Time from apheresis to infusion | 70 d (36-275) |
High marrow burden was defined as ≥50% plasma cells in pre–cilta-cel bone marrow biopsy. High-risk cytogenetics include del(17p), t(4;14), and t(14;16). Penta-refractory disease includes refractory to lenalidomide, pomalidomide, bortezomib, carfilzomib, and daratumumab or isatuximab. Triple-refractory disease includes refractory to an immunomodulatory drug, proteasome inhibitor, and an anti-CD38 monoclonal antibody.
AL, light chain; BMPCs, bone marrow plasma cells; CNS, central nervous system; ECOG PS, ECOG performance status; SCT: stem cell transplantation.
Organ dysfunction definition are as follows: for renal insufficiency, creatinine clearance <40 mL/minute; cardiac dysfunction, left ventricular ejection fraction <45% or history of myocardial infarction in prior 6 months or stage III/IV congestive heart failure or clinically significant ventricular arrythmia; hepatic insufficiency, serum aspartate aminotransferase or alanine aminotransferase >3× upper limit of normal, serum total bilirubin >2× upper limit of normal. Cytopenias were defined as hemoglobin <8 g/dL, absolute neutrophil count <750/μL, and platelets <50 x 103/μL.
Among 236 patients who received infusion, the median age was 64 years, with 26% being ≥70 years. The majority of patients were Non-Hispanic White (76%), with Black patients and Hispanic patients constituting 11% and 8% of the cohort, respectively. High-risk cytogenetics were present in 39% of patients and EMD in 26% of patients. Among patients who received infusion, 54% would not have met the CARTITUDE-1 eligibility criteria. The median of prior LOTs was 6 (range, 2-18), 69% of patients were triple-class refractory, 30% were penta-refractory, and 14% (n = 33) were exposed to prior BCMA-TT, including antibody drug conjugate or naked antibody (ADC; n = 16), CAR T-cell therapy (n = 6), ADC and CAR T-cell therapy (n = 2), BCMA-bispecific antibody (n = 8), and ADC and bispecific (n = 1). Of the 8 patients receiving prior CAR T-cell therapy, 4 received bb2121/idecabtagene vicleucel (ide-cel), 1 commercial and 3 on trial; 4 received other investigational CAR T cells (allogeneic, n = 3; autologous, n = 1; supplemental Table 1). Two patients had prior exposure to anti-GPRC5D bispecific antibody. Renal impairment, defined as creatinine clearance <50 mL per minute or being on dialysis, was present in 34 of 236 patients (14%) who received infusion before CAR T-cell therapy, including creatinine clearance of 30 to 49 mL per minute (n = 20), creatinine clearance <30 mL per minute (n = 6), and dialysis (n = 8). Bridging therapy was administered in 78% of patients, and the ORR to bridging was 27%. Flu/Cy lymphodepletion was administered in 81% of patients, whereas other regimens included bendamustine (13%), cladribine and cyclophosphamide (3%), and cyclophosphamide alone (3%).
Patients who received an OOS/nonconforming product were more likely to have a history of autologous transplant (95% vs 82%; P = .03), receipt of prior bispecific antibody (11% vs 3%; P = .02), higher baseline ferritin (342.5 vs 187.0 ng/mL; P = .006), and higher baseline C-reactive protein (3.4 vs 1.9 mg/L; P = .008) than patients who received an in-specification/conforming CAR T-cell product (supplemental Table 2).
Safety
Table 2 describes safety outcomes. CRS was seen in 75% (grade ≥3, 5%; including 3 grade 5 events), with a median time to onset of 7 days. ICANS was seen in 14% (grade ≥3, 4%; including 1 grade 5 event) of patients, with a median time to onset of 9 days. Immune effector cell–associated hemophagocytic lymphohistiocytosis–like syndrome was seen in 5 patients (2%).
Safety with SOC cilta-cel in 236 patients
| Event∗ . | n (%) or median (range) . |
|---|---|
| CRS | |
| Any grade | 177 (75%) |
| Unknown | 1 |
| Grade ≥3 | 12 (5%) |
| Grade 1 | 115 (49%) |
| Grade 2 | 49 (21%) |
| Grade 3 | 6 (3%) |
| Grade 4 | 3 (1%) |
| Grade 5 | 3 (1%) |
| Grade unknown | 1 |
| Median time to onset from CAR T-cell therapy | 7 days (0-14; IQR, 6-8) |
| ICANS | |
| Any grade | 32 (14%) |
| Unknown | 6 |
| Grade ≥3 | 9 (4%) |
| Grade 1 | 14 (6%) |
| Grade 2 | 8 (3.5%) |
| Grade 3 | 4 (2%) |
| Grade 4 | 4 (2%) |
| Grade 5 | 1 (0.4%) |
| Grade unknown | 1 |
| Median time to onset from CAR T-cell therapy | 9 days (1-51; IQR: 7-12.5) |
| DNT | 24 (10%) |
| Seventh CNP | 11 |
| Diplopia | 4 |
| Parkinsonism | 5 (1 patient with concomitant PRES) |
| PRES | 3 (1 patient with concomitant Parkinsonism) |
| Dysautonomia | 1 |
| Polyneuropathy | 1 |
| Median time to onset from CAR T-cell therapy | 24 d (13-149) |
| IEC-HS | 5 (2%) |
| Infections (any grade; severe) | |
| Any infection | 110 (47%); 49 (46%) |
| Bacterial (any grade; severe) | 41;25 |
| Viral (any grade; severe) | 47;10 |
| Fungal (any grade; severe) | 3;3 |
| Infection with >1 pathogen type | 19;11 |
| Resource utilization | |
| Median duration hospital stay, d | 13 (0-69) |
| Intensive care unit stay, yes | 18 (8%) |
| Tocilizumab use | 141 (61%) |
| Corticosteroid use | 86 (37%) |
| Anakinra use | 27 (12%) |
| Severe hematologic toxicity in first 90 d | |
| Grade ≥3 neutropenia | 163 (80%) |
| Unknown | 33 |
| Grade ≥3 anemia | 61 (37%) |
| Unknown | 71 |
| Grade ≥3 thrombocytopenia | 94 (53%) |
| Unknown | 59 |
| Supportive care for cytopenias | |
| G-CSF | 118 (52%) |
| TPO agonist | 20 (9%) |
| Stem cell boost | 11 (5%) |
| Event∗ . | n (%) or median (range) . |
|---|---|
| CRS | |
| Any grade | 177 (75%) |
| Unknown | 1 |
| Grade ≥3 | 12 (5%) |
| Grade 1 | 115 (49%) |
| Grade 2 | 49 (21%) |
| Grade 3 | 6 (3%) |
| Grade 4 | 3 (1%) |
| Grade 5 | 3 (1%) |
| Grade unknown | 1 |
| Median time to onset from CAR T-cell therapy | 7 days (0-14; IQR, 6-8) |
| ICANS | |
| Any grade | 32 (14%) |
| Unknown | 6 |
| Grade ≥3 | 9 (4%) |
| Grade 1 | 14 (6%) |
| Grade 2 | 8 (3.5%) |
| Grade 3 | 4 (2%) |
| Grade 4 | 4 (2%) |
| Grade 5 | 1 (0.4%) |
| Grade unknown | 1 |
| Median time to onset from CAR T-cell therapy | 9 days (1-51; IQR: 7-12.5) |
| DNT | 24 (10%) |
| Seventh CNP | 11 |
| Diplopia | 4 |
| Parkinsonism | 5 (1 patient with concomitant PRES) |
| PRES | 3 (1 patient with concomitant Parkinsonism) |
| Dysautonomia | 1 |
| Polyneuropathy | 1 |
| Median time to onset from CAR T-cell therapy | 24 d (13-149) |
| IEC-HS | 5 (2%) |
| Infections (any grade; severe) | |
| Any infection | 110 (47%); 49 (46%) |
| Bacterial (any grade; severe) | 41;25 |
| Viral (any grade; severe) | 47;10 |
| Fungal (any grade; severe) | 3;3 |
| Infection with >1 pathogen type | 19;11 |
| Resource utilization | |
| Median duration hospital stay, d | 13 (0-69) |
| Intensive care unit stay, yes | 18 (8%) |
| Tocilizumab use | 141 (61%) |
| Corticosteroid use | 86 (37%) |
| Anakinra use | 27 (12%) |
| Severe hematologic toxicity in first 90 d | |
| Grade ≥3 neutropenia | 163 (80%) |
| Unknown | 33 |
| Grade ≥3 anemia | 61 (37%) |
| Unknown | 71 |
| Grade ≥3 thrombocytopenia | 94 (53%) |
| Unknown | 59 |
| Supportive care for cytopenias | |
| G-CSF | 118 (52%) |
| TPO agonist | 20 (9%) |
| Stem cell boost | 11 (5%) |
G-CSF, granulocyte colony stimulating factor; IEC-HS, immune effector cell–associated hemophagocytic lymphohistiocytosis–like syndrome; IQR, interquartile range; TPO, thrombopoietin.
In addition to missing data described in table, infection severity is missing in 4 patients. The length of hospital stay is missing in 1 patient, intensive care unit admission missing in 10 patients, tocilizumab use missing in 6, steroid use missing in 6, anakinra use missing in 4 patients, G-CSF missing in 7 patients, TPO agonist in 8 patients, and stem cell boost in 14 patients.
DNT was seen in 24 patients (10%), including seventh cranial nerve palsy (CNP) in 11 patients (5%), parkinsonism in 5 (2%; with 1 patient having other DNT features as well), diplopia in 4 (2%), posterior reversible encephalopathy syndrome (PRES) in 2, dysautonomia in 1 patient, and polyneuropathy in 1 patient (supplemental Table 3). The median time of onset of all DNTs was 24 days (range, 13-149) and for parkinsonism was 30 days (range, 13-69). Treatment for CNP and diplopia included steroids and intravenous immunoglobulin (IVIG). Patients with parkinsonism received more extensive therapy. DNT resolved in 13 of 24 patients (54%) at the last follow-up (seventh nerve palsy, n = 6; diplopia, n = 4; dysautonomia, n = 1; polyneuropathy, n = 1) and at least significantly improved in 1 patient (PRES/parkinsonism). Of the remaining 11 patients, 5 patients are alive with ongoing toxicity at the last follow-up. Three patients have died due to DNT (parkinsonism, n = 1; PRES, n = 1; cerebral edema/PRES, n = 1); 2 patients have died due to other causes (infection, n = 1; myeloma, n = 1); and information was missing in 1 patient. The median duration of DNT in patients with CNP/diplopia was 64 days (range, 1-599), with resolution seen in 67% of patients. Only 1 of the 5 patients with parkinsonism showed improvement in symptoms, but later died from other causes.
We investigated the association of baseline patient characteristics with toxicity for grade ≥2 CRS, any-grade ICANS, and DNT in both univariate and multivariable analyses (supplemental Tables 4-6). High marrow burden (≥50% plasma cells), poor performance status (ECOG ≥2), higher R-ISS stage, high baseline ferritin, and history or presence of PCL, amyloidosis, and Waldenstrom macroglobulinemia were associated with CRS grade ≥2 in univariate analysis. In a multivariable model, poor performance status and high baseline ferritin were predictors of developing grade ≥2 CRS. Similarly, for ICANS, high marrow burden, poor performance status, >4 prior LOTs, penta-refractory status, higher baseline ferritin, and not meeting the CARTITUDE-1 eligibility criteria were associated with a higher likelihood of developing ICANS on univariate analysis. On multivariable analysis, poor performance status and penta-refractory status were associated with a higher risk of any-grade ICANS. When evaluating the risk factors for DNT, any-grade ICANS and steroid use were associated with a higher risk of DNT on univariable analysis (P < .05; supplemental Table 6). Due to the small number of events for DNT, we did not perform a multivariable analysis identifying the predictors of DNT. Supplemental Table 7 describes ICANS and DNT in 12 patients with underlying central nervous system pathology, with 4 cases of ICANS (33%) including 2 cases of grade ≥2 ICANS and 2 cases of DNT (17%; 1 case of parkinsonism and 1 of Bell palsy).
Infections were seen in 47% of patients, of whom 46% (n = 49) had severe infections. Most common infections were viral and bacterial, with fungal infections seen in only 5 patients. A significant proportion of patients continued to experience grade ≥3 cytopenias at day 30, with cell counts improving in the majority of patients by day 90 after CAR T-cell therapy. Grade ≥3 cytopenias at day 30 were seen in 70% of patients, including grade ≥3 neutropenia in 59% and grade ≥3 thrombocytopenia in 49% of patients. At days 60 and 90 after CAR T-cell therapy, grade ≥3 neutropenia was seen in 20% and 11% and thrombocytopenia in 22% and 14% of patients, respectively. Granulocyte colony-stimulating factor was used in 52%, thrombopoietin agonists in 9%, and stem cell rescue in 5% of patients (Table 2; supplemental Table 8).
Second primary malignancies (SPMs) were seen in 20 patients (8.5%) who received infusion. SPMs excluding nonmelanoma skin cancers were seen in 5.5% (n = 13), including myeloid malignancies/acute leukemia in 1.3% of patients (n = 3). Peripheral T-cell lymphoma was seen in 1 patient. Other SPMs included T-large granular lymphocytic leukemia (n = 2), colorectal cancer (n = 2), breast cancer (n = 1), prostate cancer (n = 3), and melanoma (n = 1).
At the last follow-up, 50 patients had died, 23 (10%) due to nonrelapse mortality (NRM). Causes of death for NRM included infections (n = 12; including progressive multifocal leukoencephalopathy in 1), CRS (n = 3), CRS and infection (n = 1), DNT (n = 3), immune effector cell–associated hemophagocytic lymphohistiocytosis–like syndrome (n = 2), ICANS (n = 1), and SPM (n = 1).
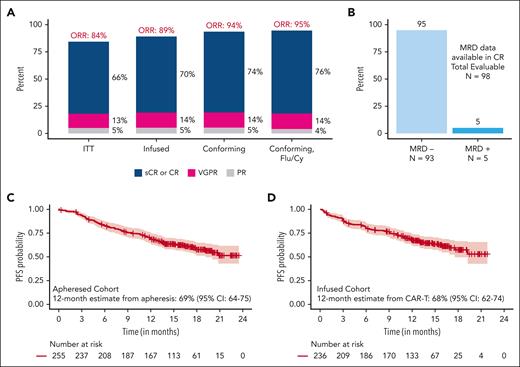
Response to therapy
Figure 2 and supplemental Table 9 show response rates in all patients and in patients who received a conforming product. Intention-to-treat ORR in all 255 patients was 84%, with a CR or better rate of 66%. Among patients who received CAR T-cell therapy (N = 236), the ORR was 89%, and the CR or better rate was 70%. MRD data were available in 98 patients with CR or better, of whom 93 patients (95%) were MRD negative. ORR and CR rates among patients receiving a conforming product (n = 192) were 94% and 74%, respectively; and they were 95% and 76% among those receiving a conforming product with Fly/Cy lymphodepletion (n = 152), respectively. Response deepened over time (from day 30 to day 90 and best response) in all patients who received cilta-cel infusion and subgroups of patients receiving conforming product with Flu/Cy lymphodepletion (supplemental Figure 1).
Efficacy with SOC cilta-cel. (A) Best response in all patients who underwent apheresis, patients who received cilta-cel infusion, patients who received cilta-cel infusion with conforming product, and patients who received cilta-cel infusion with conforming product and Flu/Cy lymphodepletion. (B) MRD negativity rates among patients who received infusion and were in CR with MRD data available. (C) PFS in all patients who underwent apheresis (n = 255). (D) PFS in patients receiving cilta-cel infusion (n = 236).
Efficacy with SOC cilta-cel. (A) Best response in all patients who underwent apheresis, patients who received cilta-cel infusion, patients who received cilta-cel infusion with conforming product, and patients who received cilta-cel infusion with conforming product and Flu/Cy lymphodepletion. (B) MRD negativity rates among patients who received infusion and were in CR with MRD data available. (C) PFS in all patients who underwent apheresis (n = 255). (D) PFS in patients receiving cilta-cel infusion (n = 236).
On univariate analysis for factors associated with response, male sex, poor performance status, R-ISS stage III disease, >4 prior LOTs, prior BCMA-TT, lower cell dose, and elevated baseline ferritin were associated with a lower probability of best ORR. Patients with poor performance status, EMD, a higher R-ISS stage, prior BCMA-TT, penta-refractory status, elevated C-reactive protein, and elevated ferritin were less likely to achieve a best response of CR or better. We did not investigate nonconforming products as a variable (supplemental Table 10). On multivariable analysis (Table 3), the patient characteristics associated with a lower likelihood of achieving ORR were prior BCMA-TT (P = .002), higher baseline ferritin levels (P = .001), White race (P = .02), and male sex (P = .01). Additionally, a lower odds of achieving a best response of CR or better was observed for patients with prior BCMA-TT (P = .002), penta-refractory disease (P = .005), high-risk cytogenetics (P = .05), presence of EMD (P = .03), and a poor ECOG performance status at lymphodepletion (P = .002) in multivariable models.
Multivariable analysis for factors associated with ORR, CR rate, PFS, and OS after SOC cilta-cel
| Characteristic . | OR (95% CI) . | P value . |
|---|---|---|
| Best ORR | ||
| Prior BCMA-TT, yes vs no | 0.19 (0.06-0.54) | .002 |
| Ferritin, ≥400 vs <400 ng/mL | 0.20 (0.07-0.51) | .001 |
| Race and ethnicity, Non-White vs White | 6.99 (1.71-49.20) | .02 |
| Sex, male vs female | 0.21 (0.06-0.63) | .01 |
| Best CR or better | ||
| Prior BCMA-TT, yes vs no | 0.20 (0.07-0.54) | .002 |
| Penta-refractory, yes vs no | 0.32 (0.14-0.70) | .005 |
| ECOG PS, 2-4 vs 0-1 | 0.16 (0.05-0.51) | .002 |
| EMD, yes vs no | 0.41 (0.18-0.90) | .03 |
| High-risk cytogenetics, yes vs no | 0.48 (0.22-1.00) | .05 |
| Characteristic . | OR (95% CI) . | P value . |
|---|---|---|
| Best ORR | ||
| Prior BCMA-TT, yes vs no | 0.19 (0.06-0.54) | .002 |
| Ferritin, ≥400 vs <400 ng/mL | 0.20 (0.07-0.51) | .001 |
| Race and ethnicity, Non-White vs White | 6.99 (1.71-49.20) | .02 |
| Sex, male vs female | 0.21 (0.06-0.63) | .01 |
| Best CR or better | ||
| Prior BCMA-TT, yes vs no | 0.20 (0.07-0.54) | .002 |
| Penta-refractory, yes vs no | 0.32 (0.14-0.70) | .005 |
| ECOG PS, 2-4 vs 0-1 | 0.16 (0.05-0.51) | .002 |
| EMD, yes vs no | 0.41 (0.18-0.90) | .03 |
| High-risk cytogenetics, yes vs no | 0.48 (0.22-1.00) | .05 |
| . | HR (95% CI) . | P value . |
|---|---|---|
| PFS | ||
| Prior BCMA-TT, yes vs no | 1.65 (0.94-2.89) | .08 |
| Ferritin, ≥400 vs <400 ng/mL | 2.99 (1.86-4.80) | <.001 |
| High-risk cytogenetics, yes vs no | 1.90 (1.20-3.02) | .006 |
| EMD, yes vs no | 1.96 (1.19-3.23) | .009 |
| OS | ||
| Ferritin, ≥400 vs <400 ng/mL | 3.35 (1.81-6.19) | <.001 |
| High-risk cytogenetics, yes vs no | 2.57 (1.40-4.72) | .005 |
| EMD, yes vs no | 1.88 (1.04-3.42) | .04 |
| . | HR (95% CI) . | P value . |
|---|---|---|
| PFS | ||
| Prior BCMA-TT, yes vs no | 1.65 (0.94-2.89) | .08 |
| Ferritin, ≥400 vs <400 ng/mL | 2.99 (1.86-4.80) | <.001 |
| High-risk cytogenetics, yes vs no | 1.90 (1.20-3.02) | .006 |
| EMD, yes vs no | 1.96 (1.19-3.23) | .009 |
| OS | ||
| Ferritin, ≥400 vs <400 ng/mL | 3.35 (1.81-6.19) | <.001 |
| High-risk cytogenetics, yes vs no | 2.57 (1.40-4.72) | .005 |
| EMD, yes vs no | 1.88 (1.04-3.42) | .04 |
High-risk cytogenetics includes del(17p), t(4;14), and t(14;16). Penta-refractory disease includes refractory to lenalidomide, pomalidomide, bortezomib, carfilzomib, and daratumumab or isatuximab.
HR, hazard ratio; OR, odds ratio.
PFS and OS
Among all patients who underwent apheresis (N = 255), the median PFS was not reached, with the 12-month PFS estimate from apheresis being 69% (95% confidence interval [CI], 64-75). Among patients who received CAR T-cell infusion (N = 236), the median PFS was not reached; with the 12-month PFS estimate from CAR T-cell therapy being 68% (95% CI, 62-74; Figure 2). Among patients receiving a conforming product and those receiving a conforming product with Flu/Cy lymphodepletion, the 12-month PFS estimates were 72% and 73%, respectively (supplemental Figure 2). The median OS was also not reached, and the 12-month OS estimate in patients who received infusion was 82% (95% CI, 77-87).
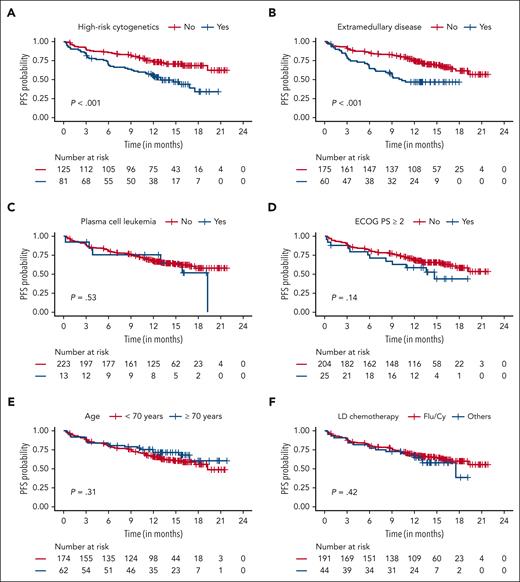
As shown in Figures 3 and 4 and supplemental Figure 2, on univariate analysis, patients with high-risk cytogenetics, EMD, prior BCMA therapy, poor performance status (ECOG ≥2), and overall ineligibility for CARTITUDE-1 trial had worse PFS. Age (<70 vs ≥70 years), presence of PCL, and type of lymphodepletion chemotherapy did not impact PFS. Although not statistically significant (P = .06), patients who did not need bridging therapy had better PFS, likely a surrogate for less aggressive disease biology. Response to bridging did not affect PFS. On multivariable analysis, high baseline ferritin levels, high-risk cytogenetics, and EMD were independently associated with inferior PFS. Prior BCMA-TT was also associated with inferior PFS but not statistically significant (P = .08). Only the presence of high baseline ferritin levels, high-risk cytogenetics, and EMD were associated with inferior OS on multivariable analysis (Table 3)
PFS in patients receiving SOC cilta-cel based on patient and treatment characteristics. (A) High-risk cytogenetics. (B) Extramedullary disease. (C) Plasma cell leukemia. (D) ECOG performance status (PS). (E) Age (≥70 years vs <70 years). (F) Lymphodepletion (LD) chemotherapy (Flu/Cy and others).
PFS in patients receiving SOC cilta-cel based on patient and treatment characteristics. (A) High-risk cytogenetics. (B) Extramedullary disease. (C) Plasma cell leukemia. (D) ECOG performance status (PS). (E) Age (≥70 years vs <70 years). (F) Lymphodepletion (LD) chemotherapy (Flu/Cy and others).
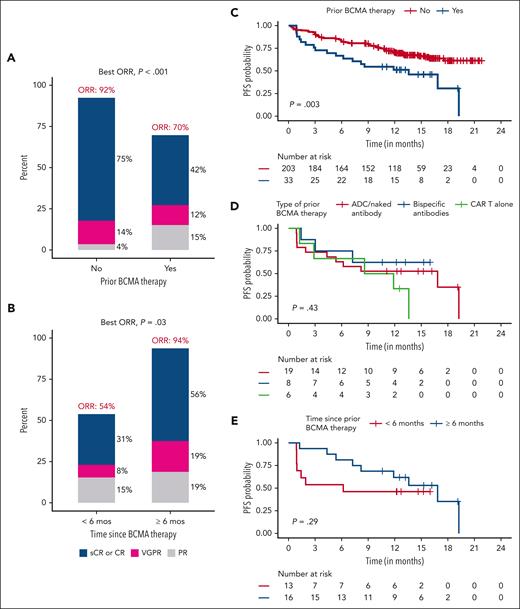
Efficacy of SOC cilta-cel in patients with prior BCMA-TT based on patient and treatment characteristics. (A) Response based on prior BCMA targeted therapy (yes/no). (B) Response based on time from prior BCMA-TT (<6 months vs ≥6 months). (C) PFS based on prior BCMA-directed therapy. (D) PFS based on types prior BCMA-directed therapy. (E) PFS based on the time from last BCMA therapy (<6 months vs ≥6 months).
Efficacy of SOC cilta-cel in patients with prior BCMA-TT based on patient and treatment characteristics. (A) Response based on prior BCMA targeted therapy (yes/no). (B) Response based on time from prior BCMA-TT (<6 months vs ≥6 months). (C) PFS based on prior BCMA-directed therapy. (D) PFS based on types prior BCMA-directed therapy. (E) PFS based on the time from last BCMA therapy (<6 months vs ≥6 months).
Prior BCMA-TT
As described above, prior BCMA-TT had a significant impact on ORR, CR, and survival outcomes. The median PFS among patients receiving prior BCMA therapy was 13.6 months. To investigate this further, we evaluated the time from last BCMA-TT exposure among 29 patients with available data. The median time from last BCMA therapy was 7.1 months, with 16 of 29 patients (55%) having the last BCMA therapy exposure ≥6 months before CAR T-cell infusion. ORR and CR rates in patients with ≥6 months vs <6 months from last exposure were 94% vs 54% (P = .03) and 56% vs 31% (P = .2), respectively. The median PFS was 16.8 months in patients with ≥6 months from last BCMA therapy exposure and 6.2 months in patients with <6 months from last exposure (P = .29; Figure 4; supplemental Table 1).
Alternate lymphodepletion
As shown in Figure 3F and supplemental Table 11, there was no significant difference in ORR (90% vs 86%; P = .6), CR rate (72% vs 61%; P = .2), or PFS (median not reached vs 18 months; P = .4): 6 in patients receiving Flu/Cy lymphodepletion vs alternate lymphodepletion.
Disease burden at CAR T-cell infusion
Response rates and PFS were similar in patients with “low burden/responding disease” (defined as not needing bridging therapy or PR or better to bridging) vs “higher disease burden group.” Interestingly, the low burden/responding group had better OS (P = .05; supplemental Methods; supplemental Figure 4; supplemental Table 12).
CARTITUDE-1 eligible patients
We conducted a subgroup analysis of 108 patients who were eligible for CARTITUDE-1, as described in detail in the supplement. The ORR and CR rates in this group were 93% and 74%, respectively, and the 12-month estimates for PFS and OS were 76% (95% CI, 67-85) and 85% (95% CI, 78-92), respectively. The NRM rate was 7%, and the rates of other toxicities were along the lines of that observed for the entire cohort (supplemental Tables 12-15).
Discussion
This large, multicenter, real-world evidence study is, to our knowledge, the first to report on outcomes in patients with RRMM receiving SOC cilta-cel. Our data indicate that cilta-cel administration in the real world is feasible and effective, although there is a need for better toxicity management.
Successful manufacturing and timely infusion are important aspects of delivering CAR T-cell therapy to patients. The manufacturing failure rate with first apheresis was 6%, with an overall manufacturing failure rate of 1%. In CARTITUDE-1 clinical trial, no patient discontinued treatment due to manufacturing failure, although data on success rate of first manufacturing are not available. First-attempt manufacturing failure rate with SOC ide-cel was comparable at 6%.9 In this cohort, 19% of patients received an OOS nonconforming CAR T-cell product. It is notable that the FDA specification for cilta-cel is narrower than the product release criteria used in CARTITUDE-1. The OOS rate for the clinical trial in package insert was 18%.14 Prior autologous transplant and bispecific antibody therapy use were associated with a higher likelihood of receiving an OOS product, as were markers of aggressive disease and inflammation. This should be investigated further in larger studies, particularly for patients with bispecific antibody exposure to optimize sequencing of immunotherapies. The median vein-to-vein time of almost 10 weeks is considerable for patients with RRMM and longer than that of CARTITUDE-1 (29 days).
We observed a favorable efficacy profile, despite 56% of patients not being trial eligible and 92.5% of patients who underwent apheresis received cilta-cel, compared with 86% in the CARTITUDE-1 trial. Patients receiving SOC cilta-cel had a numerically greater aggressive disease burden than the CARTITUDE-1 population, including high-risk cytogenetics (40% vs 24%) and PCL (6% vs 0%). EMD was also more common (26% vs 13%).4,8 Despite this, SOC cilta-cel resulted in high response rates, with ORR and CR rate of 89% and 70% in patients who received infusion, respectively, with 95% of patients in CR being MRD negative among patients with available data. The ORR and CR rates with SOC conforming products were 94% and 74%, respectively, and in the CARTITUDE-1–eligible patients, they were 93% and 74%, respectively. In comparison, the ORR and CR rates in CARTITUDE-1 were 98% and 82.5%, respectively. Estimated 12-month PFS and OS in patients who received infusion in this study were 68% and 82%, respectively. In the initial publication from CARTITUDE-1, the 12-month PFS estimate was 77%, and the 12-month OS estimate was 89%. In the final analysis from CARTITUDE-1, the median PFS is 34.9 months, whereas the median OS is still not reached.7 Although it is difficult to make direct comparisons, the response rates and estimated PFS in our cohort appears to be somewhat lower than that of the CARTITUDE-1 population, likely due to differences in patient selection, because half of the patients in our cohort were trial ineligible, and such patients had inferior PFS. Receipt of nonconforming products in 19% of patients may also be associated with lower efficacy. A clinical trial to understand outcomes with nonconforming cilta-cel is ongoing.
The incidence and the timing of CRS and ICANS were very similar to that seen with cilta-cel in CARTITUDE-1 clinical trial. The rate of DNT was also similar, but this was a heterogeneous group with the most common presentation in our cohort being seventh CNP. Other presentations included diplopia without an identified CNP, parkinsonism, and PRES. Parkinsonism was numerically less common in our cohort than that seen in CARTITUDE-1 (2% vs 6%), consistent with what has been observed with other clinical trials of cilta-cel.5 The biological underpinnings of DNT are poorly understood. One hypothesis is that this high affinity CAR T cell binds to low level BCMA-expressing cells in the central nervous system as suggested by infiltrating T cells noted in caudate nucleus on autopsy of a patient with parkinsonism after cilta-cel.15 Therapies for Parkinson disease are ineffective in treating CAR T-cell–related parkinsonism. Treatments directed at reducing CAR T-cell burden may be considered, including high-dose steroids and chemotherapy. Recently, a case report described resolution in parkinsonism after cilta-cel with the use of cyclophosphamide and steroids.16 Seventh CNP was observed in 5% of our cohort and was the most common DNT seen in the earlier line CARTITUDE-4 trial (9%).5 Patients who developed CNPs on CARTITUDE clinical trials were observed to have greater CAR T-cell expansion.17 Although optimal treatment of CNP remains unknown, most patients in our cohort and on CARTITUDE clinical trials received treatment with a short course of steroids, often in combination with IV immunoglobulin. Resolution or improvement was seen in approximately half of the patients in our cohort and 90% of patients in CARTITUDE trials, although improvement was slow over several weeks in both cohorts.
We observed a significant NRM rate of 10% among the patients who received infusion, primarily from infections and CRS, although deaths due to DNTs were also seen. Not surprisingly, this is higher than the 6% NRM rate seen in the CARTITUDE-1 study, likely due to the higher proportion of patients with comorbidities and poor performance status in our cohort.4,8 The NRM rate in the CARTITUDE-1–eligible patients in our cohort was 7%. Nevertheless, this highlights the need for aggressive supportive care for the management of CRS, ICANS, and infections and need to find effective treatment strategies for parkinsonian neurotoxicity. We also observed a high rate of SPMs with 13 months of follow-up after CAR T-cell therapy. The overall rate of SPMs was 8.5%, with nonmelanoma skin cancers seen in 5.5% of patients, myelodysplastic syndrome/myeloid malignancies in 1.3%, and 1 patient with T-cell lymphoma. These numbers are likely to increase with longer follow-up.
With increasing immunotherapy options for patients with RRMM, including 2 FDA-approved CAR T-cell therapies (cilta-cel and ide-cel) and 3 bispecific antibodies against BCMA (teclistamab and elranatamab) or GPRC5D (talquetamab), treatment selection and sequencing are critical. Prior BCMA-TT was associated with inferior survival outcomes in our cohort, particularly for patients who received prior BCMA-TT within 6 months of cilta-cel, for whom the median PFS was only 6.2 months compared with 16.8 months for patients who received prior BCMA-TT ≥6 months before cilta-cel infusion. This is consistent with other reports of CAR T-cell therapy after prior BCMA-TT. Data from cohort C of CARTITUDE-2 trial demonstrated only a 60% response rate with cilta-cel after prior BCMA-directed ADC or bispecific antibodies and a median PFS of 9 months, with a median PFS of 5.3 months after bispecific antibodies.18 We have previously reported lower response rate and PFS with ide-cel in patients with prior BCMA exposure.19 In both published studies, shorter interval from last BCMA-TT was associated with inferior outcomes, with patients receiving CAR T-cell therapy within 6 months of prior BCMA-TT having lower response rates.
Strengths of our study include a large, multicenter cohort of patients treated at 16 centers with in-depth data on outcomes, collected using a uniform data collection form, and a median of 13 months of follow-up from CAR T-cell therapy, allowing for enough time for response adjudication and development of short and intermediate term adverse events. Limitations include adjudication of response per investigator discretion, without central review and lack of mandated imaging studies and likely underestimation of late complications beyond 1 year, particularly SPMs. Despite these limitations, our study provides informative data on cilta-cel use in clinical practice, with high response rates in a heavily treated population with aggressive disease, although highlighting the need for close monitoring of toxicity.
In summary, cilta-cel administration is feasible in a heterogeneous real-world population of patients with RRMM with comorbidities, resulting in deep and durable responses. Close surveillance for late complications such as SPMs remains crucial. Future efforts should focus on measures to decrease NRM, mitigate and manage DNT, and a priori identification of patients at risk of developing SPMs.
Acknowledgments
S.S. was supported by the Stanford Clinical and Translational Science KL2 Career Development Award program (award number KL2 TR003143) and Stanford Cancer Institute/American Cancer Society Pilot Grant 2022. This work was in part supported by the International Myeloma Society Young Investigator Award, the Moffitt Cancer Center National Institutes of Health (NIH), National Cancer Institute (NCI) Core Grant (P30-CA076292), and a generous donation from the Hyer family. D.K.H., M.A., and C.F. are supported by the Pentecost Family Myeloma Research Center. F.L.L. is supported by the Leukemia and Lymphoma Society as a Clinical Scholar and the NIH, NCI (grants R01CA244328, P30-CA076292).
Authorship
Contribution: S.S., K.K.P., L.C.P., M.J., Y.L., and D.K.H. contributed to the conception and design; S.S., K.K.P., L.C.P., M.J., Y.L., and D.K.H. contributed to data analysis and interpretation; S.S., D.K.H., and L.C.P. drafted the manuscript; and all authors contributed to the critical review of the manuscript, contributed to the provision of study material of patients or collection and assembly of data, and approved the final manuscript.
Conflict-of-interest disclosure: S.S. reports consulting or advisory fees from Janssen, Bristol Myers Squibb (BMS), Legend, Magenta Therapeutics, Sanofi, Pfizer, Takeda, Kite, AbbVie, and Regeneron and research funding from Janssen, Magenta Therapeutics, Allogene Therapeutics, Novartis, and BMS. K.K.P. reports consulting or advisory role fees from BMS, Janssen, Pfizer, Arcellx, and Karyopharm Therapeutics; research funding from BMS, Poseida Therapeutics, Takeda, Janssen, Cellectis, Nektar, AbbVie/Genentech, Precision Biosciences, and Allogene Therapeutics; and travel, accommodations, and expenses from BMS. L.C.P. reports research funding from BMS. D.W.S. reports consulting or advisory role fees from Sanofi, GlaxoSmithKline (GSK), BMS, Legend Biotech, Janssen, Pfizer, Bioline, AstraZeneca, Arcellx, and AbbVie. S.A. reports honoraria from Janssen and research funding from GSK, Amgen, Karyopharm Therapeutics, Janssen, and BMS. C.F. reports consulting or advisory role fees from Sanofi and stock/other ownership in Affimed Therapeutics. J.K. reports honoraria from OncLive. C.L.F. reports honoraria/consulting fees from BMS, Seattle Genetics, Celgene, AbbVie, Sanofi, Incyte, Amgen, and ONK Therapeutics/Janssen and research funding from BMS, Janssen, and Roche/Genentech. F.L.L. reports consulting or advisory role fees from Allogene, Amgen, Bluebird Bio, BMS/Celgene, Calibr, Cellular Biomedicine Group, Cowen, EcoR1, Emerging Therapy Solutions, Gerson Lehman Group, GammaDelta Therapeutics, Iovance, Janssen, Kite (a Gilead Company), Legend Biotech, Novartis, Umoja, and Wugen; research funding from Allogene, Kite, and Novartis; and patents, royalties, and other intellectual property in the field of cellular immunotherapy. M.A. reports consulting or advisory role fees from BMS and Janssen and speakers’ bureau fees and honoraria from Janssen. J.M. reports honoraria from Kite Pharma, Juno Therapeutics, AlloVir, Magenta Therapeutics, and EcoR1 Capital; speakers’ bureau fees from Kite/Gilead; research funding from Novartis, Fresenius Biotech, Astellas Pharma, Bellicum Pharmaceuticals, Novartis, Gamida Cell, Pluristem Therapeutics, Kite Pharma, and AlloVir; honoraria from Kite, AlloVir, Juno Therapeutics, and Magenta Therapeutics; and travel, accommodations, and expenses from Kita Pharma. S.W. is a current employee of BMS. D.K.H. reports honoraria from OncLive and Multiple Myeloma Hub; research funding from BMS and Adaptive Biotech; and consulting or advisory role fees from BMS, Janssen, and Pfizer. The remaining authors declare no competing financial interests.
Correspondence: Surbhi Sidana, Division of Blood and Marrow Transplantation and Cellular Therapy, Stanford University School of Medicine, 780 Welch Rd CJ250C, Stanford, CA 94305; email: surbhi.sidana@stanford.edu; and Doris K. Hansen, Department of Blood and Marrow Transplant and Cellular Immunotherapy, H. Lee Moffitt Cancer Center and Research Institute, 12902 USF Magnolia Dr, CSB-7 BMT, Tampa, FL 33612; email: doris.hansen@moffitt.org.
References
Author notes
S.S., K.K.P., and L.C.P. are joint first authors.
M.J., Y.L., and D.K.H. are joint senior authors.
The data that support the findings of this study are available on reasonable request from the corresponding authors, Surbhi Sidana (surbhi.sidana@stanford.edu) and Doris K. Hansen (doris.hansen@moffitt.org).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal