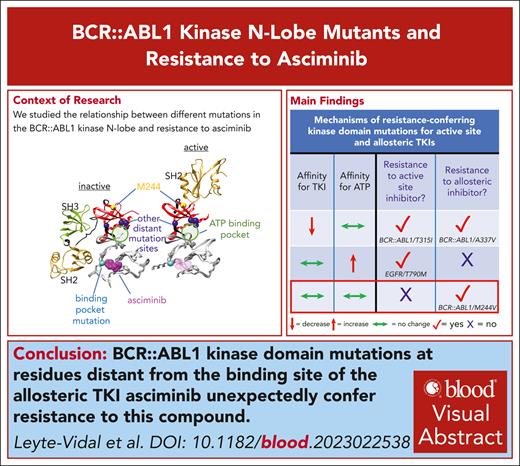
Key Points
BCR::ABL1 M244V confers clinical resistance to asciminib; M244V and other kinase N-lobe mutations confer resistance to asciminib in vitro.
Mutations in the BCR::ABL1 kinase domain may impair the durability of response to combinations of asciminib and ATP-competitive TKIs.
Visual Abstract
Secondary kinase domain mutations in BCR::ABL1 represent the most common cause of resistance to tyrosine kinase inhibitor (TKI) therapy in patients with chronic myeloid leukemia. The first 5 approved BCR::ABL1 TKIs target the adenosine triphosphate (ATP)–binding pocket. Mutations confer resistance to these ATP-competitive TKIs and those approved for other malignancies by decreasing TKI affinity and/or increasing ATP affinity. Asciminib, the first highly active allosteric TKI approved for any malignancy, targets an allosteric regulatory pocket in the BCR::ABL1 kinase C-lobe. As a non–ATP-competitive inhibitor, the activity of asciminib is predicted to be impervious to increases in ATP affinity. Here, we report several known mutations that confer resistance to ATP-competitive TKIs in the BCR::ABL1 kinase N-lobe that are distant from the asciminib binding pocket yet unexpectedly confer in vitro resistance to asciminib. Among these is BCR::ABL1 M244V, which confers clinical resistance even to escalated asciminib doses. We demonstrate that BCR::ABL1 M244V does not impair asciminib binding, thereby invoking a novel mechanism of resistance. Molecular dynamic simulations of the M244V substitution implicate stabilization of an active kinase conformation through impact on the α-C helix as a mechanism of resistance. These N-lobe mutations may compromise the clinical activity of ongoing combination studies of asciminib with ATP-competitive TKIs.
Introduction
Tyrosine kinase inhibitors (TKIs) have improved outcomes for patients with chronic myeloid leukemia (CML)1 and several other malignancies. Most TKIs target the adenosine triphosphate (ATP)–binding site nestled in the cleft between the kinase N- and C-lobes, in a type I manner, where the aspartate residue in the conserved Asp-Phe-Gly (DFG) motif at the base of the kinase activation loop points inward (DFG-in) or type II (DFG-out) kinase conformation. Examples include the first 5 approved BCR::ABL1 TKIs (type I: dasatinib, bosutinib; type II: imatinib, nilotinib, and ponatinib). Because of the highly conserved nature of ATP-binding pockets, these TKIs exhibit varying degrees of kinase selectivity and toxicity. There is considerable interest in developing allosteric inhibitors, which may achieve superior selectivity and tolerability. Inhibitors that bind allosteric sites adjacent to the ATP-binding pocket (eg, trametinib, cobimetinib) are defined as type III inhibitors. Type IV inhibitors target allosteric pockets removed from the ATP-binding site.2 The prototypic type IV TKI asciminib has recently demonstrated considerable efficacy and tolerability.3,4 Asciminib is approved for patients with chronic phase CML with resistance/intolerance to 2 prior TKIs and is being investigated in the frontline setting. Regulation of kinase activity of the native ABL1B isoform is achieved by myristoylation of its N-terminus, which subsequently binds to a myristoyl-binding pocket in the kinase C-lobe and stabilizes an inactive kinase conformation through favoring interactions between the SRC Homology 3 (SH3) domain, the SH2-kinase domain linker, and the N-lobe (N-lobe/SH3), and the SH2 domain and the C-lobe (C-lobe/SH2).5,6 Asciminib targets this myristoyl-binding pocket and thereby mimics a feature of physiological ABL1 inhibition in BCR::ABL1 through stabilization of N-lobe/SH3 and C-lobe/SH2 interactions.7
Secondary kinase domain mutations are well-recognized causes of on-target TKI resistance.8-11 Clinically detected resistance-conferring kinase domain mutations impact relative affinities toward ATP and TKI, shifting the balance toward ATP, typically by diminishing kinase binding/occupancy,12 and less commonly through increasing ATP affinity.13 Resistance-conferring mutations for prior BCR::ABL1 TKIs are largely concentrated in close spatial proximity to amino acid positions that line the ATP-binding pocket.14 To date, the limited number of clinically resistant BCR::ABL1 kinase domain mutations for asciminib include substitutions in the myristoyl-binding pocket (eg, A337V/T, I502L),3 and C-lobe substitutions of a residue close to the ATP-binding pocket and αC-helix (F359C/I/V).15 Here, we describe several mutations in the kinase N-lobe that unexpectedly confer resistance to asciminib and propose a novel resistance mechanism.
Study design
Cell viability studies
BCR::ABL1-transformed Ba/F3 cells were assessed for sensitivity to TKI (asciminib; Selleckchem), as previously described.8
Western immunoblots
Biochemical assessments of asciminib inhibition were performed, as previously described,8 using anti-ABL1 phosphorylated Tyr412 (Abcam), anti-ABL1 (BD Biosciences), phosphorylated STAT5, STAT5, and glyceraldehyde-3-phosphate dehydrogenase (Cell Signaling).
Protein expression/purification
ABL1 SH3-SH2-kinase domain unit (SH3-SH2-KD) proteins (wild type and M244V; residues 63-512, splice form 1A numbering) were expressed in Escherichia coli and purified as previously described.16
Isothermal titration calorimetry
Asciminib (100 μM) was titrated in 19 steps (0.4 μL for the first and 2 μL for the other steps) to the ABL1 proteins (10 μM) in the calorimetry cell in 50 mM Tris-HCl, pH 7.5, 500 mM NaCl, 5% glycerol (MicroCal PEAQ-ITC [Malvern Pananlytical] instrument). MicroCal software was used to determine thermodynamic parameters.
Cellular thermal shift assays
A total of 3 × 107 Ba/F3 cells transduced with BCR::ABL1 isoforms were exposed to 10 μM asciminib or vehicle for 2 hours, resuspended in 750 μL of phosphate-buffered saline, divided into 12 50-μL aliquots, exposed to a temperature gradient (37°C-52.8°C) for 3 minutes, and placed on ice for 3 minutes. Lysates were prepared and subjected to immunoblotting. Anti-ABL1 (BD biosciences) was used to assess BCR::ABL1 protein stability; GAPDH served as a loading control.
Molecular dynamic simulations
Mutations were introduced into the native structural model (5MO4), prepared with Schrödinger Protein Preparation Wizard, and minimized using Amber Molecular Dynamics Suite in 2-step minimization: restrained and unrestrained, for 500 steps of steepest descent and 500 steps of conjugate gradients. Models were heated up to 300 K and equilibrated in 3 steps: restrained + heating, lower restraints, and unrestrained equilibration for 1 ns. Equilibrated models were run for 3 independent 500-ns production runs. Representative structures shown were chosen on the basis of root-mean-square deviation analysis of trajectories.
Deidentified samples were used.
Results and discussion
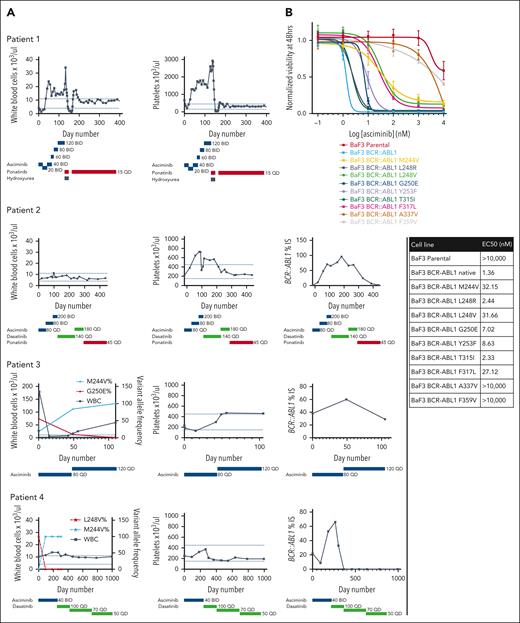
Four TKI-pretreated patients with CML at 2 institutions displayed unanticipated asciminib resistance. Two harbored predominant M244V substitutions in the absence of other detectable kinase domain mutations before initiating asciminib. Neither achieved an objective response to asciminib despite dose escalation to as much as 200 mg twice daily, the dose currently recommended only for patients with the BCR::ABL1 T315I mutation. At the end of asciminib treatment, M244V persisted as the sole detectable kinase domain substitution by Sanger sequencing in both cases. Ponatinib subsequently achieved durable responses in both patients. Two additional patients, 1 with a minor M244V subclone and 1 without any grossly detectable M244V by next-generation sequencing, achieved transient responses with asciminib before disease progression. In both cases, the M244V substitution rapidly established clonal dominance. One patient subsequently achieved a durable response with dasatinib, whereas the other experienced rapid disease transformation and death before receiving alternative TKI treatment (Figure 1A).
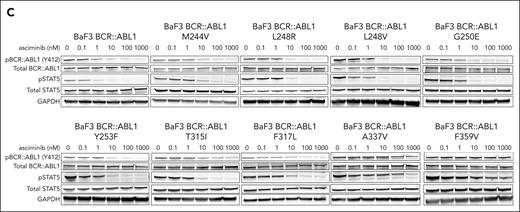
BCR::ABL1 M244V and other N-lobe mutations are associated with resistance to asciminib. (A) Hematologic and molecular parameters of 4 patients with preexisting or rapidly emerging M244V substitutions. Dotted lines represent normal reference range for hematologic parameters. Dose (mg) and duration of TKI therapy is listed below each graph. BCR::ABL1 transcript levels were not assessed for patient 1, as this patient had never had an appreciable molecular response during 10 years of prior TKI therapy. (B) Normalized viability of Ba/F3 cells transduced with BCR::ABL1 isoforms depicted in increasing concentrations of asciminib after 48 hours of treatment. Data represent 3 independent experiments. Error bars represent standard error of the mean. EC50 values are represented in the table. (C) Representatives of 3 immunoblots of lysates of Ba/F3 cells expressing the isoforms listed in increasing concentrations of asciminib. Cells were lysed after 2 hours of asciminib exposure. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; pSTAT5, phosphorylated STAT5.
BCR::ABL1 M244V and other N-lobe mutations are associated with resistance to asciminib. (A) Hematologic and molecular parameters of 4 patients with preexisting or rapidly emerging M244V substitutions. Dotted lines represent normal reference range for hematologic parameters. Dose (mg) and duration of TKI therapy is listed below each graph. BCR::ABL1 transcript levels were not assessed for patient 1, as this patient had never had an appreciable molecular response during 10 years of prior TKI therapy. (B) Normalized viability of Ba/F3 cells transduced with BCR::ABL1 isoforms depicted in increasing concentrations of asciminib after 48 hours of treatment. Data represent 3 independent experiments. Error bars represent standard error of the mean. EC50 values are represented in the table. (C) Representatives of 3 immunoblots of lysates of Ba/F3 cells expressing the isoforms listed in increasing concentrations of asciminib. Cells were lysed after 2 hours of asciminib exposure. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; pSTAT5, phosphorylated STAT5.
To determine if BCR::ABL1 M244V confers asciminib resistance in vitro, cell viability assays were performed in BCR::ABL1-transduced Ba/F3 cells. M244V conferred ∼2 logs greater asciminib resistance relative to native BCR::ABL1, and ∼1 log greater resistance than BCR::ABL1 T315I. We assessed additional mutations in the kinase N-lobe in the vicinity of M244 that confer resistance to ATP-competitive TKIs, many of which have not been previously assessed for asciminib sensitivity. These included mutations in the kinase P-loop (L248R, L248V, G250E, and Y253F), hinge region (T315I, F317L, and F359V), and myristoyl-binding region (A337V). Four classes of asciminib resistance were observed: mild (T315I, L248R), moderate (G250E, Y253F), high (M244V, L248V, and F317L), and complete (A337V, F359V) (Figure 1B). Western immunoblot analysis demonstrated a strong correlation between biochemical resistance in cell-based assessments and resistance in cellular proliferation assays (Figure 1C).
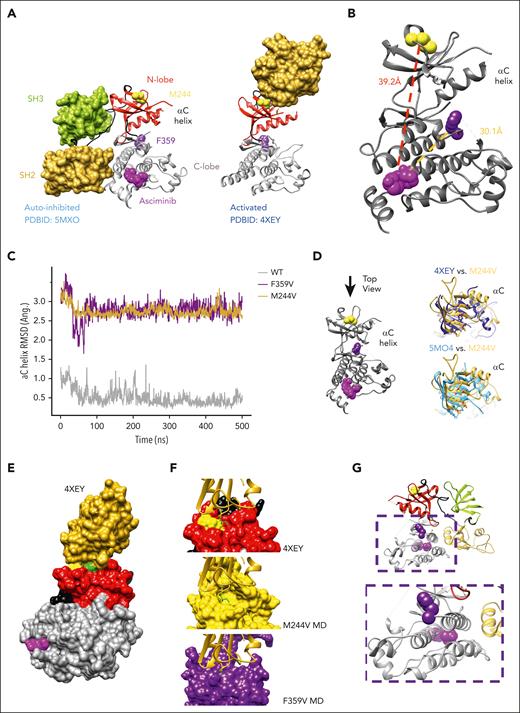
We focused our attention on 2 mutations that are clinically resistant to asciminib and not in the myristoyl-binding pocket: M244V and F359V. Given that these substitutions are not equivalently resistant to all other BCR::ABL1 TKIs, their resistance to asciminib cannot be ascribed resistance to increased ATP affinity. To gain further insights into potential mechanisms of resistance to asciminib, we examined autoinhibited ABL1 (PDBID: 5MO4)7 and activated ABL1 complexes (PDBID: 4XEY),17 which reveal different interactions between the kinase domain and SH3/SH2 domains (Figure 2A). Although the autoinhibited conformation exhibits interactions that span both lobes of the kinase domain and the SH3-SH2 domains (Figure 2A, left), the activated conformation involves interactions of only the top of the N-lobe and the SH2 domain (Figure 2A, right). Notably, M244V lies in the N-lobe near the interface with the SH2 domain that is critical for the activated conformation16; F359V lies in the C-lobe at the opposite end of the αE helix, which forms part of the myristoyl-binding pocket (Figure 2B). We hypothesized that M244V is unlikely to directly impact asciminib binding. Rather, it is possible that its mechanism of resistance involves stabilization of the activated kinase conformation (N-lobe/SH2) relative to the autoinhibited conformation (N-lobe/SH3, C-lobe/SH2). The F359V mutation could directly impact asciminib binding given that it is located on helix αE, at the opposite end of the myristoyl-binding pocket, but it could also impact the stability of the autoinhibitory interaction between the kinase domain and the SH3-SH2 domains.
Structural hypotheses and computational studies of asciminib resistance mechanisms for M244V and F359V. (A) Structural models of autoinhibited ABL1-SH2/SH3 complex (left; PDBID: 5MO4) with asciminib bound, and activated ABL1-SH2 complex (right; PDBID: 4XEY). Crystallographic active site ligands are omitted for clarity. M244 is shown as yellow spheres, and F359 is shown as purple spheres. (B) Relative position and distance between points of mutation and asciminib binding pocket. (C) αC helix RMSD for wild type (WT), M244V, and F359V simulations with respect to the average structure from the WT simulation. (D) Rearrangements of top of the N-terminal kinase domain, which interfaces SH2 domain in activated structure, for M244V compared with activated structure (top, dark blue) and autoinhibited structure (bottom, light blue). (E) Surface representation of ABL1 kinase N-lobe-SH2 interaction in the activated ABL1 conformation (PDBID: 4XEY). Colors: N-lobe (red), C-lobe (gray), hinge (black), SH2 (gold), asciminib (pink), M244 (yellow), F359 (purple), and I164 (green). (F) Molecular dynamic snapshots of M244V exhibit a well-formed complementary pocket for I164 (bright green) in the SH2 domain, which defines the molecular interaction surface of the kinase N-lobe with the SH2 domain (middle). Compare with similar observation for crystallographic model of activated conformation (top), and contrast with MD snapshots from F359V condition for which no such complementary pocket is significantly observed (bottom). (G) C-lobe α helix bundle (gray) with F359V on one end (purple) and asciminib bound at other end (pink). SH2 domain (yellow) interactions in the autoinhibited conformation could play a role in mechanisms that impact the arrangement of the C-lobe helix bundle.
Structural hypotheses and computational studies of asciminib resistance mechanisms for M244V and F359V. (A) Structural models of autoinhibited ABL1-SH2/SH3 complex (left; PDBID: 5MO4) with asciminib bound, and activated ABL1-SH2 complex (right; PDBID: 4XEY). Crystallographic active site ligands are omitted for clarity. M244 is shown as yellow spheres, and F359 is shown as purple spheres. (B) Relative position and distance between points of mutation and asciminib binding pocket. (C) αC helix RMSD for wild type (WT), M244V, and F359V simulations with respect to the average structure from the WT simulation. (D) Rearrangements of top of the N-terminal kinase domain, which interfaces SH2 domain in activated structure, for M244V compared with activated structure (top, dark blue) and autoinhibited structure (bottom, light blue). (E) Surface representation of ABL1 kinase N-lobe-SH2 interaction in the activated ABL1 conformation (PDBID: 4XEY). Colors: N-lobe (red), C-lobe (gray), hinge (black), SH2 (gold), asciminib (pink), M244 (yellow), F359 (purple), and I164 (green). (F) Molecular dynamic snapshots of M244V exhibit a well-formed complementary pocket for I164 (bright green) in the SH2 domain, which defines the molecular interaction surface of the kinase N-lobe with the SH2 domain (middle). Compare with similar observation for crystallographic model of activated conformation (top), and contrast with MD snapshots from F359V condition for which no such complementary pocket is significantly observed (bottom). (G) C-lobe α helix bundle (gray) with F359V on one end (purple) and asciminib bound at other end (pink). SH2 domain (yellow) interactions in the autoinhibited conformation could play a role in mechanisms that impact the arrangement of the C-lobe helix bundle.
To investigate these hypotheses, we performed molecular dynamic simulations to generate structural models for the M244V and F359V mutants. We focused our attention on the reorganization at the N-lobe (especially the αC helix) induced by both mutants and compared them with both crystallographic structural models. Preliminarily, the simulations suggest M244V and F359V mutations alter the αC helix conformation (Figure 2C), which interacts with the SH2 domain in the active conformation, potentially in ways favoring SH2 docking to the N-lobe in the active state at the expense of the autoinhibited state induced by asciminib binding. N-lobe structural reorganization consistent with N-lobe-SH2 domain interactions was observed for the M244V mutant (Figure 2D-E). Molecular dynamic snapshots demonstrate a well-formed complementary pocket for I164 in SH2, which defines the molecular interaction surface with M244V in the kinase N-lobe (Figure 2F). Further computational studies that consider the SH3-SH2 domains are needed for more rigorous characterization. Our findings support a model whereby asciminib binding is largely unaffected by M244V, and resistance is mediated by alterations of the relative stability of biological conformations. F359V could conceivably impact asciminib binding because of its location at the opposite end of the α-helix bundle that causes the allosteric pocket, although our simulations did not show significant deformation of the helix bundle (Figure 2G). Hence, it is likely that changes in the SH2 domain in an autoinhibited-like conformation may also play a role in F359V-mediated resistance, further highlighting the need for more sophisticated molecular simulations and structural studies.
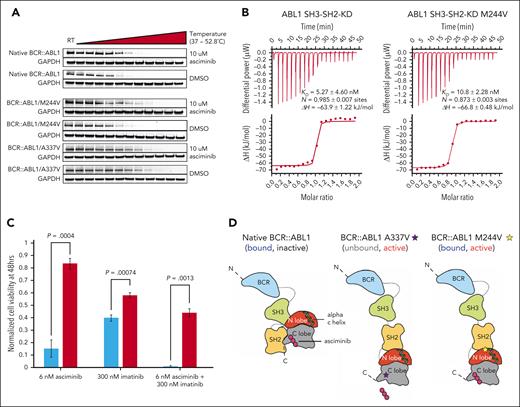
To begin to test these models for our index N-lobe mutation, we first performed cellular thermal shift assays, which rely on the principle of compounds conferring thermal stability to proteins with which they interact18 (Figure 3A). We found that the M244V mutant is stabilized to a similar degree as native BCR::ABL1, whereas the myristoyl-binding pocket A337V mutant displayed no stabilization. We next recombinantly expressed and purified BCR::ABL1 protein fragments spanning the SH3, SH2, and kinase domains (amino acids 63-512, ABL1A numbering). These are the core domains that are required for BCR::ABL1/ABL1B kinase regulation and can recapitulate an asciminib allosteric inhibitory mechanism.6,19 Isothermal titration calorimetry showed that asciminib binds the ABL1 SH3-SH2-KD wild type and M244V mutant with similar binding affinities (5 and 10 nM, respectively; Figure 3B). Collectively, these results demonstrate that the M244V mutation does not impair binding of asciminib to the myristoyl pocket but may confer resistance through disruption of the induced allosteric effect of asciminib.
Mechanistic studies of asciminib resistance mechanisms for M244V and assessment of sensitivity to combined asciminib/imatinib treatment. (A) Representative of 3 cellular thermal shift assays performed in Ba/F3 cells of BCR::ABL1 isoforms indicated. (B) Isothermal titration calorimetry (ITC) measurements of recombinant ABL1 SH3-SH2-kinase domain (SH3-SH2-KD) unit native (left panel) and M244V mutant (right panel) (10 μM, respectively) with asciminib (100 μM) at 25°C. Each panel shows the raw heat signal of an ITC experiment (top) and the integrated calorimetric data of the area of each peak (bottom). The continuous line represents the best fit of the data based on a 1:1 binding model computed from the MicroCal software. A representative measurement of 2 independent experiments is shown with Kd value, stoichiometry (N), and enthalpy (ΔH) calculated from the fit. (C) Assessment of sensitivity of Ba/F3 cells harboring native BCR::ABL1 or BCR::ABL1/M244V to imatinib, asciminib, or the combination. Biological and technical triplicates were performed. (D) Proposed model of BCR::ABL1/M244V-mediated disruption of asciminib-induced allosteric modulation. DMSO, dimethyl sulfoxide; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Mechanistic studies of asciminib resistance mechanisms for M244V and assessment of sensitivity to combined asciminib/imatinib treatment. (A) Representative of 3 cellular thermal shift assays performed in Ba/F3 cells of BCR::ABL1 isoforms indicated. (B) Isothermal titration calorimetry (ITC) measurements of recombinant ABL1 SH3-SH2-kinase domain (SH3-SH2-KD) unit native (left panel) and M244V mutant (right panel) (10 μM, respectively) with asciminib (100 μM) at 25°C. Each panel shows the raw heat signal of an ITC experiment (top) and the integrated calorimetric data of the area of each peak (bottom). The continuous line represents the best fit of the data based on a 1:1 binding model computed from the MicroCal software. A representative measurement of 2 independent experiments is shown with Kd value, stoichiometry (N), and enthalpy (ΔH) calculated from the fit. (C) Assessment of sensitivity of Ba/F3 cells harboring native BCR::ABL1 or BCR::ABL1/M244V to imatinib, asciminib, or the combination. Biological and technical triplicates were performed. (D) Proposed model of BCR::ABL1/M244V-mediated disruption of asciminib-induced allosteric modulation. DMSO, dimethyl sulfoxide; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Our results demonstrate an unexpected degree of clinical and/or preclinical resistance to asciminib by substitutions in the kinase N-lobe. Asciminib should be considered contraindicated in cases that harbor M244V, one of the most commonly detected imatinib-resistant mutations in clinical isolates.20-22 In such cases, effective treatment options are dasatinib, nilotinib, bosutinib, and ponatinib. Evidence of clinical asciminib resistance is required for other N-lobe substitutions. In patients with asciminib resistance, it is imperative to evaluate for mutations outside of the myristoyl-binding pocket. Ongoing clinical approaches combining asciminib with type I or type II TKIs may be hampered by resistance-conferring N-lobe substitutions. We have found that the M244V mutant retains resistance to the combination of asciminib and imatinib in vitro (Figure 3C). Recent work suggests the presence of communication between the N-lobe and myristoyl-binding pocket,23 and although we cannot formally rule out that such interactions may be contributing to asciminib resistance, our work implicates a novel mechanism of resistance to the emerging class of type IV TKIs, whereby allostery (ie, interdomain interaction) is primarily impacted, rather than the relative affinities of TKI (eg, A337V) or ATP (Figure 3D).
Acknowledgments
The authors thank Bogdan Popescu for assistance with troubleshooting experiments and technical advice, and Ben Cravatt for helpful discussions.
This work was supported in part by an American Society of Hematology Minority Hematology Graduate Award to A.L.-V. and the European Treatment and Outcome Study for CML to I.B.L. and O.H. N.P.S. acknowledges the Edward S. Ageno family for continued support.
Authorship
Contribution: A.L.-V., D.G.R., M.P.J., Y.S., I.B.L., O.H., R.D., and N.P.S. designed the study and analyzed and interpreted the data; D.G.R. and M.P.J. performed molecular dynamic simulations; D.R. and N.P.S. provided clinical information; A.L.-V., R.D., I.B.L., S.P., F.H., K.B.M., and A.M. performed wet laboratory experiments; A.L.-V., D.G.R., M.P.J., Y.S., and N.P.S. wrote the first manuscript draft; and all authors critically revised and approved the final version of the article.
Conflict-of-interest disclosure: N.P.S. has received funding from Bristol Myers Squibb Oncology for the conduct of clinical research. D.G.R. has served on an advisory board, steering committee, and as a consultant for Novartis Pharmaceuticals. O.H. received funding from Novartis through the European Treatment and Outcome Study for CML; and speaker’s honoraria from Novartis. The remaining authors declare no competing financial interests.
Correspondence: Neil P. Shah, Division of Hematology/Oncology, Department of Medicine, University of California, San Francisco, CA; email: neil.shah@ucsf.edu.
References
Author notes
Data are available on request from the corresponding author, Neil P. Shah (neil.shah@ucsf.edu).
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal