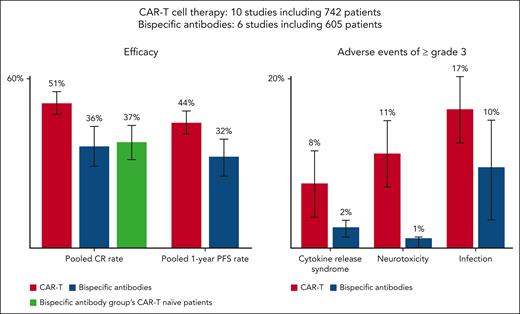
In this issue of Blood, Kim and colleagues report on a timely meta-analysis comparing the safety and efficacy of chimeric antigen receptor (CAR) T-cell therapy and bispecific antibodies for relapsed/refractory large B-cell lymphoma in the third-line setting and beyond.1 Pooled complete remission and progression-free survival rates were significantly better for CAR T cells than for bispecific antibodies, although this greater efficacy came with an increased incidence of adverse events (including cytokine release syndrome, neurotoxicity, and infections) in the CAR T group (see figure).
CAR-T therapy shows better efficacy than bispecific antibody in treating relapsed/refractory large B-cell lymphoma, but with more severe side effects. CAR-T, chimeric antigen receptor T cell; CR, complete remission; PFS, progression free survival.
CAR-T therapy shows better efficacy than bispecific antibody in treating relapsed/refractory large B-cell lymphoma, but with more severe side effects. CAR-T, chimeric antigen receptor T cell; CR, complete remission; PFS, progression free survival.
The knowledge that CD19-targeting CAR T-cell therapy can potentially cure patients with relapsed/refractory large B-cell lymphoma approximately 40% of the time2 with just one treatment motivates clinicians and patients to obtain CAR T-cell therapy, despite the known, often severe side effects that are usually reversible with optimal and immediate management. Axicabtagene ciloleucel,2 tisagenlecleucel,3 and lisocabtagene maraleucel4 have been approved by the US Food and Drug Administration for relapsed/refractory large B-cell lymphoma in the third-line setting and beyond, whereas axicabtagene ciloleucel and lisocabtagene maraleucel are approved for the second-line setting as well. Unfortunately, factors such as disease kinetics, patient comorbidities, socioeconomic/geographic limitations, and resource allocations often make it difficult for CAR T-cell therapy to be used widely.
Bispecific antibodies are an off-the-shelf way to harness the body’s T-cell immune system to fight B-cell lymphoma in vivo without having to perform leukapheresis, ex vivo transduction of autologous T cells, and manufacturing of CAR T cells before the lymphoma can actually be treated (typically a 2- to 4-week process). Initial approvals of the CD20/CD3-targeting bispecific antibodies glofitamab5 and epcoritamab6 came in 2023 for diffuse large B-cell lymphoma as third-line or higher therapy or for patients ineligible for CD19-targeting CAR T-cell therapy. Mosunetuzumab7 and odronextamab8 have also shown success in relapsed/refractory large B-cell lymphoma, but are not yet approved. All these bispecific antibodies can be used more widely geographically but involve repeated injections or infusions, often indefinitely; can still cause cytokine release syndrome and other side effects; and have not yet demonstrated cure potential. To improve the efficacy of bispecific antibodies in large B-cell lymphoma, studies have now been designed to combine bispecifics with novel targeted therapies and/or chemoimmunotherapy, even in the first-line setting. These studies also are exploring the use of bispecific antibodies for a fixed duration. Likewise, efforts to further improve efficacy of CAR T-cell therapy with a more favorable safety profile have led to the development and exploration of novel CAR T-cell targets and costimulation methods. One particularly interesting approach is direct administration of vectors into the body with directed in vivo transduction and CAR T-cell formation to save time and costs while enhancing wider access to these products.9,10
Although Kim et al’s meta-analysis shows that CAR T-cell therapy in the third-line and beyond scenario has superior efficacy to bispecific antibody treatment with higher complete remission and progression-free survival rates, it comes with a greater incidence of grade 3 and higher adverse events. Because many patients still relapse, the race is on to come up with the optimal novel therapy/combination that will confer even higher cures rates with lower toxicity and global access for patients with this deadly disease.
Conflict-of-interest disclosure: T.S. is a speaker for Bristol Myers Squibb (BMS), AstraZeneca, and BeiGene; is an advisory board member for BMS, AstraZeneca, BeiGene, Gilead, and AbbVie; and has received has grant funding from BMS.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal