Key Points
Similar to clinical trial results, fostamatinib has demonstrated a high efficacy rate for ITP in daily clinical practice conditions.
Fostamatinib is a well-tolerated drug with a very low rate of thrombotic events associated with its use.
Visual Abstract
Fostamatinib, a recently approved Syk inhibitor used in adult primary immune thrombocytopenia (ITP), has been shown to be safe and effective in this disorder. However, clinical trial results may not be similarly reproduced in clinical practice. Here, 138 patients with ITP (both primary and secondary) from 42 Spanish centers who had been treated with fostamatinib were evaluated prospectively and retrospectively. The median age of our cohort (55.8% women) was 66 years (interquartile range [IQR], 56-80). The median time since ITP diagnosis at fostamatinib initiation was 51 months (IQR, 10-166). The median number of therapies before fostamatinib initiation was 4 (IQR, 2-5), including eltrombopag (76.1%), romiplostim (57.2%), and IV immunoglobulins (44.2%). Fifty-eight patients (42.0%) had signs/symptoms of bleeding in the month before treatment initiation. Seventy-nine percent of patients responded to fostamatinib with 53.6% complete responses (platelet count > 100 × 109/L). Eighty-three patients (60.1%) received fostamatinib monotherapy, achieving a high response rate (85.4%). The proportion of time in response during the 27-month period examined was 83.3%. The median time to platelet response was 11 days (IQR, 7-21). Sixty-seven patients (48.5%) experienced adverse events, mainly grade 1 to 2; the commonest of which were diarrhea (n = 28) and hypertension (n = 21). One patient had deep venous thrombosis, and one patient developed acute myocardial infarction. Fostamatinib was shown to be effective with good safety profile in patients with primary and secondary ITP across a wide age spectrum in this real-world study.
Introduction
Immune thrombocytopenia (ITP) is an acquired autoimmune disease characterized by antibody–mediated platelet destruction and inadequate platelet production.1 The clinical course of ITP and its symptomatology are highly variable.2,3 There are no specific criteria defining the diagnosis, and ITP remains a diagnosis of exclusion.4,5
The understanding of the pathogenesis of thrombocytopenia in ITP has increased greatly in recent years. Classically, platelet destruction by anti-platelet antibodies was considered the main cause of the disease.6-9 However, a decrease in platelet production and T-cell–mediated effects have subsequently been described.10-16
In Spain, there are no consistent epidemiological data on this disease. The estimated incidence in adults is 1.6 to 6.6 new cases of chronic ITP per 100 000 inhabitants per year.17 The incidence increases with age, and recent epidemiological data suggest that the incidence in adults is approximately equal in both sexes, except in middle adulthood (30-60 years) when the disease is more prevalent in women.18 Factors contributing to bleeding risk include: platelet count, age, lifestyle, and other factors.19-21 Corticosteroids (prednisone or dexamethasone) remain the standard first-line treatment and IV immunoglobulins (IVIGs) are reserved for use in combination with steroids in case of bleeding.22-24
Some medical treatments show robust evidence when used as subsequent (second-line) therapies. One example is fostamatinib, which is considered as an option for second-line treatment in ITP.5,25,26 Fostamatinib is a Syk kinase inhibitor that decreases Fc receptor–mediated clearance of autoantibody-coated platelets in the spleen. In 2 randomized, double-blind trials of severe heavily pretreated patients with ITP with a median disease duration of 8.5 years, the overall response rate was 43% in the fostamatinib arm vs 14% in the placebo arm. A stable response rate was seen in 18% in the fostamatinib arm and 2% in the placebo arm.27
The most common side effects associated with fostamatinib use are gastrointestinal side effects (nausea and diarrhea) and hypertension. The rate of thromboembolic events (TEEs) in patients receiving long-term ITP treatment with fostamatinib was lower than previously observed when using thrombopoietin receptor agonists.28
The starting dose of fostamatinib is 100 mg twice daily, which may be increased if platelet counts are inadequate, to 150 mg twice daily after 4 weeks. If there is no response after 4 weeks at the higher dose, fostamatinib should be discontinued. However, these formal pharmaceutical studies may not reflect what happens in real-world practice, and, to our knowledge, no real-world case series have been published to date that have analyzed the effectiveness and safety of fostamatinib beyond clinical trials.29-33 The aim of this study is to determine the effectiveness and safety of fostamatinib in ITP in everyday clinical practice.
Methods
Patients and study design
A total of 148 adult patients with ITP (aged ≥18 years) from 42 Spanish centers who had been treated with fostamatinib were prospectively and retrospectively evaluated. Patients who received fostamatinib in the past but are no longer receiving it were included in the study retrospectively, whereas all other patients were included prospectively (even if data were collected from the patient's medical records) in an observational and noninterventional manner.
Each center collected clinical and biological data from all patients with ITP who met the inclusion criteria. Researchers reviewed the main characteristics of each patient in the study by means of a predetermined case report form. Patient and disease characteristics, including age, sex, type of therapies before starting fostamatinib, months since diagnosis at fostamatinib initiation, platelet counts (at diagnosis, and before and during fostamatinib treatment), dose and duration of fostamatinib treatment, and adverse events (AEs) during the treatment, were recorded.
Fostamatinib was administered at doses approved by the European Medicines Agency. Primary ITP was defined as a platelet count of <100 × 109/L in the absence of other causes or disorders that might be associated with thrombocytopenia.4 Secondary ITP was defined according to international standards.4 The stages of primary ITP (newly diagnosed, persistent, and chronic ITP) were also defined according to classic definitions.4
End points
Fostamatinib effectiveness was evaluated based on platelet responses, bleeding resolution, and the percentage of patients able to reduce or discontinue their doses of concurrent ITP treatment. Complete response was defined as a platelet count of ≥100 × 109/L. Response was defined as a platelet count of ≥30 × 109/L and at least twice the baseline count, with a concurrent resolution of bleeding symptoms and the absence of any rescue intervention during the preceding 8 weeks. No response was defined as a platelet count of <30 × 109/L or less than twice the baseline count.4
Treatment failure was defined as no clinical improvement after 12 weeks of fostamatinib treatment.34 Rescue treatment was defined as the need to increase the dose of a concomitant treatment to fostamatinib at higher levels of the baseline dose.4 We evaluated and classified the AEs with fostamatinib according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.
Ethical aspects
The study was performed in accordance with the guidelines of the institutional review boards of the participating centers and the standards of the Helsinki Declaration. It was approved by the Hospital Universitario de Burgos Ethics Committee (protocol code FOSTAMES) and was authorized as a postauthorization observational study by the Spanish Medicines and Health Products Agency. Written informed consent was obtained from participating patients.
Statistical analysis
A descriptive statistical analysis was carried out in Excel (Microsoft Corp, Redmond, WA). Normally and nonnormally distributed continuous variables were summarized as the mean and standard deviation and as the median and interquartile range (IQR), respectively. Quantitative data were compared using the Mann-Whitney U test. Patients with a platelet response, a complete platelet response, and a relapse after response were assessed based on number (percentages). The response rate according to the different variables analyzed was evaluated using percentages only. Statistical significance was concluded for P value <.05. All statistical analyses were performed using SPSS version 19.0 for Windows (SPSS, Chicago, IL).
Results
Patient characteristics
We identified 148 patients who had received fostamatinib. Of these, 10 patients were excluded as ITP diagnosis was not confirmed. Thus, the study included 138 patients who had received fostamatinib for a minimum of 1 month.
The main demographic and hematologic features of the 138 patients who received fostamatinib are presented in Table 1. Most (n = 126 [91.3%]) were evaluated prospectively. A total of 122 had primary ITP, 15 secondary ITP (4 lymphoproliferative disorders, 3 monoclonal gammopathy of undetermined significance, 3 immunodeficiencies, 2 systemic lupus erythematosus, 2 antiphospholipid syndrome, and 1 ITP secondary to COVID-19) and 1 Evans syndrome. The clinical behavior of secondary ITP is different to primary ITP, and these cases may respond less well to treatment. We have included them here because this is a real-world study with a more heterogeneous population than that seen in pharmaceutical company registration studies. The median age of our cohort was 66 years (IQR, 56-80). The majority of patients were women (77 [55.8%]). At diagnosis, 28 patients (20.2%) had a Charlson comorbidity index ≥1, the median platelet count was 13 × 109/L (IQR, 5× 109/L to 26 × 109/L), and 95 patients (68.8%) had bleeding. When fostamatinib treatment was started, the median time since diagnosis of ITP was 51 months (IQR, 10-166). The median number of therapies before administration of fostamatinib was 4 (IQR, 2-5), including eltrombopag (76.1%), romiplostim (57.2%), IVIG (44.2%), rituximab (29.0%), and avatrombopag (9.4%). Nineteen patients (13.8%) underwent splenectomy. Eighty-three patients (60.1%) were treated with fostamatinib in monotherapy during the course of ITP. Most of the concomitant treatments consisted of corticosteroids (44.6%), romiplostim (8.6%), or combinations of both drugs (8.6%). The median platelet count at the time of initiation of fostamatinib treatment was 21 × 109/L (IQR, 8 × 109/L to 47 × 109/L), whereas 58 patients (42.0%) had signs/symptoms of bleeding in the month before treatment initiation (See Table 1).
Patient characteristics
| Variable . | Total (N = 138) . |
|---|---|
| Age, y, median [Q1;Q3] | 66 [56;80] |
| Men/women (n) | 61/77 |
| Charlson comorbidity index >1, n (%) | 28 (20.2) |
| Months with ITP, median [Q1;Q3] | 51 [10;165] |
| Platelet count at diagnosis, median [Q1;Q3], ×109/L | 13 [5;26] |
| Bleeding at diagnosis, n (%) | 95 (68.8) |
| Type of primary ITP, n (%) | 122 (88.4) |
| Newly diagnosed | 15 (12.3) |
| Persistent | 21 (17.2) |
| Chronic | 86 (70.5) |
| Type of secondary ITP, n (%) | 16 (11.6) |
| Neoplastic disease | 7 (43.7) |
| Autoimmune disorders | 4 (25) |
| Immunodeficiencies | 3 (18.8) |
| Viral infections | 1 (6.2) |
| Evans syndrome | 1 (6.2) |
| Past ITP treatments, median [Q1;Q3] | 4 [2;5] |
| Eltrombopag, n (%) | 105 (76.1) |
| Romiplostim, n (%) | 79 (57.2) |
| IVIG, n (%) | 61 (44.2) |
| Rituximab, n (%) | 40 (29.0) |
| Splenectomy, n (%) | 19 (13.8) |
| Avatrombopag, n (%) | 13 (9.4) |
| Platelet count at fostamatinib treatment initiation, median [Q1;Q3], ×109/L | 21 [8;47] |
| Bleeding at fostamatinib treatment initiation, n (patients; %) | 58 (42.0) |
| Bruising, n (episodes; %) | 39 (28.3) |
| Petechiae, n (episodes; %) | 32 (23.2) |
| Epistaxis, n (episodes; %) | 6 (4.3) |
| Gingivorrhages, n (episodes; %) | 6 (4.3) |
| Bullae in oral cavity, n (episodes; %) | 5 (3.6) |
| Digestive bleeding (high or low), n (episodes; %) | 4 (2.9) |
| Menorrhagia, n (episodes; %) | 2 (1.45) |
| Hematuria, n (episodes; %) | 1 (0.72) |
| Bleeding episodes during fostamatinib treatment, n (patients; %) | 20 (14.5) |
| Bruising, n (episodes; %) | 12 (8.7) |
| Petechiae, n (episodes; %) | 10 (7.2) |
| Epistaxis, n (episodes; %) | 5 (3.6) |
| Gingivorrhages, n (episodes; %) | 3 (2.1) |
| Bullae in oral cavity, n (episodes; %) | 3 (2.1) |
| Digestive bleeding (high or low), n (episodes; %) | 2 (1.45) |
| Menorrhagia, n (episodes; %) | 1 (0.72) |
| Concomitant treatment, n (%) | 56 (40.6) |
| Corticoids | 25 (44.6) |
| Romiplostim | 5 (8.6) |
| Corticoids plus romiplostim | 5 (8.6) |
| Immunoglobulins | 4 (7.1) |
| Corticoids plus immunoglobulins | 4 (7.1) |
| Eltrombopag | 3 (5.3) |
| Avatrombopag | 3 (5.3) |
| Triple drug combinations | 2 (3.5) |
| Corticoids plus other different drugs | 2 (3.5) |
| Variable . | Total (N = 138) . |
|---|---|
| Age, y, median [Q1;Q3] | 66 [56;80] |
| Men/women (n) | 61/77 |
| Charlson comorbidity index >1, n (%) | 28 (20.2) |
| Months with ITP, median [Q1;Q3] | 51 [10;165] |
| Platelet count at diagnosis, median [Q1;Q3], ×109/L | 13 [5;26] |
| Bleeding at diagnosis, n (%) | 95 (68.8) |
| Type of primary ITP, n (%) | 122 (88.4) |
| Newly diagnosed | 15 (12.3) |
| Persistent | 21 (17.2) |
| Chronic | 86 (70.5) |
| Type of secondary ITP, n (%) | 16 (11.6) |
| Neoplastic disease | 7 (43.7) |
| Autoimmune disorders | 4 (25) |
| Immunodeficiencies | 3 (18.8) |
| Viral infections | 1 (6.2) |
| Evans syndrome | 1 (6.2) |
| Past ITP treatments, median [Q1;Q3] | 4 [2;5] |
| Eltrombopag, n (%) | 105 (76.1) |
| Romiplostim, n (%) | 79 (57.2) |
| IVIG, n (%) | 61 (44.2) |
| Rituximab, n (%) | 40 (29.0) |
| Splenectomy, n (%) | 19 (13.8) |
| Avatrombopag, n (%) | 13 (9.4) |
| Platelet count at fostamatinib treatment initiation, median [Q1;Q3], ×109/L | 21 [8;47] |
| Bleeding at fostamatinib treatment initiation, n (patients; %) | 58 (42.0) |
| Bruising, n (episodes; %) | 39 (28.3) |
| Petechiae, n (episodes; %) | 32 (23.2) |
| Epistaxis, n (episodes; %) | 6 (4.3) |
| Gingivorrhages, n (episodes; %) | 6 (4.3) |
| Bullae in oral cavity, n (episodes; %) | 5 (3.6) |
| Digestive bleeding (high or low), n (episodes; %) | 4 (2.9) |
| Menorrhagia, n (episodes; %) | 2 (1.45) |
| Hematuria, n (episodes; %) | 1 (0.72) |
| Bleeding episodes during fostamatinib treatment, n (patients; %) | 20 (14.5) |
| Bruising, n (episodes; %) | 12 (8.7) |
| Petechiae, n (episodes; %) | 10 (7.2) |
| Epistaxis, n (episodes; %) | 5 (3.6) |
| Gingivorrhages, n (episodes; %) | 3 (2.1) |
| Bullae in oral cavity, n (episodes; %) | 3 (2.1) |
| Digestive bleeding (high or low), n (episodes; %) | 2 (1.45) |
| Menorrhagia, n (episodes; %) | 1 (0.72) |
| Concomitant treatment, n (%) | 56 (40.6) |
| Corticoids | 25 (44.6) |
| Romiplostim | 5 (8.6) |
| Corticoids plus romiplostim | 5 (8.6) |
| Immunoglobulins | 4 (7.1) |
| Corticoids plus immunoglobulins | 4 (7.1) |
| Eltrombopag | 3 (5.3) |
| Avatrombopag | 3 (5.3) |
| Triple drug combinations | 2 (3.5) |
| Corticoids plus other different drugs | 2 (3.5) |
The recommended starting dose of fostamatinib is 100 mg twice daily, and this was used as the starting dose in 107 patients. After initiating fostamatinib, the dose can be increased to 150 mg twice daily after 4 weeks based on platelet count and tolerability. Eighty-five patients increased fostamatinib to the maximum daily dose of 300 mg. Thirty-one patients started treatment directly with the maximum approved dose of fostamatinib, given the extreme refractoriness of these patients to previous treatments. The median time from initiation of fostamatinib to dose increase to 150 mg twice a day was 28 days (IQR, 16-39). The median duration of treatment with fostamatinib for the entire cohort was 207 days (IQR, 78-449). However, in nonresponders, the duration of treatment was only 48 days (IQR, 28-70).
Fostamatinib effectiveness
A total of 109 of the 138 patients (79.0%) obtained a platelet response. Any patient who achieved a platelet count ≥30 ×109/L and at least twice the baseline count with a concurrent resolution of bleeding symptoms and the absence of any rescue intervention during the preceding 8 weeks on a single occasion during the study was considered a responder. When fostamatinib was used as a single agent, its response rates were high (85.4%) and showed a good effectiveness profile across the range of patients in the study. A response rate of 84.6% was seen when fostamatinib was used in earlier ITP stages as second-line therapy. In contrast, overtreated patients (third ITP line or later) achieved a 78.4% response rate. Fostamatinib demonstrated similar results in primary ITP (80.3% of responses) and secondary thrombocytopenia (68.7%). Statistically significant differences regarding effectiveness were observed when fostamatinib was used in monotherapy (85.4%) vs in combination (69.6%; P = .026) and with platelet counts at fostamatinib initiation higher (88.7%) (≥20 × 109/L) vs lower (67.2%) (<20 × 109/L) (P = .002).
Those patients who previously failed prior treatments attained lower platelet responses than treatment-naïve patients; for example, prior IVIG treatment (73.8% of responses) vs IVIG naïves (83.1%), avatrombopag treated (61.5%) vs not treated (80.8%), or previous failure of romiplostim (74.7%) or not (84.7%). These characteristics were examined as possible predictors of platelet response, but none proved to be statistically significant at the P value <.05 level. However, patients previously treated with rituximab showed a trend toward a lower response rate to fostamatinib (70.0%) than rituximab-naïve patients (82.6%). Surprisingly, we also observed another trend toward a better response to fostamatinib (89.5%) in patients who underwent splenectomy vs those who did not (77.3%) and higher responses in patients with a worse Charlson comorbidity index (84.4% if ≥1 vs 75% in Charlson 0 cases). Nevertheless, this response rate varied little when other variables were analyzed. Thus, 80.2% vs 79.2% of responses were observed when platelet numbers were <20 × 109/L and ≥20 × 109/L at diagnosis, respectively, whereas 76.7% of responses were recorded in younger patients (aged <65 years) vs 80.5% in our population aged ≥65 years. Men (78.7%) and women (79.2%) also achieved similar effectiveness outcomes when using fostamatinib. Similarly, in bleeding vs no bleeding at fostamatinib initiation (78.9% vs 77.5%), the use of fostamatinib in chronic ITP vs nonchronic ITP (79.2% vs 76.9%) and the condition of previous treatments with some drugs such as eltrombopag (79.0% of responses if treated vs 78.8% if not) did not correlate with significantly different platelet responses either (Table 2).
Platelet response
| Variable . | Total (n = 138) . | Statistical significance (P value) . |
|---|---|---|
| Quality of response | ||
| Patients with a platelet response, n (%) | 109 (79.0) | |
| Response stratified by variables | ||
| Age in y (<65 vs ≥65), % | 76.7 vs 80.5 | .584 |
| Sex (men vs women), % | 78.7 vs 79.2 | .939 |
| Baseline platelet count (<20 × 109/L vs ≥20 × 109/L), % | 80.2 vs 79.2 | .882 |
| Prior therapy with IVIG (yes/no), % | 73.8 vs 83.1 | .181 |
| Prior therapy with TPO-RAs (yes/no), % | 76.1 vs 80.3 | .326 |
| Prior therapy with rituximab (yes/no), % | 70.0 vs 82.6 | .098 |
| Splenectomized vs nonsplenectomized, % | 89.5 vs 77.3 | .227 |
| Bleeding at diagnosis (yes/no), % | 78.9 vs 77.5 | .653 |
| Charlson comorbidity index at diagnosis (0 vs ≥1), % | 75.0 vs 84.4 | .177 |
| Fostamatinib received in first/second line vs third line or later, % | 84.6 vs 78.4 | .601 |
| Fostamatinib use in primary ITP vs secondary ITP, % | 80.3 vs 68.7 | .413 |
| Fostamatinib monotherapy vs use in combination, % | 85.4 vs 69.6 | .026 |
| Platelet count at fostamatinib initiation (<20 × 109/L vs ≥20 × 109/L), % | 67.2 vs 88.7 | .002 |
| Fostamatinib use in chronic ITP vs nonchronic ITP, % | 79.2 vs 76.9 | .774 |
| Patients with a complete response, n (%) | 74 (53.6) | |
| Duration of response | ||
| Proportion of time with platelet response, % | 83.3 | |
| No. of patients with relapse after response, n (%) | 19 (13.8) | |
| Treatments after fostamatinib | ||
| Avatrombopag | 5 | |
| Romiplostim | 3 | |
| Steroids | 3 | |
| Steroids + immunosuppressants | 2 | |
| Immunoglobulins | 2 | |
| Eltrombopag | 1 | |
| Rituximab | 1 | |
| Cyclosporine | 1 | |
| Splenectomy | 1 | |
| Death | 9 |
| Variable . | Total (n = 138) . | Statistical significance (P value) . |
|---|---|---|
| Quality of response | ||
| Patients with a platelet response, n (%) | 109 (79.0) | |
| Response stratified by variables | ||
| Age in y (<65 vs ≥65), % | 76.7 vs 80.5 | .584 |
| Sex (men vs women), % | 78.7 vs 79.2 | .939 |
| Baseline platelet count (<20 × 109/L vs ≥20 × 109/L), % | 80.2 vs 79.2 | .882 |
| Prior therapy with IVIG (yes/no), % | 73.8 vs 83.1 | .181 |
| Prior therapy with TPO-RAs (yes/no), % | 76.1 vs 80.3 | .326 |
| Prior therapy with rituximab (yes/no), % | 70.0 vs 82.6 | .098 |
| Splenectomized vs nonsplenectomized, % | 89.5 vs 77.3 | .227 |
| Bleeding at diagnosis (yes/no), % | 78.9 vs 77.5 | .653 |
| Charlson comorbidity index at diagnosis (0 vs ≥1), % | 75.0 vs 84.4 | .177 |
| Fostamatinib received in first/second line vs third line or later, % | 84.6 vs 78.4 | .601 |
| Fostamatinib use in primary ITP vs secondary ITP, % | 80.3 vs 68.7 | .413 |
| Fostamatinib monotherapy vs use in combination, % | 85.4 vs 69.6 | .026 |
| Platelet count at fostamatinib initiation (<20 × 109/L vs ≥20 × 109/L), % | 67.2 vs 88.7 | .002 |
| Fostamatinib use in chronic ITP vs nonchronic ITP, % | 79.2 vs 76.9 | .774 |
| Patients with a complete response, n (%) | 74 (53.6) | |
| Duration of response | ||
| Proportion of time with platelet response, % | 83.3 | |
| No. of patients with relapse after response, n (%) | 19 (13.8) | |
| Treatments after fostamatinib | ||
| Avatrombopag | 5 | |
| Romiplostim | 3 | |
| Steroids | 3 | |
| Steroids + immunosuppressants | 2 | |
| Immunoglobulins | 2 | |
| Eltrombopag | 1 | |
| Rituximab | 1 | |
| Cyclosporine | 1 | |
| Splenectomy | 1 | |
| Death | 9 |
Platelet response and variables with statistically significant differences are indicated in bold. TPO-RAs, thrombopoietin receptor agonists.
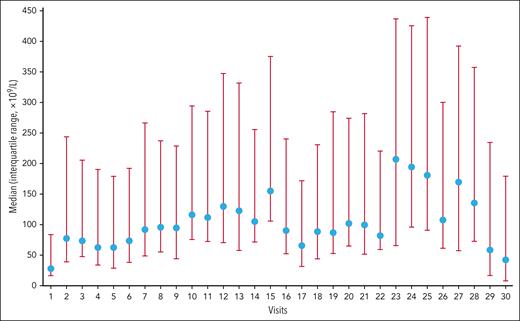
According to the protocol, patients had to undergo platelet counts every 2 weeks, and patients with stable platelet counts could undergo platelet counts every 3 weeks. The proportion of time in response during the 27-month period of the study was 83.3%, and 74 of 138 patients (53.6%) were able to attain CR. Although large variability in the platelet counts between visits was observed, the platelet increase was sustained during the period under examination, as shown in Figure 1.
Platelet count over time. The proportion of the cumulative time spent in platelet response during the 27-month period of the study was 83.3%; 74 of 138 patients (53.6%) were able to attain CR. Although large variability in the platelet counts between visits was observed, the platelet increase was sustained during the period under examination. The blue circles are the median platelet counts, and red bars are the error intervals.
Platelet count over time. The proportion of the cumulative time spent in platelet response during the 27-month period of the study was 83.3%; 74 of 138 patients (53.6%) were able to attain CR. Although large variability in the platelet counts between visits was observed, the platelet increase was sustained during the period under examination. The blue circles are the median platelet counts, and red bars are the error intervals.
Twenty-seven of 109 patients (24.8%) discontinued fostamatinib due to lack of effectiveness after receiving treatment for a median of 54 days (IQR, 16-108). Eighty-four (80%) and 69 of responders (65.7%) maintained a sustained response at 3 and 6 months, respectively. Four patients suffered a loss to follow-up that prevents us from assessing their sustained response rate. Only 12 patients were retrospectively evaluated. Of these, 8 patients responded to treatment, with 6 patients maintaining response at 3 months and 4 at 6 months. Nonresponders had received a median of 8 lines of treatment for their ITP, with no clear response to any treatment for the disease.
The median time to platelet response was 11 days (IQR, 7-21). The time to platelet response was similar independently of variables analyzed, with 2 exceptions. Firstly, statistically significant differences were observed between the patients who had received a prior therapy with rituximab (n = 28), who needed 32.0 days to obtain a platelet response, vs 12.8 days only for those who did not (P = .000). Secondly, fostamatinib needed 15.0 days to respond when used as a single agent, whereas it needed 22.4 days when used in combination (P = .076). These findings may reflect the fact that fostamatinib combination therapies were predominantly used for heavily pretreated and refractory patients, and even in this setting, fostamatinib demonstrated high effectiveness rates (69.6%; Table 3). The best platelet response obtained with fostamatinib treatment had a median platelet count of 120 × 109/L (IQR, 49 × 109/L to 211 × 109/L). The median time from initiation of fostamatinib to maximum response to treatment was 49 days (IQR, 14-113). Effectiveness data were compared in the primary and secondary ITP population, with similar results for fostamatinib in both patient cohorts. We only observed statistically significant differences in the time required to achieve platelet response, which was much longer in the secondary ITP cohort: 11 days (IQR,7-21) in primary ITP and 21 days (9-22) in secondary ITP (P = .044; Table 4).
Time to platelet response
| Variable . | Total (n = 138) . | Statistical significance (P value) . |
|---|---|---|
| No. of days to platelet response, median (IQR) | 11 (7-21) | |
| No. of days to platelet response stratified by variables | ||
| Age (<65 y vs ≥65 y), d | 16.1 vs 18.7 | .531 |
| Sex (men vs women), d | 17.7 vs 18.2 | .921 |
| Baseline platelet count (<20 × 109/L vs ≥20 × 109/L), d | 18.1 vs 17.7 | .937 |
| Prior therapy with IVIG (yes/no), d | 17.8 vs 17.9 | .981 |
| Prior therapy with TPO-RAs (yes/no), d | 18.4 vs 17.0 | .514 |
| Prior therapy with rituximab (yes/no), d | 32.0 vs 12.8 | .000 |
| Splenectomized vs nonsplenectomized, d | 13.2 vs 18.5 | .370 |
| Bleeding at diagnosis (yes/no) | 19.0 vs 15.0 | .431 |
| Charlson comorbidity index at diagnosis (0 vs ≥1), d | 18.4 vs 17.1 | .752 |
| Fostamatinib received in first/second line vs third line or later, d | 14.0 vs 18.2 | .538 |
| Fostamatinib use in primary ITP vs secondary ITP, d | 17.6 vs 19.5 | .786 |
| Fostamatinib monotherapy vs use in combination, d | 15.0 vs 22.4 | .076 |
| Platelet count at fostamatinib initiation (<20 × 109/L vs ≥20 × 109/L), d | 20.8 vs 14.7 | .128 |
| Fostamatinib use in chronic ITP vs nonchronic ITP, d | 19.0 vs 16.7 | .877 |
| Variable . | Total (n = 138) . | Statistical significance (P value) . |
|---|---|---|
| No. of days to platelet response, median (IQR) | 11 (7-21) | |
| No. of days to platelet response stratified by variables | ||
| Age (<65 y vs ≥65 y), d | 16.1 vs 18.7 | .531 |
| Sex (men vs women), d | 17.7 vs 18.2 | .921 |
| Baseline platelet count (<20 × 109/L vs ≥20 × 109/L), d | 18.1 vs 17.7 | .937 |
| Prior therapy with IVIG (yes/no), d | 17.8 vs 17.9 | .981 |
| Prior therapy with TPO-RAs (yes/no), d | 18.4 vs 17.0 | .514 |
| Prior therapy with rituximab (yes/no), d | 32.0 vs 12.8 | .000 |
| Splenectomized vs nonsplenectomized, d | 13.2 vs 18.5 | .370 |
| Bleeding at diagnosis (yes/no) | 19.0 vs 15.0 | .431 |
| Charlson comorbidity index at diagnosis (0 vs ≥1), d | 18.4 vs 17.1 | .752 |
| Fostamatinib received in first/second line vs third line or later, d | 14.0 vs 18.2 | .538 |
| Fostamatinib use in primary ITP vs secondary ITP, d | 17.6 vs 19.5 | .786 |
| Fostamatinib monotherapy vs use in combination, d | 15.0 vs 22.4 | .076 |
| Platelet count at fostamatinib initiation (<20 × 109/L vs ≥20 × 109/L), d | 20.8 vs 14.7 | .128 |
| Fostamatinib use in chronic ITP vs nonchronic ITP, d | 19.0 vs 16.7 | .877 |
Variables with statistically significant differences or with a tendency to be statistically significant are indicated in bold.
Efficacy comparison between primary and secondary ITP
| Variable . | Primary ITP (n = 122) . | Secondary ITP (n = 16) . | Statistical significance (P value) . |
|---|---|---|---|
| Quality of response | |||
| Patients with a platelet response, n (%) | 98 (80.3%) | 11 (68.7%) | .537 |
| Response stratified by variables | |||
| Fostamatinib monotherapy vs use in combination | |||
| Fostamatinib monotherapy | 63 (87.5%) | 6 (66.7%) | .234 |
| Fostamatinib use in combination | 34 (69.4%) | 5 (71.4%) | .913 |
| Platelet count at fostamatinib initiation (<20 × 109/L vs ≥20 × 109/L) | |||
| <20 × 109/L | 40 (69%) | 3 (50%) | .385 |
| ≥20 × 109/L | 55 (90.2%) | 8 (80%) | .313 |
| Patients with a complete platelet response, n (%) | 67 (55.4%) | 7 (43.8%) | .381 |
| Number of days to platelet response, median (IQR) | 11 (7-21) | 21 (9-22) | .044 |
| Number of days to platelet response stratified by variables | |||
| Prior therapy with rituximab | |||
| Yes | 21 (14-36) | 22 (21-54) | .126 |
| No | 9 (7-15) | 14 (8-20) | .874 |
| Fostamatinib monotherapy vs use in combination | |||
| Monotherapy | 11 (7-19) | 21 (11-22) | .438 |
| Combination | 14 (8-28) | 17 (10-22) | .109 |
| Variable . | Primary ITP (n = 122) . | Secondary ITP (n = 16) . | Statistical significance (P value) . |
|---|---|---|---|
| Quality of response | |||
| Patients with a platelet response, n (%) | 98 (80.3%) | 11 (68.7%) | .537 |
| Response stratified by variables | |||
| Fostamatinib monotherapy vs use in combination | |||
| Fostamatinib monotherapy | 63 (87.5%) | 6 (66.7%) | .234 |
| Fostamatinib use in combination | 34 (69.4%) | 5 (71.4%) | .913 |
| Platelet count at fostamatinib initiation (<20 × 109/L vs ≥20 × 109/L) | |||
| <20 × 109/L | 40 (69%) | 3 (50%) | .385 |
| ≥20 × 109/L | 55 (90.2%) | 8 (80%) | .313 |
| Patients with a complete platelet response, n (%) | 67 (55.4%) | 7 (43.8%) | .381 |
| Number of days to platelet response, median (IQR) | 11 (7-21) | 21 (9-22) | .044 |
| Number of days to platelet response stratified by variables | |||
| Prior therapy with rituximab | |||
| Yes | 21 (14-36) | 22 (21-54) | .126 |
| No | 9 (7-15) | 14 (8-20) | .874 |
| Fostamatinib monotherapy vs use in combination | |||
| Monotherapy | 11 (7-19) | 21 (11-22) | .438 |
| Combination | 14 (8-28) | 17 (10-22) | .109 |
Platelet response and complete platelet response variables with statistically significant differences are indicated in bold.
Given that the cutoff point used by most clinical trials when conducting their studies is 50 ×109/L, we have proceeded to assess the response rate with this new threshold. Under this new response criterion, a platelet response rate was obtained in 99 of our patients (71.7%).
Successful and durable (>9 months) discontinuation of fostamatinib was observed in 5 patients only (4 primary ITP and 1 monoclonal gammopathy of undetermined significance–associated secondary ITP), with a median age of 53 years (IQR, 43-68). Four patients were male, and 4 patients had a prior complete response before discontinuation. Two patients had persistent ITP, and 3 had chronic ITP, with 3 previous treatment lines as a median (IQR, 2-3).
Fostamatinib safety
Of the 138 patients, 67 (48.5%) experienced ≥1 AEs during treatment with fostamatinib. Notably, 18 patients were receiving fostamatinib in combination with other drugs. AEs were mainly grade 1 to 2 in severity (Table 5), with an equal frequency in older (aged ≥65 years) and younger patients; 33 and 34 events, respectively. The most common AEs reported during fostamatinib treatment were diarrhea (n = 28) and hypertension (n = 21). Most of these resolved with symptomatic treatment: antidiarrheal and antihypertensive drugs, respectively.
AEs with fostamatinib
| Variable . | n (%) . |
|---|---|
| Any adverse event | 94 (100) |
| Number of grade 3-4 events | 13 (13.8) |
| Diarrhea | 28 (29.8) |
| Hypertension | 21 (22.3) |
| Neutropenia grade 3-4 | 10 (10.6) |
| Headaches | 8 (8.5) |
| Hepatobiliary laboratory abnormalities | 7 (7.4) |
| Dyspepsia/epigastralgia | 4 (4.25) |
| Depression | 2 (2.1) |
| Blurred vision/macular lesion | 2 (2.1) |
| Nausea/vertigo | 1 (1.06) |
| Anorexia | 1 (1.06) |
| Asthenia | 1 (1.06) |
| Erythromelalgia | 1 (1.06) |
| Cold sores | 1 (1.06) |
| Hypophosphatemia | 1 (1.06) |
| Pruritus | 1 (1.06) |
| Muscle cramps | 1 (1.06) |
| Mouth sores | 1 (1.06) |
| Myalgia | 1 (1.06) |
| Venous thromboembolism | 1 (1.06) |
| Acute myocardial infarction | 1 (1.06) |
| Variable . | n (%) . |
|---|---|
| Any adverse event | 94 (100) |
| Number of grade 3-4 events | 13 (13.8) |
| Diarrhea | 28 (29.8) |
| Hypertension | 21 (22.3) |
| Neutropenia grade 3-4 | 10 (10.6) |
| Headaches | 8 (8.5) |
| Hepatobiliary laboratory abnormalities | 7 (7.4) |
| Dyspepsia/epigastralgia | 4 (4.25) |
| Depression | 2 (2.1) |
| Blurred vision/macular lesion | 2 (2.1) |
| Nausea/vertigo | 1 (1.06) |
| Anorexia | 1 (1.06) |
| Asthenia | 1 (1.06) |
| Erythromelalgia | 1 (1.06) |
| Cold sores | 1 (1.06) |
| Hypophosphatemia | 1 (1.06) |
| Pruritus | 1 (1.06) |
| Muscle cramps | 1 (1.06) |
| Mouth sores | 1 (1.06) |
| Myalgia | 1 (1.06) |
| Venous thromboembolism | 1 (1.06) |
| Acute myocardial infarction | 1 (1.06) |
Only 13 patients (7.7%) had to discontinue fostamatinib due to toxicity or serious (grade 3-4) AEs: 4 patients discontinued treatment due to severe diarrhea refractory to antidiarrheal treatment (loperamide); 4 others for hypertension (1 of these patients suffered a severe cerebral hemorrhage in the context of an episode of severe hypertension and died afterward). Three patients stopped fostamatinib for grade 3 to 4 neutropenia and 2 others for severe migraine (1 patient had a previous history of headache).
Nine patients in our cohort died during the study (Table 6). There were 6 deaths among responders and 3 among nonresponders. Only 1 acute myocardial infarct and 1 thrombotic event was observed in our study. The thrombotic episode was resolved without complications.
Deaths with fostamatinib
| Age and sex . | History . |
|---|---|
| 76; female | Eight prior lines of therapy; achieved complete response response with the combination of fostamatinib + avatrombopag; lost response 4 mo after starting this combination and thereafter needed frequent rescue treatment with IVIG. She subsequently died of a cerebral hemorrhage with no response of ITP to therapies. |
| 81; male | Known hypertensive patient who, before initiation of treatment with fostamatinib, was already receiving antihypertensive therapy; developed an episode of severe hypertension resulting in a severe cerebral hemorrhage. |
| 87; male | COVID-associated pneumonia with 56 000 platelets per μL at the time of death. |
| 84; male | Cardiovascular history with ITP in complete response; died after an acute myocardial infarction. |
| 93; female | Deterioration of general condition with good control of ITP. |
| 88; female | Deterioration of general condition with good control of ITP. |
| 75; male | Ten prior lines of therapy; no response of ITP to any treatment; unknown cause of death. |
| 65; female | Patient with a pacemaker who presented with cardiorespiratory arrest attributed to deterioration of the pacemaker. |
| 82; female | She died as a result of urinary sepsis and had ITP secondary to a marginal splenic lymphoma in progression. |
| Age and sex . | History . |
|---|---|
| 76; female | Eight prior lines of therapy; achieved complete response response with the combination of fostamatinib + avatrombopag; lost response 4 mo after starting this combination and thereafter needed frequent rescue treatment with IVIG. She subsequently died of a cerebral hemorrhage with no response of ITP to therapies. |
| 81; male | Known hypertensive patient who, before initiation of treatment with fostamatinib, was already receiving antihypertensive therapy; developed an episode of severe hypertension resulting in a severe cerebral hemorrhage. |
| 87; male | COVID-associated pneumonia with 56 000 platelets per μL at the time of death. |
| 84; male | Cardiovascular history with ITP in complete response; died after an acute myocardial infarction. |
| 93; female | Deterioration of general condition with good control of ITP. |
| 88; female | Deterioration of general condition with good control of ITP. |
| 75; male | Ten prior lines of therapy; no response of ITP to any treatment; unknown cause of death. |
| 65; female | Patient with a pacemaker who presented with cardiorespiratory arrest attributed to deterioration of the pacemaker. |
| 82; female | She died as a result of urinary sepsis and had ITP secondary to a marginal splenic lymphoma in progression. |
Discussion
Fostamatinib has recently obtained regulatory approval for the treatment of chronic ITP in adults. It was approved by the US Food and Drug Administration on 17 April 2018 for patients who have not responded adequately to previous treatments and by the European Medicines Agency on 9 January 2020 for patients refractory to other treatments. Subsequently, Spain granted approval of fostamatinib on 1 September 2021.
Recently, 1 fostamatinib real-world evidence study has been published.35 However, our 27-month study in the whole Spanish population is, to our knowledge, the largest real-world study of fostamatinib treatment in ITP. Here, we describe the effectiveness and safety of fostamatinib in a group comprising 138 adult patients with ITP. Our results clearly show that fostamatinib offers a safe and highly effective treatment in this setting.
In our cohort, fostamatinib was used to treat heavily pretreated patients, obtaining similar successful results in primary and secondary ITP. Even in this refractory setting, with a median of 4 previous treatment lines, fostamatinib shows a platelet response of 79.0% when 30 × 109/L are considered as platelet threshold and an even higher response rate (85.4% of responses) when fostamatinib is used as monotherapy. Almost 40% of our patients received fostamatinib in combination achieving good effectiveness results (69.6%). Interestingly, 71.7% of patients achieved a platelet count ≥50 × 109/L. Fostamatinib was well tolerated with a good safety profile. Thus, toxicities resulting from combinations of 2 or even 3 drugs administered simultaneously were manageable, and only in a minority of cases (n = 13) did drug require discontinuation.
Fostamatinib was shown to act rather quickly, with a median time to platelet response of 11 days, and its effect was durable over time, with stable platelet count throughout the study. It should be noted that the time to dose increase in our study was the same as that recommended in the fostamatinib data sheet; that is, 28 days. On the contrary, although it is recommended to wait until 12 weeks of treatment with fostamatinib before considering the patient as refractory, in our study population and following manufacturer’s instructions, the drug was discontinued after 48 days.
Overall, our real-world routine clinical practice results are much better than those reported in the first analyses of clinical trial results.27,28,36 Numerous differences with regard to response criteria and patient characteristics can justify the large response rate disparity observed between our study (79% of responses) and response rates of the 2 phase 3 FIT 1 and FIT 2 studies (37% and 48%, respectively).27 Thus, in the pivotal studies, they used very strict response criteria. These included as a primary efficacy end point the need for a stable response, defined as a platelet count ≥50 × 109/L on at least 4 of 6 visits without the need for rescue therapy during weeks 14 to 24. Several differences in the patients enrolled can also be found when comparing our study and the FIT1 and FIT2 trails. In FIT1/2 studies, the recruitment involved 34% of patients who underwent splenectomy, 6% with persistent ITP, and 94% with chronic ITP, with a median of 8.7 years (IQR, 0.3-53) since diagnosis. On the contrary, here, we had 13.8% of patients who underwent splenectomy, 12.3%, 17.2%, and 70.5% of newly diagnosed, persistent, and chronic ITP, respectively, and a median of 4.25 years (IQR, 0.83-13.75) since ITP diagnosis.
For all the above reasons, we do believe it is necessary to analyze the results of the clinical trials in depth; when the 5-year effectiveness results were reviewed, it was found that 70% of patients responded to fostamatinib, achieving a platelet count ≥30 × 109/L.28,36 Here, the median platelet count of the study patients increased over time and remained stable between 50 × 109/L to 150 × 109/L during the time of treatment. Our results are consistent with this long-term treatment results.
Of interest is the finding of Boccia et al, who conducted an post hoc analysis of response rates to fostamatinib when used in early lines of treatment, who observed platelet responses of 78% when fostamatinib was used as second and third lines of treatment.37,38 Here, we report 84.6% of responses when fostamatinib was used as second-line therapy, and 78.4% of responses were observed with fostamatinib use in third ITP line or later. Our results are consistent with the good results for fostamatinib obtained in early ITP stages.
Statistically significant differences in the platelet response rate were observed in patients who initiated fostamatinib with low platelet counts (<20 ×109/L) and those with platelet counts ≥20 ×109/L (67.2% vs 88.7%, respectively; P = .002). A greater response was observed when fostamatinib was used alone (85.4%) vs 69.6% of responses for fostamatinib treatment combinations. Statistically significant differences were observed between both treatment schedules (P = .026). In our study, low platelet counts appear to predict response to fostamatinib. This possible role of platelet count at fostamatinib treatment initiation as predictor of response has also been postulated with other drugs such as eltrombopag.39
Patients who had received rituximab before fostamatinib had a lower platelet response rate with a much longer time to response than those who had not. Further studies are required to confirm these data.
In our series, only 5 patients were able to achieve a successful and durable discontinuation of fostamatinib. We consider that fostamatinib, unlike eltrombopag, is not a candidate drug for discontinuation.40-44
Fostamatinib was well tolerated in our study under real-world conditions, as well as in clinical trials.28 Here, 67 patients (48.5%) developed 1 or multiple AEs with only 13 AEs of grade 3 to 4 (7.7%). Diarrhea and hypertension were the most frequent. In comparison, in FIT trials with 146 patients evaluated, adverse events were reported in 89% of patients, with 69% of these mild to moderate. Serious AEs occurred in 45 (31%) patients. As expected, given the nature of our study design, the AE rate of the study is clearly lower than that reported in the clinical trials that led to the approval of the drug.28 It should be added that the median age of our sample was 66 years, compared with 53 years in the pivotal studies.27,28 We would expect that our older population would be associated with a higher rate of adverse effects than reported in clinical trials, but this was not the case.
In our study, adverse effects (n = 94) were observed with equal frequency in young and older patients. In addition, the management of most of these was simple, requiring only symptomatic treatment. This figure is similar to that published by our group in chronic ITP and eltrombopag.45
In our study, we only observed 2 TEEs: 1 acute myocardial infarction in a patient with cardiovascular history and 1 deep venous thrombosis under the combination of fostamatinib, prednisone, and azathioprine in a patient with ITP in CR. In FIT trials, only 1 minor TEE (a mild transient ischemic attack) was reported. In contrast, in the EXTEND study, although eltrombopag failed to document platelet activation,46 6% of patients experienced TEEs.47 Because of this difference in the TEE rate, we agree that most current guidelines recommend the use of fostamatinib as the drug of choice in second-line treatment for ITP in patients at high thrombotic risk.
Nine patients of our population died. Only 2 deaths may be considered related to fostamatinib use: the acute myocardial infarction and the episode of severe hypertension that resulted in a severe cerebral hemorrhage. For this reason, we recommend strict monitoring of blood pressure after initiation of fostamatinib. Four multirefractory patients died of nontreatment-related causes, 2 other patients died of deterioration of general condition with good control of the disease, and 1 man died of COVID-associated pneumonia, with the disease also under control. In contrast, in the FIT trials, none of the 4 fatalities was considered by the investigator to be related to treatment.28 Again, we would like to emphasize the high average age of our study population, which undoubtedly influences the observed death rate.
Although the vast majority of patients have been followed in a single prospective manner, the main limitations of our study are the retrospective multicenter analytical approach to a certain percentage of patients and the possibility of significant selection bias. The failure to use the Bonferroni correction in our statistical analyses is also a limitation. Despite these constraints, this study demonstrates, in line with clinical trials, the high effectiveness and good tolerability of fostamatinib.
In summary, in daily clinical practice, fostamatinib is highly effective and well tolerated in unselected patients with primary or secondary ITP.
Acknowledgment
This research was funded by a Grifols Research grant. Grifols did not have any role in the design, execution, analysis, or interpretation of the study.
Authorship
Contribution: T.J.G.-L. contributed to conception and design; all authors contributed to data collection; T.J.G.-L. contributed to statistical analysis; all authors contributed to analysis and interpretation of results; T.J.G.-L., and D.P. contributed to writing the article (text and tables); and all authors provided the final approval for the article.
Conflict-of-interest disclosure: T.J.G.-L. has received research grants from Amgen and Novartis; and speaker honoraria from Amgen, Novartis, Sobi, Grifols, and Argenx. M.L.L. reports grants and personal fees from Amgen and from Terumo SA; and personal fees from Alexion, Amgen, Argenx BV, Celgene, GlaxoSmithKline, Grifols, Novartis, Sobi, and UCB Biopharma. R.J.-B. has received speaker or adviser honoraria from Amgen, CSL Behring, Grifols, Novo Nordisk, Pfizer, Roche, and Sobi. D.M.-C. has participated in advisory activities in collaboration with Sobi. I.S. has carried out teaching and scientific advisory activities in collaboration with Sobi, CSL Behring, Novo Nordisk, Takeda, Bayer, Pfizer, Boehringher Ingelheim, Bristol Myers Squibb, Leo Pharma, Daiichi Sankyo, and Sanofi. D.P. reports research support from Amgen and Novartis; honoraria from Amgen, Novartis, Sobi, UCB, and Argenx; and consultancies with UCB, MedImmune, Ono, Sobi, Argenx, and Takeda. The remaning authors declare no competing financial interests.
Correspondence: Tomás José González-López, Servicio de Hematología, Hospital Universitario de Burgos, Avenida Islas Baleares s/n, 09006 Burgos, Spain; email: tjgonzalez@saludcastillayleon.es.
References
Author notes
Data are available on request from the corresponding author, Tomás José González-López (tjgonzalez@saludcastillayleon.es).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal