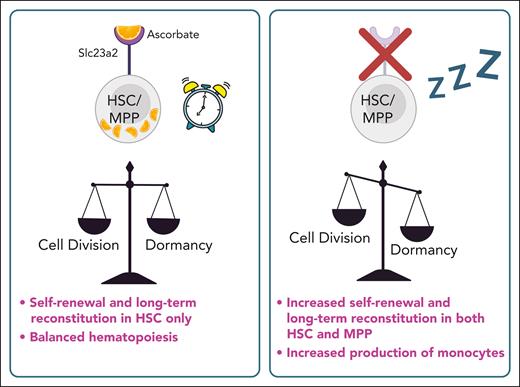
In this issue of Blood, Comazzetto et al1 demonstrate that vitamin C deprivation promotes stem cell quiescence and functionally reprograms multipotent hematopoietic progenitors (MPPs) to acquire hematopoietic stem cell (HSC)-like properties, such as enhanced self-renewal and reconstitution potential (see figure).
Intracellular vitamin C deprivation via Slc23a2 promotes HSC quiescence and long-term reconstitution. Created with BioRender.com.
Intracellular vitamin C deprivation via Slc23a2 promotes HSC quiescence and long-term reconstitution. Created with BioRender.com.
Ascorbate (vitamin C) is a potent regulator of stem cell function and acts as a suppressor of leukemia.2,3 Two studies published in 2017 in the journals Cell2 and Nature3 demonstrated that vitamin C suppresses leukemogenesis through its ability to modulate Tet2 function. Ascorbate acts as a cofactor for Tet2, enhancing its enzymatic activity. Tet2 is involved in the conversion of 5-methylcytosine to 5-hydroxymethylcytosine, a key step in the DNA demethylation process. In HSC, the loss of Tet2 promotes self-renewal4 and contributes to the development of myeloid neoplasia.4,5
In humans, vitamin C is obtained through the diet, and mice synthesize it in the liver. The enzymatic action of L-gulono-γ-lactone oxidase allows for the study of vitamin C’s role in murine stem cell function by eliminating ascorbate uptake.3 Previous seminal work conducted by the Morrison group showed that bone marrow (BM) cells from Gul-–/– mice exhibit higher reconstitution upon BM transplantation (BMT).3 Metabolomic profiling on HSCs and MPPs found that ascorbate is one of the most enriched metabolites,3 correlating with abundant expression of the sodium-dependent vitamin C transporter Slc23a2 compared with most differentiated hematopoietic cells.3
In the current study, Comazzetto investigated the role of Slc23a2 in hematopoiesis using a conditional mouse model to directly delete the Slc23a2 gene in the hematopoietic system, followed by with transplantation and elegant pulse-chase experiments. This approach leads to the inhibition of the vitamin C uptake in BM cells without affecting ascorbate plasma levels. Slc23a2 deficiency leads to reduced HSC numbers and an increased frequency of common myeloid progenitors and accumulation of monocytes in the BM without affecting other lineages.
Using competitive BMT, the authors show Slc23a2-deficient progenitors displayed higher reconstitution rates, particularly in myeloid and B lineages in both primary and secondary recipients. Notably, isolated phenotypically identified MPPs showed a significant boost in long-term multilineage reconstitution during Slc23a2 deficiency, with this potential conserved in secondary recipients. RNA-seq analysis confirmed that the loss of Slc23a2 did not alter the expression of the key phenotypic markers but showed reduced expression of genes that control cell division. Single-cell analyses did not reveal different clustering patterns due to Slc23a2 deficiency but confirmed decreased expression of proliferation-associated genes, such as Mki67. 5-Bromo-2′-deoxyuridine (BrdU) pulse-chase experiments demonstrated reduced BrdU incorporation in HSCs and MPPs of Slc23a2-deficient mice, suggesting that ascorbate deficiency promotes quiescence and boosts their reconstitution potential upon BMT. Thus, the main finding is that ascorbate-depleted MPPs acquired HSC properties such as self-renewal and long-term reconstitution potential.
Furthermore, the authors employed Col1a1-H2B-GFP;Rosa26-M2-rtTA mice to track labeled cells after doxycycline exposure. In this experiment, HSCs and MPPs in Slc23a2-deficient mice retained the H2B-GFP mark, indicating a state of quiescence in vivo. They isolated quiescent H2B-GFPhigh and actively dividing HSCs (H2B-GFPneg) from control and knockout animals for BMT experiments. As expected, the dormant ascorbate-depleted HSCs demonstrated the highest engraftment rates, while dividing HSCs also showed robust contributions. Interestingly, only MPPs that became dormant due to Slc23a2 loss exhibited long-term reconstitution.
The interplay between vitamin C and Tet2 function is crucial for HSC hemostasis. In this study, the authors compared single Slc23a2 and Tet2 mutants, as well as double mutants. Interestingly, Tet2 deficiency did not replicate the cellular changes in the BM. Although Tet2-deficient HSCs exhibited a previously reported myeloid bias,4,5Slc23a2-deficient cells showed a significant B-cell contribution, with double mutants showing comparable levels to Slc23a2 single mutants. Furthermore, the enhanced reconstitution potential observed in MPPs was exclusive to Slc23a2 deficiency, as Tet2 did not enhance reconstitution during MPPs BMT, suggesting Tet2-independent mechanisms.
Tet2 is one of the most frequently mutated genes in clonal hematopoiesis (CH), typically exhibiting a heterozygous mutation pattern.6 Individuals with CH have higher probability of progression to myeloid neoplasia. Vitamin C treatment can enhance the function of the unaffected Tet2 allele, thereby blocking the aberrant self-renewal.2 These observations have inspired clinical trials (NCT03433781, NCT03418038, NCT03682029) to explore the benefit of oral vitamin C interventions for clonal cytopenias of undetermined significance and low-risk myelodysplastic syndrome (MDS). Moreover, several reports showed that patients with MDS or acute myeloid leukemia have lower vitamin C levels compared with healthy controls.7,8 Additional research has shown a reduced expression of ascorbate transporters in leukemic cells, suggesting a potential link between vitamin C deficiency and leukemogenesis. Although some dietary interventions involving vitamin C have shown variable outcomes in myeloid neoplasms,9 these broad responses may be influenced by genetic variations such as polymorphisms in ascorbate transporters that have been associated with chronic pathologies.
Plasma levels of ascorbate can vary with age due to several factors including reduced dietary intake, impaired absorption, and altered ascorbate metabolism.10 Nonetheless, epidemiological studies revelated that individuals with low plasma ascorbate levels have higher risk of mortality from all causes including cancer, particularly among men.10 Furthermore, it is crucial to explore whether mutations in vitamin C transporters contribute to CH or if epigenetic silencing cooperates with clonal expansion and malignancy. Future studies should also investigate the double mutant (Slc23a2 and Tet2) in the context of aging and inflammation to determine whether these mice develop myeloid neoplasms more rapidly, which could provide valuable insights into the mechanisms driving age-related hematological disorders and the fine-tuning role vitamin C.
Ultimately, a comprehensive understanding of the interplay between vitamin C levels and HSC function could reveal new therapeutic strategies for myeloid leukemia and suggest ways to enhance quiescent states and self-renewal potential during BMT. Such insights may pave the way for the development of small molecules or genetic interventions aimed at optimizing HSC functionality, by targeting the vitamin C pathway.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal