Key Points
CAR T-cell therapy demonstrates superior efficacy to bispecific antibody in DLBCL treatment, with higher CR rates.
CAR T-cell therapy exhibits higher incidence of grade ≥3 adverse events than bispecific antibody.
Visual Abstract
This meta-analysis evaluates the efficacy and safety of chimeric antigen receptor (CAR) T-cell therapy and bispecific antibodies for relapsed/refractory diffuse large B-cell lymphoma (R/R DLBCL). We searched MEDLINE, Embase, and Cochrane databases until July 2023 for trials assessing CAR T-cell therapies and CD20×CD3 bispecific antibodies as third or subsequent lines in R/R DLBCL. Random-effects models estimated the complete response (CR) rate and secondary outcomes, with meta-regressions adjusting for relevant covariates. Sixteen studies comprising 1347 patients were included in the pooled analysis. The pooled CR rate for bispecific antibodies was 0.36 (95% confidence interval [CI], 0.29-0.43), compared with 0.51 (95% CI, 0.46-0.56) for CAR T-cell therapy (P < .01). This superiority persisted when comparing the CAR T-cell–naive patients within the bispecific antibody group, with a CR rate of 0.37 (95% CI, 0.32-0.43). Multivariable meta-regression also revealed better efficacy of CAR T cells with adjustment for the proportion of double-hit lymphoma. The pooled 1-year progression-free survival rate mirrored these findings (0.32 [95% CI, 0.26-0.38] vs 0.44 [95% CI, 0.41-0.48]; P < .01). For adverse events of grade ≥3, the bispecific antibody had incidences of 0.02 (95% CI, 0.01-0.04) for cytokine release syndrome, 0.01 (95% CI, 0.00-0.01) for neurotoxicity, and 0.10 (95% CI, 0.03-0.16) for infections. The CAR T cell had rates of 0.08 (95% CI, 0.03-0.12), 0.11 (95% CI, 0.06-0.17), and 0.17 (95% CI, 0.11-0.22), respectively, with significant differences observed in the first 2 categories. In summary, CAR T-cell therapy outperformed bispecific antibody in achieving higher CR rates, although with an increase in severe adverse events.
Introduction
Diffuse large B-cell lymphoma (DLBCL) represents the most common subtype of non-Hodgkin lymphoma worldwide, making up ∼20% of all lymphoid malignancies.1 Although most patients with DLBCL may experience long-term survival after frontline immunochemotherapy R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) or comparable treatments, 10% to 15% of patients have primary refractory disease, and 20% to 25% experience relapse after an initial response.2 Among patients with relapsed or refractory (R/R) DLBCL who are considered to be suitable for autologous stem-cell transplantation (ASCT), two-thirds could not proceed to transplantation after salvage chemotherapy3 or experience a relapse soon after ASCT.4 The prognosis remains dismal for patients who are ineligible for aggressive salvage chemotherapy as a second-line treatment and for those who have undergone at least 2 prior systemic treatment.4,5
Various classes of novel therapies have been developed and approved for patients with R/R DLBCL who have received at least 2 prior lines of therapy to address the need for more potent interventions. These include antibody-drug conjugates such as polatuzumab vedotin, targeting CD79b and used in conjunction with bendamustine and rituximab,6 as well as loncastuximab tesirine, which targets CD19.7 Other additions include tafasitamab, an anti-CD19 monoclonal antibody, paired with lenalidomide,8 and the oral exportin 1 inhibitor selinexor.9 Yet, even with these substantial advancements, the most important progression in R/R DLBCL therapy has been the emergence of T-cell–mediated therapies, encompassing chimeric antigen receptor (CAR) T-cell therapy10-13 and T-cell–engaging bispecific antibodies.14,15 Both therapies have changed the treatment paradigm due to their potential in harnessing the body's immune system to target malignant cells, offering hope for patients who do not respond to conventional treatments.
Although axicabtagene ciloleucel (axi-cel) and lisocabtagene maraleucel (liso-cel) have recently been approved for the second-line treatment of R/R DLBCL, CAR T-cell therapy and bispecific antibodies are widely used and recommended as third-line or subsequent therapies for patient who have not previously received the same treatment.16 Although both treatments demonstrate significant potential, the absence of direct comparative studies hinders the clinical decision-making process. Given the difficulties in evaluating and contrasting the effectiveness of these 2 T-cell–mediated therapies through randomized trials, a pooled analysis of existing studies and a comprehensive comparison of the therapies might offer valuable clinical insights. Considering the current uncertainties, our study undertakes a comparative meta-analysis of CAR T-cell therapy and bispecific antibody treatment. By evaluating both their efficacy and adverse event profiles, we aim to offer a clearer perspective on their therapeutic value in third- or later-line treatments for R/R DLBCL, aiding clinicians in making informed decisions for their patients.
Methods
Systematic literature review
The systematic review and meta-analysis were conducted following the guidelines outlined by Preferred Reporting Items for Systematic Reviews and Meta-Analyses. An extensive literature search was performed on MEDLINE, Embase, and the Cochrane Central Register of Controlled Trial databases, limiting entries to those in English up until 21 February 2024. The primary keywords included “B-cell lymphoma,” “chimeric antigen receptor,” and "bispecific antibodies,” as well as derivatives of each keyword. More details on the search methodology are described in the supplemental Methods, available on the Blood website. Moreover, meeting abstracts from the American Society of Clinical Oncology, American Society of Hematology, European Society for Medical Oncology, and European Hematology Association, as well as the references from relevant articles, were manually reviewed to find additional pertinent studies.
Eligibility criteria
Prospective interventional clinical trials that determined a therapeutic dose and evaluated the efficacy of CD20×CD3 bispecific monoclonal antibodies or CAR T-cell therapy for R/R DLBCL were selected for the meta-analysis. Phase 1 study, in which the recommended dose for phase 2 was confirmed and its efficacy evaluated, was also included. Studies with the following characteristics were excluded: (1) evaluated the efficacy of therapies as first-line or second-line treatment; (2) mainly comprised patients with central nervous system lymphoma, B-cell cutaneous lymphoma, HIV-related lymphoma, or primary mediastinal lymphoma; (3) studied the effectiveness of radiotherapy only; (4) had no information on previous treatments; (5) studied a pediatric population; (6) evaluated the efficacy of retreatment using therapies with the same mechanism of action; and (7) assessed dual or other targeting CAR T-cell therapy or bispecific antibody.
Data extraction and quality assessment
The following records from the included studies were independently extracted by 2 authors (J.K. and J.C.): trial name, trial identifier, published journal, publication year, name of the first author, trial phase, the nation where the trial was conducted, treatment regimens, number of patients, and median follow-up duration. Histology information on the proportions of patients with de novo DLBCL, transformed lymphoma, primary mediastinal DLBCL, germinal center B-cell like subtype, and double-hit or triple-hit lymphoma were collected. Clinical data included the median age (with range), median number of prior lines of therapies (with range), allowance for bridging chemotherapy (in CAR T-cell studies), proportions of patients who were aged ≥65 years, of male sex, at stage III/IV, with high lactate dehydrogenase levels, bulky disease, ≥3 previous lines of treatment, with International Prognostic Index scores of 0 to 1, 2 to 3, or 4 to 5, those who had undergone prior ASCT, those refractory to their last treatment, and those who had received prior CAR T-cell therapy (in bispecific antibody studies) were also obtained. Efficacy outcomes including complete response (CR) rate and 1-year progression-free survival (PFS) rate, as well as grade ≥3 adverse events outcomes including cytokine release syndrome (CRS), neurologic events, and infection, were also extracted. In the bispecific antibody trials, CR rates for the CAR T-cell–naive population were gathered additionally. Two authors (J.K. and S.J.K.) independently assessed the potential assessed risk of bias of the studies included using the methodological index for nonrandomized studies (MINORS).17 The global ideal score was 16 for noncomparative studies and 24 for comparative studies.
Data synthesis and analyses
The primary outcome was CR rate. The secondary outcomes were 1-year PFS rate and grade ≥3 adverse events of CRS, neurologic events, and infection, which were analyzed according to the treatments. CR rates for the CAR T-cell–naive population in the bispecific antibody group compared with the CAR T-cell group were also analyzed. Using random-effects models, the pooled primary and secondary outcomes along with corresponding 95% confidence intervals (CIs) and P values were calculated. This model was a meta-analysis of single-arm proportion, and the calculations were derived using the inverse variance method. To assess primary and secondary outcomes according to the treatment category, subgroup analyses were performed using the Q test. A meta-regression analysis was also conducted to evaluate potential moderators influencing the CR rate and adjust for those variables. These included median age, the proportion of patients with transformed lymphoma, double-hit or triple-hit lymphoma, those aged ≥65 years, at stage III to IV, with ≥3 previous lines of treatment, those who had undergone prior ASCT, and those refractory to the prior last treatment. Variables with a P value <.1 in the univariate meta-regression were included in the multivariate meta-regression analysis. Meta-regression was executed using the metareg function, using a mixed-effects model with standard settings. Heterogeneity was evaluated using the τ2 and I2 statistics. Sensitivity analyses were conducted in 2 ways: (1) based on bias assessment, specifically by removing studies with a MINORS score of <14; and (2) by excluding 1 study at a time and analyzing its effect on the main summary estimate to assess whether any single study exerted a dominant effect. Two-sided statistical tests were used, and P values ≤.05 were considered significant. Quantitative pooled analysis and meta-regression modeling were carried out using R studio software (version 2022.07.1; R Foundation for Statistical Computing, Vienna, Austria).
Results
Literature search
The initial database search yielded 3012 records. Once duplicates were eliminated and study titles and abstracts were screened, 269 potentially eligible studies remained for detailed review. As outlined in “Methods,” further exclusion criteria were applied to derive the final 16 studies, 6 studies14,15,18-21 involving bispecific antibodies and 1010-13,22-27 with CAR T-cell therapy, comprising 1347 patients included in the pooled analysis (Figure 1).
Characteristics of studies
The baseline characteristics of the 16 studies are detailed in Table 1 and supplemental Table 1. All studies were phase 2 trials, except for one13 with a seamless design and another27 that was a phase 1 trial assessing anakinra with CAR T-cell therapy. The publication years ranged from 2017 to 2023. Regimens of 6 bispecific antibody trials included epcoritamab,15,21 glofitamab,14,20 mosunetuzumab,19 and odronexatamb.18 Ten CAR T-cell trials comprised 2 studies with tisagenlecleucel (tisa-cel),11,12 4 with axi-cel,10,23,26,27 2 with liso-cel,13,25 1 with relmacabtagene,22 and 1 combining axi-cel and tisa-cel.24 Among the total 16 studies, the median number of previous systemic treatments was 2 in 5 trials,18,20,22,25,26 with 2 of these trials being in the bispecific antibody group and 3 in the CAR T-cell group. The median number of prior treatments was 3 in the remaining 11 trials. In the CAR T-cell trials, 2 studies10,23 did not permit bridging chemotherapy before CAR T-cell infusion. In the bispecific antibody trials, 2 studies18,21 excluded patients who had previously received CAR T-cell therapy, whereas the other 4 trials included patients with a history of CAR T-cell therapy, ranging from 20.0% to 38.9% of their populations.
Baseline characteristics of included studies
| Trial name . | Authors (trial identifier) . | Phase . | Regimen . | No. . | Median age (range), y . | DH/TH lymphoma, % . | Stage III/IV, % . | Median no. of previous therapy (range) . | Prior ASCT, % . | Refractory to last prior treatment, % . |
|---|---|---|---|---|---|---|---|---|---|---|
| Bispecific antibody | ||||||||||
| EPCORE NHL-1 | Thieblemont et al15 (NCT03625037) | 2 | Epcoritamab | 157 | 64 (20-83) | 0.131 | 0.752 | 3 (2-11) | 0.197 | 0.828 |
| NR | Dickinson et al14 (NCT03075696) | 2 | Glofitamab | 154 | 66 (21-90) | 0.129 | 0.753 | 3 (2-7) | 0.182 | 0.857 |
| NR | Bartlett et al18 (NCT02500407) | 2 | Mosunetuzumab | 88 | 66.5 (24-96) | 0.193 | 0.841 | 3 (2-13) | 0.17 | 0.795 |
| ELM-2 | Poon et al17 (NCT03888105) | 2 | Odronextamab | 140 | 66 (24-88) | 0.193 | 0.80 | 2 (2-8) | 0.157 | 0.864 |
| NR | Song et al20 (NCT04657302) | 2 | Glofitamab | 30 | 57.5 (20-82) | NR | 0.90 | 2 (2-6) | 0.10 | 0.90 |
| EPCORE NHL-3 | Izutsu et al21 (NCT04542824) | 2 | Epcoritamab | 36 | 68.5 (44-89) | 0.0 | 0.778 | 3 (2-8) | 0.194 | 0.806 |
| CAR T cell | ||||||||||
| NR | Schuster et al11 (NCT02030834) | 2 | Tisa-cel | 14 | 58 (25-77) | 0.214 | 0.643 | 3 (1-8) | 0.5 | 0.86 |
| ZUMA-1 | Neelapu et al10 (NCT02348216) | 2 | Axi-cel | 111 | 58 (51-64 [IQR]) | 0.095 | 0.775 | 3 (2-4) | 0.189 | 0.604 |
| JULIET | Schuster et al12 (NCT02445248) | 2 | Tisa-cel | 115 | 56 (46-84) | 0.27 | 0.765 | 3 (2-6) | 0.487 | 0.548 |
| TRANSCEND NHL 001 | Abramson et al13 (NCT02631044) | Seamless | Liso-cel | 269 | 63 (54-70) | 0.134 | NR | 3 (2-4) | 0.335 | 0.673 |
| RELIANCE | Ying et al19 (NCT04089215) | 2 | Relmacabtagene | 59 | 56 (18-75) | 0.051 | NR | 2 (2-7) | 0.102 | 0.814 |
| NR | Kato et al20 (JapicCTI-183914) | 2 | Axi-cel | 16 | 58 (44-70) | 0.067 | 0.5 | 3 (NR) | 0.375 | 0.625 |
| NR | Park et al21 (NCT04148430) | 2 | Axi-cel/tisa-cel | 27 | 62 (25-77) | NR | 0.61 | 3 (2-5) | 0.19 | 0.71 |
| NR | Jacobson et al26 (NCT03954106) | 2 | Axi-cel | 25 | 68 (31-78) | NR | NR | 2 (1-6) | 0.20 | 0.84 |
| NR | Strati et al27 (NCT04432506) | 1 | Axi-cel | 20 | 58 (26-81) | NR | 0.95 | 3 (2-8) | 0.05 | 1.00 |
| OUTREACH | Godwin et al25 (NCT03744676) | 2 | Liso-cel | 82 | 66 (28-86) | 0.18 | NR | 2 (2-6) | 0.16 | 0.91 |
| Trial name . | Authors (trial identifier) . | Phase . | Regimen . | No. . | Median age (range), y . | DH/TH lymphoma, % . | Stage III/IV, % . | Median no. of previous therapy (range) . | Prior ASCT, % . | Refractory to last prior treatment, % . |
|---|---|---|---|---|---|---|---|---|---|---|
| Bispecific antibody | ||||||||||
| EPCORE NHL-1 | Thieblemont et al15 (NCT03625037) | 2 | Epcoritamab | 157 | 64 (20-83) | 0.131 | 0.752 | 3 (2-11) | 0.197 | 0.828 |
| NR | Dickinson et al14 (NCT03075696) | 2 | Glofitamab | 154 | 66 (21-90) | 0.129 | 0.753 | 3 (2-7) | 0.182 | 0.857 |
| NR | Bartlett et al18 (NCT02500407) | 2 | Mosunetuzumab | 88 | 66.5 (24-96) | 0.193 | 0.841 | 3 (2-13) | 0.17 | 0.795 |
| ELM-2 | Poon et al17 (NCT03888105) | 2 | Odronextamab | 140 | 66 (24-88) | 0.193 | 0.80 | 2 (2-8) | 0.157 | 0.864 |
| NR | Song et al20 (NCT04657302) | 2 | Glofitamab | 30 | 57.5 (20-82) | NR | 0.90 | 2 (2-6) | 0.10 | 0.90 |
| EPCORE NHL-3 | Izutsu et al21 (NCT04542824) | 2 | Epcoritamab | 36 | 68.5 (44-89) | 0.0 | 0.778 | 3 (2-8) | 0.194 | 0.806 |
| CAR T cell | ||||||||||
| NR | Schuster et al11 (NCT02030834) | 2 | Tisa-cel | 14 | 58 (25-77) | 0.214 | 0.643 | 3 (1-8) | 0.5 | 0.86 |
| ZUMA-1 | Neelapu et al10 (NCT02348216) | 2 | Axi-cel | 111 | 58 (51-64 [IQR]) | 0.095 | 0.775 | 3 (2-4) | 0.189 | 0.604 |
| JULIET | Schuster et al12 (NCT02445248) | 2 | Tisa-cel | 115 | 56 (46-84) | 0.27 | 0.765 | 3 (2-6) | 0.487 | 0.548 |
| TRANSCEND NHL 001 | Abramson et al13 (NCT02631044) | Seamless | Liso-cel | 269 | 63 (54-70) | 0.134 | NR | 3 (2-4) | 0.335 | 0.673 |
| RELIANCE | Ying et al19 (NCT04089215) | 2 | Relmacabtagene | 59 | 56 (18-75) | 0.051 | NR | 2 (2-7) | 0.102 | 0.814 |
| NR | Kato et al20 (JapicCTI-183914) | 2 | Axi-cel | 16 | 58 (44-70) | 0.067 | 0.5 | 3 (NR) | 0.375 | 0.625 |
| NR | Park et al21 (NCT04148430) | 2 | Axi-cel/tisa-cel | 27 | 62 (25-77) | NR | 0.61 | 3 (2-5) | 0.19 | 0.71 |
| NR | Jacobson et al26 (NCT03954106) | 2 | Axi-cel | 25 | 68 (31-78) | NR | NR | 2 (1-6) | 0.20 | 0.84 |
| NR | Strati et al27 (NCT04432506) | 1 | Axi-cel | 20 | 58 (26-81) | NR | 0.95 | 3 (2-8) | 0.05 | 1.00 |
| OUTREACH | Godwin et al25 (NCT03744676) | 2 | Liso-cel | 82 | 66 (28-86) | 0.18 | NR | 2 (2-6) | 0.16 | 0.91 |
DH, double-hit; IQR, interquartile range; TH, triple-hit.
The risk of bias assessment is summarized in supplemental Table 2. The overall score was also calculated to compare the quality of each study and to conduct the sensitivity analysis. The score ranged from 12 to 16 out of 16. The main factors that lowered total scores were item 4 (“end points appropriate to the aim of the study”), item 5 (“unbiased assessment of the study end point”), and item 8 (“prospective calculation of study size”).
Pooled analyses
The overall pooled proportion of CR was 0.45 (95% CI, 0.40-0.51). There was significant difference in CR rate between the bispecific antibody and CAR T-cell therapy (P < .01); 0.36 (95% CI, 0.29-0.43) in the bispecific antibody group and 0.51 (95% CI, 0.46-0.56) in the CAR T-cell group (Figure 2A). Even when limiting the bispecific antibody group to patients who had not previously undergone CAR T-cell treatment, there was a significant difference between the 2 groups (Figure 2B). The overall pooled proportion of 1-year PFS was 0.40 (95% CI, 0.35-0.44), and a notable difference in the PFS rate also existed between the 2 groups; 0.32 (95% CI, 0.26-0.38) for the bispecific antibody and 0.44 (95% CI, 0.41-0.48) for the CAR T cell, with a significance level of P value <.01, as shown in supplemental Figure 1.
Pooled CR rate by the treatment category. (A) Whole population and (B) CAR T-cell–naive population.
Pooled CR rate by the treatment category. (A) Whole population and (B) CAR T-cell–naive population.
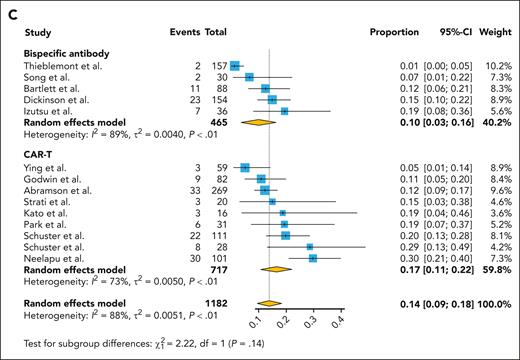
In the analysis related to the adverse events, the incidence of grade ≥3 CRS was 0.02 (95% CI, 0.01-0.04) in the bispecific antibody group and was significantly higher at 0.08 (95% CI, 0.03-0.12) in the CAR T-cell group (Figure 3A). For neurologic events of grade ≥3, the rate was 0.01 (95% CI, 0.00-0.01) in the bispecific antibody group, whereas the CAR T-cell group presented a significantly elevated rate of 0.11 (95% CI, 0.06-0.17; Figure 3B). As for infections of grade ≥3, the bispecific antibody group reported an incidence of 0.10 (95% CI, 0.03-0.16), with the CAR T-cell group again showing a higher incidence at 0.17 (95% CI, 0.11-0.22; Figure 3C).
Pooled grade ≥3 adverse events rate by the treatment category. (A) CRS, (B) neurotoxicity, and (C) infection.
Pooled grade ≥3 adverse events rate by the treatment category. (A) CRS, (B) neurotoxicity, and (C) infection.
A mixed-effects meta-regression model was conducted to evaluate whether the observed heterogeneity was affected by variables such as patient characteristics or study factors and to adjust for these moderators (Table 2). Univariate meta-regression demonstrated that the CAR T-cell therapy (vs bispecific antibody) was the significant moderator associated with CR rate. Variables with a P value <0.1 in the univariate meta-regression included the median age (supplemental Figure 2), the proportion of patients with transformed lymphoma (supplemental Figure 3), and those with double-hit or triple-hit lymphoma (supplemental Figure 4). The multivariable meta-regression model, which incorporated these variables, ultimately revealed that CAR T-cell therapy was significantly better than bispecific antibody (odds ratio, 1.21; P < .001) with adjustment for the proportion of double-hit or triple-hit lymphoma (Table 2).
Meta-regression analysis using study-level characteristics in relation to CR rate
| Variables . | Univariate analysis . | Multivariate analysis . | ||||||
|---|---|---|---|---|---|---|---|---|
| Coefficient . | Standard error . | 95% CI . | P value . | Coefficient . | Standard error . | 95% CI . | P value . | |
| CAR T-cell therapy∗ | 0.1554 | 0.0372 | 0.0824-0.2283 | <.0001 | 0.1901 | 0.0408 | 0.1102-0.2700 | <.0001 |
| Median age, y | −0.0107 | 0.0060 | −0.0224 to −0.0010 | .0734 | 0.0057 | 0.0059 | −0.0059 to 0.0173 | .3366 |
| Transformed lymphoma, % | −0.6213 | 0.3430 | −1.2937 to 0.0510 | .0701 | −0.0547 | 0.2811 | −0.6056 to 0.4961 | .8456 |
| Double-hit or triple-hit lymphoma, % | −0.6870 | 0.3953 | −1.4618 to −0.0877 | .0822 | −0.8101 | 0.2459 | −1.2920 to −0.3282 | .001 |
| Age ≥65 y, % | −0.1665 | 0.1911 | −0.5410 to 0.2080 | .3835 | NA | NA | NA | NA |
| Stage III-IV, % | −0.1739 | 0.3433 | −0.8468 to −0.4989 | .6124 | NA | NA | NA | NA |
| ≥3 previous lines of treatment, % | −0.3759 | 0.3389 | −1.0403 to 0.2884 | .2673 | NA | NA | NA | NA |
| Prior ASCT, % | −0.1020 | 0.2379 | −0.5682 to 0.3642 | .6681 | NA | NA | NA | NA |
| Refractory to last prior treatment, % | −0.0382 | 0.2334 | −0.4956 to 0.4193 | .8701 | NA | NA | NA | NA |
| Variables . | Univariate analysis . | Multivariate analysis . | ||||||
|---|---|---|---|---|---|---|---|---|
| Coefficient . | Standard error . | 95% CI . | P value . | Coefficient . | Standard error . | 95% CI . | P value . | |
| CAR T-cell therapy∗ | 0.1554 | 0.0372 | 0.0824-0.2283 | <.0001 | 0.1901 | 0.0408 | 0.1102-0.2700 | <.0001 |
| Median age, y | −0.0107 | 0.0060 | −0.0224 to −0.0010 | .0734 | 0.0057 | 0.0059 | −0.0059 to 0.0173 | .3366 |
| Transformed lymphoma, % | −0.6213 | 0.3430 | −1.2937 to 0.0510 | .0701 | −0.0547 | 0.2811 | −0.6056 to 0.4961 | .8456 |
| Double-hit or triple-hit lymphoma, % | −0.6870 | 0.3953 | −1.4618 to −0.0877 | .0822 | −0.8101 | 0.2459 | −1.2920 to −0.3282 | .001 |
| Age ≥65 y, % | −0.1665 | 0.1911 | −0.5410 to 0.2080 | .3835 | NA | NA | NA | NA |
| Stage III-IV, % | −0.1739 | 0.3433 | −0.8468 to −0.4989 | .6124 | NA | NA | NA | NA |
| ≥3 previous lines of treatment, % | −0.3759 | 0.3389 | −1.0403 to 0.2884 | .2673 | NA | NA | NA | NA |
| Prior ASCT, % | −0.1020 | 0.2379 | −0.5682 to 0.3642 | .6681 | NA | NA | NA | NA |
| Refractory to last prior treatment, % | −0.0382 | 0.2334 | −0.4956 to 0.4193 | .8701 | NA | NA | NA | NA |
NA, not applicable.
Vs bispecific antibody.
Given that several trials were conducted with slightly varying indications and distinct patient baseline characteristics, as well as the subtly differing mechanisms of drugs within the same category, we performed a separate quantitative analysis confined to trials with US Food and Drug Administration (FDA)–approved regimens. In this analysis, the bispecific antibody group consisted of epcoritamab and glofitamab, whereas the CAR T-cell group comprised tisa-cel, axi-cel, and liso-cel. The pooled CR rate for bispecific antibodies and CAR T cells was 0.40 (95% CI, 0.36-0.45) and 0.51 (95% CI, 0.46-0.57), respectively, indicating a significant difference in efficacy (supplemental Figure 5A). When focusing solely on CAR T-cell–naive patients within the bispecific antibody group, the CR rate for bispecific antibodies was reported at 0.43 (95% CI, 0.37-0.49), with a P value of 0.04 when comparing the 2 groups (supplemental Figure 5B). The pooled 1-year PFS rate differed significantly between CAR T cell vs bispecific antibody with FDA approval: 0.37 (95% CI, 0.32-0.42) for bispecific antibodies and 0.45 (95% CI, 0.41-0.48) for CAR T cell, with significance at P value of .02 (supplemental Figure 6). In the same analysis of FDA-approved agents, the frequency differences in 3 adverse events of grade ≥3 also showed results similar to the primary analysis (supplemental Figure 7).
Sensitivity analysis using the MINORS revealed that the difference in CR rate between the 2 groups was maintained after excluding studies with a relatively high risk of bias (MINORS score <14; supplemental Figure 8). In another sensitivity analysis using the “leave-one-out” method, no single study significantly influenced the overall pooled outcome or the subgroup differences in efficacy between the 2 groups (supplemental Table 3).
Discussion
This meta-analysis highlighted distinct differences in efficacy and safety profiles between bispecific antibodies and CAR T-cell therapies in R/R DLBCL. The pivotal outcome, CR rate, showed a superiority of CAR T cell over bispecific antibodies. Interestingly, even when bispecific antibody–treated cohorts were adjusted to exclude patients with prior CAR T-cell therapy, the gap in efficacy remained significant. Furthermore, meta-regression analysis factored in variables such as patient age and disease aggressiveness (double-hit or triple-hit lymphoma), eventually demonstrating CAR T-cell therapy's enhanced efficacy even after adjusting for these potential confounders. However, this superior efficacy came with an increased incidence of adverse events in the CAR T-cell group, high-grade CRS, neurologic events, and severe infections. When the analysis was specifically focused on FDA-approved treatments, the observed trends in therapeutic effectiveness persisted, underscoring the dominant role of CAR T-cell therapy in R/R DLBCL management, albeit with necessary caution regarding its safety profile.
CAR T-cell therapy, which modifies a patient's T cells to target the CD19 antigen on cancer cells, has emerged as an innovative treatment for R/R lymphomas. Three CAR T-cell constructs, tisa-cel, axi-cel, and liso-cel, have initially received FDA approval for third-line treatment of R/R DLBCL based on their promising results in pivotal trials. Specifically, in the ZUMA-1 trial, patients infused with axi-cel reported an overall response rate (ORR) of 82% and a CR rate of 54%.10 In the TRANSCEND trial using liso-cel, the ORR was 74% with a CR rate of 54%,13 and the JULIET trial, in which tisa-cel was administered, observed an ORR of 52% and a CR rate of 40%.12 Notably, long-term findings revealed potential cures in a subset of patients, with the ZUMA-1 trial showing a 4-year overall survival rate of 44%28 and the JULIET trial documenting that 39% of patients sustained remission during a median follow-up of 40.3 months.29 These encouraging outcomes suggest that CAR T cell could potentially shift the treatment paradigm for high-risk R/R DLBCL, positioning it as a second-line treatment option.
The advent and authorization of CAR T-cell therapies revealed the potential of T-cell–mediated treatments for B-cell malignancies, marking a notable progression in managing patients with R/R DLBCL. Nonetheless, careful patient selection and robust health care coordination are essential with CAR T cells. The treatment process could be hindered by the logistical hurdles involved in manufacturing a tailored product for individual patient, alongside limited access to specialized treatment facilities, and potential safety concerns such as severe CRS and immune effector cell–associated neurotoxicity syndrome (ICANS).11 Consequently, there's a demand for off-the-shelf agents that offer durable remissions and enhanced tolerability. This demand is particularly pronounced for patients with rapidly progressing disease needing immediate intervention and those of old age or with substantial comorbidities, who could not tolerate intensive therapies.
From this background, another type of monoclonal antibodies known as bispecific antibodies has been developed. Bispecific antibodies are engineered monoclonal antibodies targeting 2 distinct antigens.30 In the context of antilymphoma immunotherapy, bispecific antibodies contain a domain that targets a tumor antigen and another domain aimed at an effector cell, predominantly the CD3 portion of the T-cell receptor complex.31 Currently, bispecific antibodies are being developed that target CD20 on B cells and engage CD3 on T cells, in a 1:1 or 2:1 CD20:CD3 antigen binding fragment format.32 These treatments exhibited remarkable efficacy for R/R DLBCL, with known response rates ranging from 35% to 88% and CR rates of 29% to 42% in CAR T-cell–naive patients. The efficacy in the CAR T-cell exposure group was also commendable, with a CR rate of 34% for epcoritamab in patients who had previously received CAR T-cell therapy and a CR rate of 35% for glofitamab in those with prior CAR T-cell therapy. In our analysis, we found that the pooled CR rate for the whole bispecific antibody group was 36%, whereas it was 37% for the CAR T-cell–naive group. Despite the CAR T-cell–naive group having fewer previous lines of treatment, there was a significant difference in CR rate when compared with the CAR T-cell group, suggesting that the overall tumor response is presumed to be superior in the CAR T-cell group. Given that relapses after CAR T-cell therapy are frequent and treatment options for this group are notably limited,33 the significance of bispecific antibodies in this situation is evidently perceived to be crucial.
Prominent toxic effects associated with CAR T-cell therapy encompass CRS, ICANS, and impaired humoral immunity leading to an increased risk of infection.10,12,13 CRS is the most prevalent, reported in 42% to 93% of patients, with grade ≥3 events emerging in ∼2% to 22% of patients. Bispecific antibodies may also trigger CRS and neurologic toxicities, although seemingly at lower rates and severity, even though the toxicity data are still preliminary. In our analysis, adverse events of grade ≥3 relating to CRS, ICANS, and infection were lower with bispecific antibodies than CAR T cells, with significance observed in the first 2 categories. The occurrence of CRS with grade ≥3 events was documented in 1% to 8% of patients treated with bispecific antibodies, and across the trials, most CRS events were found to occur during the initial 2 cycles.14,15,19 Other severe side effects, grade ≥3 ICANS and infections, were also low, with the former ranging from 0% to 3% and the latter from 1% to 19%. The lower incidence of these adverse events in bispecific antibodies than CAR T cells could be attributed to the different mechanisms of action used by these therapies.34 Bispecific antibody therapy offers a level of dosing control that is not available with CAR T cells, and the dosing and cessation of treatment could be more easily adjusted, which can aid in the management and prevention of adverse events. Additionally, the effects of CAR T-cell therapy may persist for an extended period after infusion, potentially prolonging the exposure to toxic effects. In contrast, bispecific antibodies have a shorter half-life, and their effects diminish more rapidly when the treatment is stopped, potentially resulting in a lower risk of prolonged or late-onset adverse events. In summary, it appears that bispecific antibodies have a distinct advantage concerning several well-known side effects, although the mature data on adverse events should be thoroughly reviewed.
This study encountered some inherent limitations. First, being a study-level meta-analysis of a single-arm study, we were unable to extensively investigate patient-level confounders and mediators. Second, although the analysis was adjusted for various factors that could affect the primary outcome, regression analysis was not used when comparing CAR T-cell–naive patients receiving bispecific antibody therapy with those receiving CAR T-cell therapy or when analyzing studies with FDA approval. Third, although significant heterogeneity was observed within each group according to various results, further analysis for heterogeneity, such as subgroup analysis within the same group, was not performed. Despite these limitations, our meta-analysis was conducted using robust statistical methodologies with a strict set of inclusion criteria, enabling us to confirm the relative efficacy of T-cell–mediated therapies for R/R DLBCL.
In summary, this pooled analysis revealed that CAR T-cell therapy exhibited a higher CR rate than bispecific antibody therapy in the third- or subsequent-line management of R/R DLBCL, albeit with a concurrent increase in the rates of severe adverse events.
Acknowledgments
The authors thank Sooyoung Park (Medical Information and Media Services, Samsung Medical Center, Seoul, Korea) for expert support in designing the literature search.
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health and Welfare, Seoul, Republic of Korea (grant number, HI22C0999), and Inha University Research Grant.
Authorship
Contribution: J.K., W.S.K., and S.J.K. designed the research; J.K., J.C., M.H.L., and S.E.Y. contributed data; J.K., J.C., S.E.Y., and S.J.K. collected and interpreted data; J.K. and S.J.K. analyzed data; J.K., M.H.L., W.S.K., and S.J.K. wrote the manuscript; and all authors approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Seok Jin Kim, Division of Hematology-Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, #81, Irwon-ro, Gangnam-Gu, Seoul 06351, Korea; email: kstwoh@skku.edu.
References
Author notes
The data used in this study, both generated and analyzed, were retrieved or estimated from the primary sources cited in this article.
The data extracted for the meta-analysis can be obtained upon request from the corresponding author, Seok Jin Kim (kstwoh@skku.edu).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal