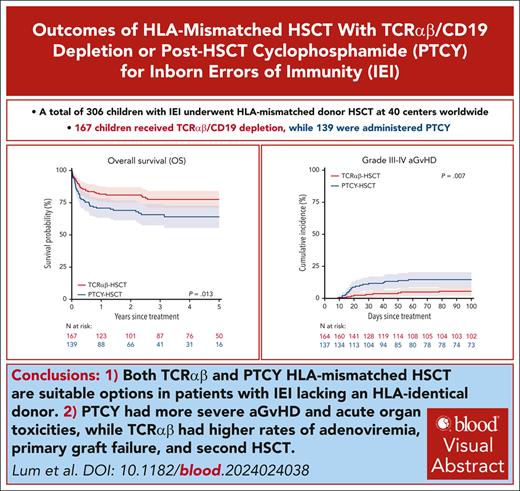
Key Points
Both TCRαβ and PTCY HLA-mismatched HSCT are suitable options in patients with IEIs lacking an HLA-identical donor.
PTCY had more severe aGvHD and acute organ toxicities, whereas TCRαβ had higher rates of adenoviremia, primary graft failure, and second HSCT.
Visual Abstract
HLA-mismatched transplants with either in vitro depletion of CD3+ T-cell receptor (TCR)αβ/CD19 (TCRαβ) cells or in vivo T-cell depletion using posttransplant cyclophosphamide (PTCY) have been increasingly used for patients with inborn errors of immunity (IEIs). We performed a retrospective multicenter study via the EBMT registry on 306 children with IEIs undergoing their first transplant between 2010 and 2019 from an HLA-mismatched donor using TCRαβ (n = 167) or PTCY (n = 139). The median age for hematopoietic stem cell transplantation (HSCT) was 1.2 years (range, 0.03-19.6 years). The 3-year overall survival (OS) was 78% (95% confidence interval (CI), 71-84) after TCRαβ and 66% (57-74) after PTCY (P = .013). Pre-HSCT morbidity score (hazard ratio [HR], 2.27; 1.07-4.80, P = .032) and non-busulfan/treosulfan conditioning (HR, 3.12; 1.98-4.92, P < .001) were the only independent predictors of unfavorable OS. The 3-year event-free survival (EFS) was 58% (50%-66%) after TCRαβ and 57% (48%-66%) after PTCY (P = .804). The cumulative incidence of severe acute graft-versus-host disease (GvHD) was higher after PTCY (15%, 9%-21%) than TCRαβ (6%, 2%-9%, P = .007), with no difference in chronic GvHD (PTCY, 11%, 6%-17%; TCRαβ, 7%, 3%-11%, P = .173). The 3-year GvHD-free EFS was 53% (44%-61%) after TCRαβ and 41% (32%-50%) after PTCY (P = .080). PTCY had significantly higher rates of veno-occlusive disease (14.4% vs TCRαβ 4.9%, P = .009), acute kidney injury (12.7% vs 4.6%, P = .032), and pulmonary complications (38.2% vs 24.1%, P = .017). Adenoviremia (18.3% vs PTCY 8.0%, P = .015), primary graft failure (10% vs 5%, P = .048), and second HSCT (17.4% vs 7.9%, P = .023) were significantly higher in TCRαβ. In conclusion, this study demonstrates that both approaches are suitable options in patients with IEIs, although they are characterized by different advantages and outcomes.
Introduction
Inborn errors of immunity (IEIs), previously referred to as primary immunodeficiencies, comprise a heterogeneous group of disorders, due to single-gene mutations that result in defects in the development and/or function of the immune system. Advanced molecular and immunological diagnostic methods have identified 485 single-gene IEI to date.1 Allogeneic hematopoietic stem cell transplantation (HSCT) is the standard of care for infants with severe combined immunodeficiency (SCID) and has also developed from a treatment of last resort into a consolidated treatment able to correct the defects of immunity in children, as well as adolescents and young adults, with non-SCID IEIs.2-6 Depending on the ethnic background, there are ∼25% to 60% of patients for whom no HLA-matched donor can be found.7 Mismatched related or unrelated HSCT can be performed with various T-cell depletion (TCD) strategies, which have been developed over the years to overcome the HLA barrier and reduce the risk of graft-versus-host disease (GvHD) and graft rejection. Historically, E-rosette lectin and ex vivo CAMPATH-1M anti-CD52 antibodies were used in the 1980s before CD34+ stem cell positive selection became the preferred method in the 1990s.8 Although these methods can achieve profound T-cell depletion, which is essential to prevent fatal GvHD, high rates of engraftment failure and delayed immune reconstitution render the patients at risk for potentially life-threatening infections. Hence, HLA-mismatched donor HSCT for IEIs was historically limited by poorer outcomes compared with matched donor HSCT and was therefore reserved for patients with IEI, such as those with SCID, with very urgent indications and conditions with selective advantage of the donor cells that overcome the risk of rejection.
In recent years, 2 new approaches have been increasingly used: either in vitro selective depletion of CD3+ T-cell receptor (TCR)αβ/CD19+ (TCRαβ) lymphocytes from peripheral blood stem cells (PBSC) or in vivo depletion of alloreactive T cells in unmanipulated grafts using posttransplant cyclophosphamide (PTCY). The TCRαβ method has enabled selective depletion of the T-lymphocyte receptor αβ-bearing cells, which are responsible for GvHD occurrence, and of CD19+ (B) cells, associated with Epstein Barr virus (EBV)-driven posttransplant lymphoproliferative disease.9 The TCRαβ-depleted cellular product contains high numbers of CD34+ progenitors, TCR-γδ+ T lymphocytes, monocytes, dendritic cells, and natural killer cells, which provide engraftment-facilitating effects with a low risk of GvHD and protection against pathogens.10 The PTCY strategy, pioneered by the Johns Hopkins group, is based on the concept of cyclophosphamide being selectively toxic to proliferating lymphocytes. When PTCY is administered within 96 hours of an unmanipulated graft, it depletes alloreactive cells from the donor and recipient while potentially sparing regulatory T cells, facilitating engraftment and preventing GvHD.11,12 Both strategies have been increasingly used in patients with IEIs and both have shown promising results.13-18 They have conceptional differences and potential advantages over each other; for example, TCRαβ allowing avoidance of post-HSCT immunosuppressive drugs and PTCY being feasible even in less-advanced health care settings.
A prospective study comparing the outcomes of these 2 approaches has not yet been attempted and would be very challenging to conduct. To compare outcomes in patients with IEIs given HLA-mismatched donor HSCT from either TCRαβ or PTCY, we performed a multicenter, retrospective analysis on behalf of the Inborn Errors Working Party (IEWP) of the European Society for Blood and Marrow Transplantation (EBMT).
Patients and methods
Data source
This retrospective study was approved by the study review board of the IEWP of the EBMT (study number 8427017). Data were retrieved from the EBMT registry, and an additional study-specific questionnaire was completed by the study centers. Informed consent for registration and data collection within the EBMT database was obtained from all patients, their parents, or legal guardians according to the ethical principles of the Declaration of Helsinki.
Study participants and transplantation procedures
All patients with IEI who received their first mismatched family or unrelated donor HSCT between 2010 and 2019 were eligible for this study. As no patient was transplanted in 2010 in the data, the study period for this analysis was 2011 to 2019. A mismatched donor was defined as having at least 1 allele disparity at HLA A, B, C, DQ, or DR loci. Patients who received additional cellular therapy after transplant as part of the planned transplant protocol were excluded.
Definition and end points
The main outcomes of interest were overall survival (OS), event-free survival (EFS), GvHD-free, event-free survival (GEFS), and cumulative incidence of GvHD. OS was defined as survival from first HSCT to last follow-up or death. EFS was defined as survival without graft failure or second procedures (CD34+ stem cell boost, donor lymphocyte infusion [DLI]) and a conditioned second transplant). GEFS was defined as survival without graft failure, second procedures, conditioned second transplant, grade III-IV acute GvHD, or chronic GvHD, whichever occurred first. Other end points assessed were engraftment kinetics (time to neutrophil engraftment was defined as the first day of achieving a neutrophil count ≥ 0.5 × 109/L for 3 consecutive days; time of platelet engraftment was defined as the first day of achieving a platelet count ≥ 20 × 109/L without platelet transfusion for at least 7 days), graft failure, toxicities, incidence of viremia, immune reconstitution, and degree of donor hematopoietic chimerism at the most recent assessment. Cytomegalovirus (CMV) infection was defined as CMV viremia either from primary infection or reactivation, and CMV disease as end-organ disease or CMV syndrome, according to Ljungman et al.19 Karnofsky or Lansky scales were used to assess performance scores depending on the patient’s age. The pre-HSCT morbidity score was calculated using the following variables: infections, organ damage, malignancies, lung disease, gut disease, renal impairment, and neurological complications, with each pre-HSCT morbidity scored with one point.
Statistical analysis
Continuous variables were described by their median and range, whereas categorical variables were reported with counts and percentages. The association between variables was assessed using the Wilcoxon rank sum test for continuous variables, and the χ2 test for categorical variables (Fisher exact test was used if the minimal expected frequency requirement was not met). Subgroup differences in OS, EFS, and GEFS were evaluated via the log-rank test. The pre-HSCT variables assessed in the univariate analyses (UVA) were: type of TCD (TCRαβ vs PTCY), year of HSCT (2011-2015 vs 2016-2019), age at HSCT (<5 years vs ≥5 years), diagnosis (SCID vs non-SCID IEIs), conditioning (busulfan-based vs treosulfan-based vs non-busulfan/treosulfan-based/no conditioning), organ damage, infection, autoimmunity, malignancy and pre-HSCT morbidity score (≥2 vs 0-1). All factors associated with a P value <.05 in the UVA for OS, EFS, and GEFS were included in the multivariate analyses (MVA) of the respective outcome. MVA were performed using Cox proportional hazard models. Where there was evidence of a center-specific effect on the outcome, the center was included as a random-effects (frailty) term in the multivariate model. The (fixed-effects) predictors associated with P values <0.05 were considered significant; all P values are 2-sided. Fine-and-Gray competing risk regression was used to estimate the cumulative incidence (CIN) of GvHD with graft failure and death as competing events. All statistical analyses were performed using R Statistical Software (version 4.3.0; R Core Team 2023).
Results
Patient and transplantation characteristics
Patient and transplantation characteristics are summarized in Table 1 and supplemental Table 1 (available on the Blood website). This analysis comprises data from 306 patients (TCRαβ, 54.6%; PTCY, 45.4%), collected from 40 transplant centers. In comparison with the TCRαβ group, a significantly greater proportion of patients in the PTCY group underwent transplantation between 2016 and 2019: 74.8% vs 52.7% (P < .001). The median age of HSCT for the entire cohort was 1.2 years (0.03-19.6 years), with no significant difference between TCRαβ (median 1.1, 0.03-18.0) and PTCY-HSCT (median 1.4, 0.2-19.6, P = .213). One hundred and twenty-three patients (40.2%) had SCID, and 183 patients (59.8%) had non-SCID IEIs. There was no significant difference in the ratio of patients with SCID and non-SCID IEIs between TCRαβ (SCID, 43.7%; non-SCID, 56.3%) and PTCY (SCID, 36.0%; non-SCID, 64.0%, P = .208).
Patient and transplantation characteristics
| . | Entire cohort n = 306 . | TCRαβ-HSCT n = 167 . | PTCY-HSCT n = 139 . | P value . | |||
|---|---|---|---|---|---|---|---|
| Missing data . | Results . | Missing data . | Results . | Missing data . | Results . | ||
| Year of HSCT, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | <.001∗ | |||
| 2011 to 2015 | 114 (37.3) | 79 (47.3) | 35 (25.2) | ||||
| 2016 to 2019 | 192 (62.7) | 88 (52.7) | 104 (74.8) | ||||
| Median age at HSCT (range), y | 0 (0.0) | 1.2 (0.03 - 19.6) | 0 (0.0) | 1.1 (0.03-18.0) | 0 (0.0) | 1.4 (0.2-19.6) | .213‡ |
| Diagnosis, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | .208∗ | |||
| SCID | 123 (40.2) | 73 (43.7) | 50 (36.0) | ||||
| Non-SCID IEI | 183 (59.8) | 94 (56.3) | 89 (64.0) | ||||
| Performance score, n (%) | 71 (23.2) | 28 (16.8) | 43 (30.9) | .341∗ | |||
| ≥ 80 | 187 (79.6) | 114 (82.0) | 73 (76.0) | ||||
| < 80 | 48 (20.4) | 25 (18.0) | 23 (24.0) | ||||
| Pre-HSCT infection, n (%) | 0 (0.0) | 240 (78.4) | 0 (0.0) | 122 (73.1) | 0 (0.0) | 118 (84.9) | .018∗ |
| Pre-HSCT organ damage, n (%) | 5 (1.6) | 121 (40.2) | 3 (1.8) | 47 (28.7) | 2 (1.4) | 74 (54.0) | <.001∗ |
| Pre-HSCT autoimmunity, n (%) | 27 (8.8) | 82 (29.4) | 11 (6.6) | 44 (28.2) | 16 (11.5) | 38 (30.9) | .721∗ |
| Pre-HSCT malignancy, n (%) | 0 (0.0) | 21 (6.9) | 0 (0.0) | 11 (6.6) | 0 (0.0) | 10 (7.2) | >.999 |
| Pre-HSCT morbidity score, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | .002∗ | |||
| 0 to 1 | 159 (52.0) | 101 (60.5) | 58 (41.7) | ||||
| ≥ 2 | 147 (48.0) | 66 (39.5) | 81 (58.3) | ||||
| Donor, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | >.999∗ | |||
| MMUD | 15 (4.9) | 8 (4.8) | 7 (5.0) | ||||
| MMFD | 291 (95.1) | 159 (95.2) | 132 (95.0) | ||||
| HLA matching, n (%) | 52 (17.0) | 11 (6.6) | 41 (29.5) | .141† | |||
| 9/10 | 19 (7.5) | 13 (8.3) | 6 (6.1) | ||||
| 8/10 | 7 (2.8) | 7 (4.5) | 0 (0.0) | ||||
| ≤7/10 | 217 (85.4) | 130 (83.3) | 87 (88.8) | ||||
| Other/incomplete information§ | 11 (4.3) | 6 (3.8) | 5 (5.1) | ||||
| Recipient/donor CMV serostatus, n (%) | 108 (35.3) | 41 (24.6) | 67 (48.2) | .098∗ | |||
| −/− | 29 (14.6) | 14 (11.1) | 15 (20.8) | ||||
| −/+, +/−, +/+ | 169 (85.4) | 112 (88.9) | 57 (79.2) | ||||
| Conditioning, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | <.001∗ | |||
| Busulfan-based | 112 (36.6) | 22 (13.2) | 90 (64.7) | ||||
| Treosulfan-based | 115 (37.6) | 99 (59.3) | 16 (11.5) | ||||
| Other/none | 79 (25.8) | 46 (27.5) | 33 (23.7) | ||||
| Serotherapy, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | <.001† | |||
| None | 77 (25.2) | 46 (27.5) | 31 (22.3) | ||||
| ATG only | 175 (57.2) | 113 (67.7) | 62 (44.6) | ||||
| Alemtuzumab only | 53 (17.3) | 7 (4.2) | 46 (33.1) | ||||
| ATG and alemtuzumab | 1 (0.3) | 1 (0.6) | 0 (0.0) | ||||
| Rituximab, n (%) | 0 (0.0) | 108 (35.3) | 0 (0.0) | 69 (41.3) | 0 (0.0) | 39 (28.1) | .022∗ |
| Post-HSCT GvHD prophylaxis, n (%) | 34 (11.1) | 32 (19.2) | 2 (1.4) | <.001∗ | |||
| None | 41 (15.1) | 41 (30.4) | 0 (0.0) | ||||
| CNI | 43 (15.8) | 34 (25.2) | 9 (6.6) | ||||
| CNI+MMF | 141 (51.8) | 19 (14.1) | 122 (89.1) | ||||
| MMF | 25 (9.2) | 23 (17.0) | 2 (1.5) | ||||
| Others | 22 (8.1) | 18 (13.3) | 4 (2.9) | ||||
| Stem cell source, n (%) | 2 (0.7) | 2 (1.2) | 0 (0.0) | <.001† | |||
| BM | 116 (38.2) | 3 (1.8) | 113 (81.3) | ||||
| PBSC | 182 (59.9) | 162 (98.2) | 20 (14.4) | ||||
| BM+PBSC | 6 (2.0) | 0 (0.0) | 6 (4.3) | ||||
| Median TNC (range), × 108/kg | 32 (10.5) | 10.6 (2.0-100.0) | 14 (8.4) | 12.3 (2.0-100.0) | 18 (12.9) | 8.4 (2.1-58.0) | <.001‡ |
| Median CD34+ cell dose (range), × 106/kg | 17 (5.6) | 13.0 (1.6-56.6) | 9 (5.4) | 18.4 (2.0-56.6) | 8 (5.8) | 8.7 (1.6-47.7) | <.001‡ |
| Median CD3+ cell dose, range, × 106/kg | 85 (27.8) | 25.0 (0.0-993.0) | 37 (22.2) | 19.9 (0.0-557.0) | 48 (34.5) | 60.7 (0.4-993.0) | <.001‡ |
| . | Entire cohort n = 306 . | TCRαβ-HSCT n = 167 . | PTCY-HSCT n = 139 . | P value . | |||
|---|---|---|---|---|---|---|---|
| Missing data . | Results . | Missing data . | Results . | Missing data . | Results . | ||
| Year of HSCT, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | <.001∗ | |||
| 2011 to 2015 | 114 (37.3) | 79 (47.3) | 35 (25.2) | ||||
| 2016 to 2019 | 192 (62.7) | 88 (52.7) | 104 (74.8) | ||||
| Median age at HSCT (range), y | 0 (0.0) | 1.2 (0.03 - 19.6) | 0 (0.0) | 1.1 (0.03-18.0) | 0 (0.0) | 1.4 (0.2-19.6) | .213‡ |
| Diagnosis, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | .208∗ | |||
| SCID | 123 (40.2) | 73 (43.7) | 50 (36.0) | ||||
| Non-SCID IEI | 183 (59.8) | 94 (56.3) | 89 (64.0) | ||||
| Performance score, n (%) | 71 (23.2) | 28 (16.8) | 43 (30.9) | .341∗ | |||
| ≥ 80 | 187 (79.6) | 114 (82.0) | 73 (76.0) | ||||
| < 80 | 48 (20.4) | 25 (18.0) | 23 (24.0) | ||||
| Pre-HSCT infection, n (%) | 0 (0.0) | 240 (78.4) | 0 (0.0) | 122 (73.1) | 0 (0.0) | 118 (84.9) | .018∗ |
| Pre-HSCT organ damage, n (%) | 5 (1.6) | 121 (40.2) | 3 (1.8) | 47 (28.7) | 2 (1.4) | 74 (54.0) | <.001∗ |
| Pre-HSCT autoimmunity, n (%) | 27 (8.8) | 82 (29.4) | 11 (6.6) | 44 (28.2) | 16 (11.5) | 38 (30.9) | .721∗ |
| Pre-HSCT malignancy, n (%) | 0 (0.0) | 21 (6.9) | 0 (0.0) | 11 (6.6) | 0 (0.0) | 10 (7.2) | >.999 |
| Pre-HSCT morbidity score, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | .002∗ | |||
| 0 to 1 | 159 (52.0) | 101 (60.5) | 58 (41.7) | ||||
| ≥ 2 | 147 (48.0) | 66 (39.5) | 81 (58.3) | ||||
| Donor, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | >.999∗ | |||
| MMUD | 15 (4.9) | 8 (4.8) | 7 (5.0) | ||||
| MMFD | 291 (95.1) | 159 (95.2) | 132 (95.0) | ||||
| HLA matching, n (%) | 52 (17.0) | 11 (6.6) | 41 (29.5) | .141† | |||
| 9/10 | 19 (7.5) | 13 (8.3) | 6 (6.1) | ||||
| 8/10 | 7 (2.8) | 7 (4.5) | 0 (0.0) | ||||
| ≤7/10 | 217 (85.4) | 130 (83.3) | 87 (88.8) | ||||
| Other/incomplete information§ | 11 (4.3) | 6 (3.8) | 5 (5.1) | ||||
| Recipient/donor CMV serostatus, n (%) | 108 (35.3) | 41 (24.6) | 67 (48.2) | .098∗ | |||
| −/− | 29 (14.6) | 14 (11.1) | 15 (20.8) | ||||
| −/+, +/−, +/+ | 169 (85.4) | 112 (88.9) | 57 (79.2) | ||||
| Conditioning, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | <.001∗ | |||
| Busulfan-based | 112 (36.6) | 22 (13.2) | 90 (64.7) | ||||
| Treosulfan-based | 115 (37.6) | 99 (59.3) | 16 (11.5) | ||||
| Other/none | 79 (25.8) | 46 (27.5) | 33 (23.7) | ||||
| Serotherapy, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | <.001† | |||
| None | 77 (25.2) | 46 (27.5) | 31 (22.3) | ||||
| ATG only | 175 (57.2) | 113 (67.7) | 62 (44.6) | ||||
| Alemtuzumab only | 53 (17.3) | 7 (4.2) | 46 (33.1) | ||||
| ATG and alemtuzumab | 1 (0.3) | 1 (0.6) | 0 (0.0) | ||||
| Rituximab, n (%) | 0 (0.0) | 108 (35.3) | 0 (0.0) | 69 (41.3) | 0 (0.0) | 39 (28.1) | .022∗ |
| Post-HSCT GvHD prophylaxis, n (%) | 34 (11.1) | 32 (19.2) | 2 (1.4) | <.001∗ | |||
| None | 41 (15.1) | 41 (30.4) | 0 (0.0) | ||||
| CNI | 43 (15.8) | 34 (25.2) | 9 (6.6) | ||||
| CNI+MMF | 141 (51.8) | 19 (14.1) | 122 (89.1) | ||||
| MMF | 25 (9.2) | 23 (17.0) | 2 (1.5) | ||||
| Others | 22 (8.1) | 18 (13.3) | 4 (2.9) | ||||
| Stem cell source, n (%) | 2 (0.7) | 2 (1.2) | 0 (0.0) | <.001† | |||
| BM | 116 (38.2) | 3 (1.8) | 113 (81.3) | ||||
| PBSC | 182 (59.9) | 162 (98.2) | 20 (14.4) | ||||
| BM+PBSC | 6 (2.0) | 0 (0.0) | 6 (4.3) | ||||
| Median TNC (range), × 108/kg | 32 (10.5) | 10.6 (2.0-100.0) | 14 (8.4) | 12.3 (2.0-100.0) | 18 (12.9) | 8.4 (2.1-58.0) | <.001‡ |
| Median CD34+ cell dose (range), × 106/kg | 17 (5.6) | 13.0 (1.6-56.6) | 9 (5.4) | 18.4 (2.0-56.6) | 8 (5.8) | 8.7 (1.6-47.7) | <.001‡ |
| Median CD3+ cell dose, range, × 106/kg | 85 (27.8) | 25.0 (0.0-993.0) | 37 (22.2) | 19.9 (0.0-557.0) | 48 (34.5) | 60.7 (0.4-993.0) | <.001‡ |
Boldface indicates significant P values.
ATG, antithymocyte globulin; BM, bone marrow; CNI, calcineurin inhibitors; MMF, mycophenolate mofetil; MMFD, mismatched donor; MMUD, mismatched unrelated donor; TNC, total nucleated cell dose.
Calculated via the χ2 test.
Calculated via Fisher exact test.
Calculated via the Wilcoxon rank sum test.
The other HLA matching scores (and counts in the entire cohort) are: 4/6 (n = 5); 6/8 (n = 3); 7/8 (n = 2); 9/12 (n = 1).
A significantly larger proportion of PTCY recipients had pre-HSCT infection (84.9% vs TCRαβ, 73.1%, P = .018), organ damage (54.0% vs TCRαβ, 28.7%, P < .001), and a pre-HSCT morbidity score of ≥2 (58.3% vs TCRαβ-HSCT, 39.5%, P = .002). The pretransplant performance score was comparable between the 2 groups (P = .341).
Donors were mismatched family donors (95.1%) and mismatched unrelated donors (4.9%). HLA matching was ≤7/10 in 217 patients (85.4%), 8/10 in 7 (2.8%), and 9/10 in 19 (7.5%); information on HLA-matching was incomplete in 11 patients (4.3%). There was no significant difference in the proportion of mismatched family or unrelated donors (P > .999), degree of HLA mismatch (P = .141) and donor-recipient CMV serostatus (P = .098) between the 2 groups.
Busulfan-based chemotherapy (64.7%) was the main conditioning in PTCY, whereas treosulfan-based chemotherapy (59.3%) was predominately used in TCRαβ (P < .001). The main stem cell source was marrow (81.3%) in PTCY and PBSC (98.2%) in TCRαβ (P < .001). The median CD34+ cell dose was significantly higher in TCRαβ (median 18.4 × 106/kg, 2.0-56.6 ×106/kg) than in PTCY (median 8.7 × 106/kg, 1.6-47.7 ×106/kg) (P<.001). The median TCRαβ+ T-cell dose was 2.2 × 104/kg (range, 0.0-75.0 × 104/kg) in TCRαβ recipients.
The method of T-cell depletion varied across centers and geographical regions. Only 1 of the 2 methods was performed in 33 of the 40 centers, 7 of 40 centers performed both methods. The 2 largest contributing countries by patient numbers were Brazil (n = 47; 47/47 PTCY) and Italy (n = 47; 46/47 TCRαβ; supplemental Table 2).
Engraftment and transplant-related complications
Engraftment was significantly faster after TCRαβ-HSCT (Table 2). The median time to neutrophil engraftment was 13.5 days (8.0-38.0 days) after TCRαβ-HSCT and 17.0 days (5.0-139.0 days) after PTCY-HSCT (P < .001). Platelet engraftment was achieved at a median of 12.0 days (5.0-377.0 days) after TCRαβ and 21.0 days after PTCY (9.0-613.0 days) (P < .001).
Transplant outcomes
| . | Entire cohort n = 306 . | TCRαβ-HSCT n = 167 . | PTCY-HSCT n = 139 . | P value . | |||
|---|---|---|---|---|---|---|---|
| Missing data . | Results . | Missing data . | Results . | Missing data . | Results . | ||
| Neutrophil recovery, n (%) | 12 (3.9) | 255 (86.7) | 9 (5.4) | 134 (84.8) | 3 (2.2) | 121 (89.0) | .381∗ |
| Median day to neutrophil recovery (range)¶ | 0 (0.0) | 15.0 (5.0-139.0) | 0 (0.0) | 13.5 (8.0-38.0) | 0 (0.0) | 17.0 (5.0-139.0) | <.001‡ |
| Platelet recovery, n (%) | 20 (6.5) | 230 (80.4) | 14 (8.4) | 123 (80.4) | 6 (4.3) | 107 (80.5) | >.999∗ |
| Median day to platelet recovery (range)¶ | 0 (0.0) | 16.0 (5.0-613.0) | 0 (0.0) | 12.0 (5.0-377.0) | 0 (0.0) | 21.0 (9.0-613.0) | <.001‡ |
| Graft failure, CIN (95% CI) | 46 (15.0) | 26 (15.6) | 20 (14.4) | ||||
| 1-year CIN of all graft failure | 13% (9%-17%) | 15% (9%-21%) | 11% (5%-17%) | .414§ | |||
| Day 60 CIN of primary graft failure | 8% (4%-11%) | 10% (5%-15%) | 5% (1%-9%) | .048§ | |||
| 1-year CIN of secondary graft failure | 4% (2%-7%) | 3% (0%-6%) | 6% (2%-10%) | .144§ | |||
| Secondary procedures (DLI or CD34+ stem cell boost), n (%) | 26 (8.5) | 13 (4.6) | 23 (13.8) | 9 (6.2) | 3 (2.2) | 4 (2.9) | .303∗ |
| Second conditioned HSCT, n (%) | 0 (0.0) | 40 (13.1) | 0 (0.0) | 29 (17.4) | 0 (0.0) | 11 (7.9) | .023∗ |
| Veno-occlusive disease, n (%) | 5 (1.6) | 28 (9.3) | 5 (3.0) | 8 (4.9) | 0 (0.0) | 20 (14.4) | .009∗ |
| Hemorrhagic cystitis, n (%) | 24 (7.8) | 18 (6.4) | 23 (13.8) | 5 (3.5) | 1 (0.7) | 13 (9.4) | .072∗ |
| Acute kidney injury, n (%) | 51 (16.7) | 20 (7.8) | 14 (8.4) | 7 (4.6) | 37 (26.6) | 13 (12.7) | .032∗ |
| Transplant-associated microangiopathy, n (%) | 52 (17.0) | 12 (4.7) | 14 (8.4) | 5 (3.3) | 38 (27.3) | 7 (6.9) | .229† |
| Cardiac complications, n (%) | 33 (10.8) | 24 (8.8) | 30 (18.0) | 10 (7.3) | 3 (2.2) | 14 (10.3) | .509∗ |
| Heart failure | 2 (0.7) | 2 (1.5) | 0 (0.0) | ||||
| Pericardial effusion | 7 (2.6) | 4 (2.9) | 3 (2.2) | ||||
| Pericarditis | 1 (0.4) | 0 (0.0) | 1 (0.7) | ||||
| Cardiomyopathy | 1 (0.4) | 0 (0.0) | 1 (0.7) | ||||
| Arrhythmia | 3 (1.1) | 0 (0.0) | 3 (2.2) | ||||
| Other∗∗ | 10 (3.7) | 4 (2.9) | 6 (4.4) | ||||
| Pulmonary complications, n (%) | 33 (10.8) | 85 (31.1) | 30 (18.0) | 33 (24.1) | 3 (2.2) | 52 (38.2) | .017∗ |
| Infection | 34 (12.5) | 14 (10.2) | 20 (14.7) | ||||
| Noninfectious pulmonary complications | 51 (18.7) | 19 (13.9) | 32 (23.5) | ||||
| Pneumonitis | 25 (9.2) | 9 (6.6) | 16 (11.8) | ||||
| Idiopathic lung disease/pneumonia | 6 (2.2) | 2 (1.5) | 4 (2.9) | ||||
| Diffuse alveolar hemorrhage | 2 (0.7) | 0 (0.0) | 2 (1.5) | ||||
| Pulmonary hypertension | 4 (1.5) | 2 (1.5) | 2 (1.5) | ||||
| Other†† | 14 (5.1) | 6 (4.4) | 8 (5.9) | ||||
| Encephalopathy, n (%) | 52 (17.0) | 15 (5.9) | 15 (9.0) | 8 (5.3) | 37 (26.6) | 7 (6.9) | .796∗ |
| Autoimmune cytopenia, n (%) | 4 (1.3) | 45 (14.9) | 4 (2.4) | 29 (17.8) | 0 (0.0) | 16 (11.5) | .172∗ |
| AIHA | 16 (5.3) | 10 (6.1) | 6 (4.3) | ||||
| ITP | 16 (5.3) | 9 (5.5) | 7 (5.0) | ||||
| AIN | 3 (1.0) | 2 (1.2) | 1 (0.7) | ||||
| AIHA + ITP | 3 (1.0) | 2 (1.2) | 1 (0.7) | ||||
| ITP + AIN | 1 (0.3) | 1 (0.6) | 0 (0.0) | ||||
| AIHA + ITP + AIN | 6 (2.0) | 5 (3.1) | 1 (0.7) | ||||
| CMV infection, n (%) | 34 (11.1) | 109 (40.1) | 18 (10.8) | 60 (40.3) | 16 (11.5) | 49 (39.8) | >.999∗ |
| Persistent from pre-transplant | 26 (9.6) | 18 (12.1) | 8 (6.5) | ||||
| Posttransplant CMV | 83 (30.5) | 42 (28.2) | 41 (33.3) | ||||
| CMV disease | 14 (5.1) | 6 (4.0) | 8 (6.5) | ||||
| CMV infection | 69 (25.4) | 36 (24.2) | 33 (26.8) | ||||
| Adenoviremia, n (%) | 4 (1.3) | 41 (13.6) | 3 (1.8) | 30 (18.3) | 1 (0.7) | 11 (8.0) | .015∗ |
| Persistent from pre-transplant | 6 (2.0) | 4 (2.4) | 2 (1.4) | ||||
| Posttransplant adenoviremia | 35 (11.6) | 26 (15.9) | 9 (6.5) | ||||
| Asymptomatic | 23 (7.6) | 18 (11.0) | 5 (3.6) | ||||
| Symptomatic | 12 (4.0) | 8 (4.9) | 4 (2.9) | ||||
| EBV viremia, n (%) | 1 (0.3) | 29 (9.5) | 0 (0.0) | 16 (9.6) | 1 (0.7) | 13 (9.4) | >.999∗ |
| Persistent from pre-transplant | 6 (2.0) | 2 (1.2) | 4 (2.9) | ||||
| Posttransplant EBV viremia | 23 (7.5) | 14 (8.4) | 9 (6.5) | ||||
| Asymptomatic | 17 (5.6) | 10 (6.0) | 7 (5.1) | ||||
| Symptomatic | 6 (2.0) | 4 (2.4) | 2 (1.4) | ||||
| Posttransplant lymphoproliferative disease, n (%) | 56 (18.3) | 5 (2.0) | 2 (1.2) | 3 (1.8) | 54 (38.8) | 2 (2.4) | >.999† |
| Cause of death,‡‡n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | .173† | |||
| Transplant related | 56 (69.1) | 23 (65.7) | 33 (71.7) | ||||
| Disease related | 17 (21.0) | 6 (17.1) | 11 (23.9) | ||||
| Other | 8 (9.9) | 6 (17.1) | 2 (4.3) | ||||
| . | Entire cohort n = 306 . | TCRαβ-HSCT n = 167 . | PTCY-HSCT n = 139 . | P value . | |||
|---|---|---|---|---|---|---|---|
| Missing data . | Results . | Missing data . | Results . | Missing data . | Results . | ||
| Neutrophil recovery, n (%) | 12 (3.9) | 255 (86.7) | 9 (5.4) | 134 (84.8) | 3 (2.2) | 121 (89.0) | .381∗ |
| Median day to neutrophil recovery (range)¶ | 0 (0.0) | 15.0 (5.0-139.0) | 0 (0.0) | 13.5 (8.0-38.0) | 0 (0.0) | 17.0 (5.0-139.0) | <.001‡ |
| Platelet recovery, n (%) | 20 (6.5) | 230 (80.4) | 14 (8.4) | 123 (80.4) | 6 (4.3) | 107 (80.5) | >.999∗ |
| Median day to platelet recovery (range)¶ | 0 (0.0) | 16.0 (5.0-613.0) | 0 (0.0) | 12.0 (5.0-377.0) | 0 (0.0) | 21.0 (9.0-613.0) | <.001‡ |
| Graft failure, CIN (95% CI) | 46 (15.0) | 26 (15.6) | 20 (14.4) | ||||
| 1-year CIN of all graft failure | 13% (9%-17%) | 15% (9%-21%) | 11% (5%-17%) | .414§ | |||
| Day 60 CIN of primary graft failure | 8% (4%-11%) | 10% (5%-15%) | 5% (1%-9%) | .048§ | |||
| 1-year CIN of secondary graft failure | 4% (2%-7%) | 3% (0%-6%) | 6% (2%-10%) | .144§ | |||
| Secondary procedures (DLI or CD34+ stem cell boost), n (%) | 26 (8.5) | 13 (4.6) | 23 (13.8) | 9 (6.2) | 3 (2.2) | 4 (2.9) | .303∗ |
| Second conditioned HSCT, n (%) | 0 (0.0) | 40 (13.1) | 0 (0.0) | 29 (17.4) | 0 (0.0) | 11 (7.9) | .023∗ |
| Veno-occlusive disease, n (%) | 5 (1.6) | 28 (9.3) | 5 (3.0) | 8 (4.9) | 0 (0.0) | 20 (14.4) | .009∗ |
| Hemorrhagic cystitis, n (%) | 24 (7.8) | 18 (6.4) | 23 (13.8) | 5 (3.5) | 1 (0.7) | 13 (9.4) | .072∗ |
| Acute kidney injury, n (%) | 51 (16.7) | 20 (7.8) | 14 (8.4) | 7 (4.6) | 37 (26.6) | 13 (12.7) | .032∗ |
| Transplant-associated microangiopathy, n (%) | 52 (17.0) | 12 (4.7) | 14 (8.4) | 5 (3.3) | 38 (27.3) | 7 (6.9) | .229† |
| Cardiac complications, n (%) | 33 (10.8) | 24 (8.8) | 30 (18.0) | 10 (7.3) | 3 (2.2) | 14 (10.3) | .509∗ |
| Heart failure | 2 (0.7) | 2 (1.5) | 0 (0.0) | ||||
| Pericardial effusion | 7 (2.6) | 4 (2.9) | 3 (2.2) | ||||
| Pericarditis | 1 (0.4) | 0 (0.0) | 1 (0.7) | ||||
| Cardiomyopathy | 1 (0.4) | 0 (0.0) | 1 (0.7) | ||||
| Arrhythmia | 3 (1.1) | 0 (0.0) | 3 (2.2) | ||||
| Other∗∗ | 10 (3.7) | 4 (2.9) | 6 (4.4) | ||||
| Pulmonary complications, n (%) | 33 (10.8) | 85 (31.1) | 30 (18.0) | 33 (24.1) | 3 (2.2) | 52 (38.2) | .017∗ |
| Infection | 34 (12.5) | 14 (10.2) | 20 (14.7) | ||||
| Noninfectious pulmonary complications | 51 (18.7) | 19 (13.9) | 32 (23.5) | ||||
| Pneumonitis | 25 (9.2) | 9 (6.6) | 16 (11.8) | ||||
| Idiopathic lung disease/pneumonia | 6 (2.2) | 2 (1.5) | 4 (2.9) | ||||
| Diffuse alveolar hemorrhage | 2 (0.7) | 0 (0.0) | 2 (1.5) | ||||
| Pulmonary hypertension | 4 (1.5) | 2 (1.5) | 2 (1.5) | ||||
| Other†† | 14 (5.1) | 6 (4.4) | 8 (5.9) | ||||
| Encephalopathy, n (%) | 52 (17.0) | 15 (5.9) | 15 (9.0) | 8 (5.3) | 37 (26.6) | 7 (6.9) | .796∗ |
| Autoimmune cytopenia, n (%) | 4 (1.3) | 45 (14.9) | 4 (2.4) | 29 (17.8) | 0 (0.0) | 16 (11.5) | .172∗ |
| AIHA | 16 (5.3) | 10 (6.1) | 6 (4.3) | ||||
| ITP | 16 (5.3) | 9 (5.5) | 7 (5.0) | ||||
| AIN | 3 (1.0) | 2 (1.2) | 1 (0.7) | ||||
| AIHA + ITP | 3 (1.0) | 2 (1.2) | 1 (0.7) | ||||
| ITP + AIN | 1 (0.3) | 1 (0.6) | 0 (0.0) | ||||
| AIHA + ITP + AIN | 6 (2.0) | 5 (3.1) | 1 (0.7) | ||||
| CMV infection, n (%) | 34 (11.1) | 109 (40.1) | 18 (10.8) | 60 (40.3) | 16 (11.5) | 49 (39.8) | >.999∗ |
| Persistent from pre-transplant | 26 (9.6) | 18 (12.1) | 8 (6.5) | ||||
| Posttransplant CMV | 83 (30.5) | 42 (28.2) | 41 (33.3) | ||||
| CMV disease | 14 (5.1) | 6 (4.0) | 8 (6.5) | ||||
| CMV infection | 69 (25.4) | 36 (24.2) | 33 (26.8) | ||||
| Adenoviremia, n (%) | 4 (1.3) | 41 (13.6) | 3 (1.8) | 30 (18.3) | 1 (0.7) | 11 (8.0) | .015∗ |
| Persistent from pre-transplant | 6 (2.0) | 4 (2.4) | 2 (1.4) | ||||
| Posttransplant adenoviremia | 35 (11.6) | 26 (15.9) | 9 (6.5) | ||||
| Asymptomatic | 23 (7.6) | 18 (11.0) | 5 (3.6) | ||||
| Symptomatic | 12 (4.0) | 8 (4.9) | 4 (2.9) | ||||
| EBV viremia, n (%) | 1 (0.3) | 29 (9.5) | 0 (0.0) | 16 (9.6) | 1 (0.7) | 13 (9.4) | >.999∗ |
| Persistent from pre-transplant | 6 (2.0) | 2 (1.2) | 4 (2.9) | ||||
| Posttransplant EBV viremia | 23 (7.5) | 14 (8.4) | 9 (6.5) | ||||
| Asymptomatic | 17 (5.6) | 10 (6.0) | 7 (5.1) | ||||
| Symptomatic | 6 (2.0) | 4 (2.4) | 2 (1.4) | ||||
| Posttransplant lymphoproliferative disease, n (%) | 56 (18.3) | 5 (2.0) | 2 (1.2) | 3 (1.8) | 54 (38.8) | 2 (2.4) | >.999† |
| Cause of death,‡‡n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | .173† | |||
| Transplant related | 56 (69.1) | 23 (65.7) | 33 (71.7) | ||||
| Disease related | 17 (21.0) | 6 (17.1) | 11 (23.9) | ||||
| Other | 8 (9.9) | 6 (17.1) | 2 (4.3) | ||||
Boldface indicates significant P values.
AIHA, autoimmune hemolytic anemia; AIN, autoimmune neutropenia; ATG, antithymocyte globulin; ITP, immune thrombocytopenia; MMFD, mismatched donor; MMUD, mismatched unrelated donor.
Calculated via the χ2 test.
Calculated via Fisher exact test.
Calculated via the Wilcoxon rank sum test.
Calculated via Gray test. This tests for differences in the cumulative incidence curves for the given event between the 2 treatments. groups across the entire follow-up period. (That is, if there is any difference between the cumulative incidence curves.) Therefore, it tests for more than only the difference in the cumulative incidence estimates at the indicated time point.
Reported among patients who achieved neutrophil/platelet recovery.
The other cardiac complications include: atrial thrombus, cardiac arrest, cardiogenic shock, and hypertension.
The other pulmonary complications include: lung fibrosis, alveolitis, pulmonary embolism, viral pneumonia, acute respiratory distress syndrome, right pleural effusion, relapsed pneumatocele, and respiratory failure.
Reported among patients who died.
There was no significant difference in overall graft failure between both groups (P = .414), but TCRαβ had a significantly higher day 60 CIN of primary graft failure (10%, 5%-15%) compared with PTCY (5%, 1%-9%, P = .048). A greater proportion of TCRαβ recipients (n = 29, 17.4%) received a conditioned second HSCT compared with PTCY (n = 11, 7.9%) (P = .023). There was no significant difference in the number of CD34+ boosts or DLI procedures between TCRαβ recipients (n = 9, 6.2%; CD34+ boost, 8; DLI, 1) and PTCY recipients (n = 4, 2.9%; CD34+ boost, 3; DLI; P = .303).
PTCY recipients had significantly higher rates of veno-occlusive disease (14.4% vs TCRαβ 4.9%, P = .009), acute kidney injury (12.7% vs TCRαβ 4.6%, P = .032), and pulmonary complications (38.2% vs TCRαβ 24.1%, P = .017). There was no statistically significant difference between the groups for rate of hemorrhagic cystitis (PTCY, 9.4% vs TCRαβ, 3.5%, P = .072), cardiac complications (PTCY, 10.3% vs TCRαβ, 7.3%, P = .509), transplant-associated microangiopathy (PTCY, 6.9% vs TCRαβ, 3.3%, P = .229), encephalopathy (PTCY, 6.9% vs TCRαβ, 5.3%, P = .796), and posttransplant autoimmune cytopenia (PTCY, 11.5% vs TCRαβ, 17.8%, P = .172).
The adenoviremia rate was significantly higher in TCRαβ, 18.3% compared with 8.0% in PTCY (P = .015). CMV infection rate was similar between both groups, 40.3% after TCRαβ and 39.8% after PTCY (P > .999). CMV disease occurred in 4.0% of TCR αβ and 6.5% of PTCY (P = .519).
EBV viremia occurred in 9.6% of TCRαβ and 9.4% of PTCY (P > .999). About 2% of patients from each group developed PTLD (P > .999). In TCRαβ, EBV viremia was significantly reduced in patients who received pretransplant rituximab (n = 2 of 69, 2.9%) compared with patients who did not receive rituximab (14 of 98, 14.3%, P=.028), but not in PTCY recipients (rituximab, n = 3 of 39, 7.7% vs no rituximab, n = 10 of 99 (10.1%, P > .999). Rituximab was not associated with significantly different rates of PTLD in TCRαβ (rituximab, n = 0 of 68, 0% vs no rituximab, n = 3 of 97 (3.1%, P = .269), nor in PTCY recipients (rituximab, n = 1 of 39, 2.6% vs no rituximab, n = 1 of 46 (2.2%, P > .999) (supplemental Table 3).
Overall and event-free survival
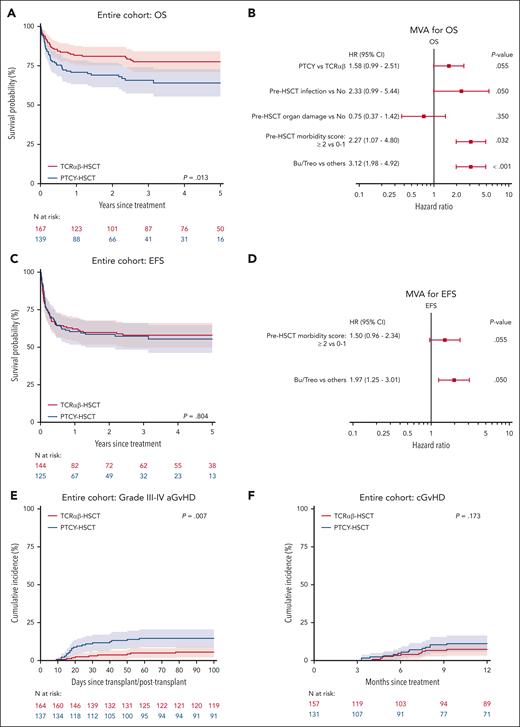
The 3-year OS was 78% (71%-84%) after TCRαβ and 66% (57%-74%) after PTCY (P = .013, Figure 1A). Conditioning regimen (P < .001), organ damage (P = .002), infection (P = .002), and pre-HSCT morbidity score (P < .001) were also significant predictors for OS in UVA (Table 3). Upon MVA, pre-HSCT morbidity score (hazard ratio [HR] 2.27, 95% confidence interval [CI], 1.07-4.80, P = .032) and non-busulfan/treosulfan (HR 3.12, 1.98-4.92, P < .001) were the only independent predictors of OS (Figure 1B). When comparing TCD methods, HR for an event in the Cox model was 1.58 (0.99-2.51, P = .055) for PTCY in reference to TCRαβ.
Overall survival, event-free survival and GvHD according to TCD methods. (A) Kaplan-Meier curves of OS stratified by TCD methods; (B) MVA for OS; (C) EFS stratified by TCD methods; (D) MVA for EFS; cumulative incidences of grade III-IV acute GvHD (E) and chronic GvHD (F) by TCD methods.
Overall survival, event-free survival and GvHD according to TCD methods. (A) Kaplan-Meier curves of OS stratified by TCD methods; (B) MVA for OS; (C) EFS stratified by TCD methods; (D) MVA for EFS; cumulative incidences of grade III-IV acute GvHD (E) and chronic GvHD (F) by TCD methods.
Univariate analysis for OS, EFS, GEFS, and acute and chronic GvHD
| . | OS (95% CI) . | EFS∗ (95% CI) . | GEFS† (95% CI) . | Grade II-IV aGvHD (95% CI) . | Grade III-IV aGvHD (95% CI) . | cGvHD (95% CI) . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1-y . | 3-y . | P value‡ . | 1-y . | 3-y . | P value‡ . | 1-y . | 3-y . | P value‡ . | Day 100 . | P value§ . | Day 100 . | P value§ . | 1-year . | P value§ . | |
| Method | .013 | .804 | .080 | .009 | .007 | .173 | |||||||||
| TCRαβ-HSCT (n = 167) | 82% (76%-88%) | 78% (71%-84%) | 62% (54%-70%) | 58% (50%-66%) | 54% (46%-62%) | 53% (44%-61%) | 16% (10%-22%) | 6% (2%-9%) | 7% (3%-11%) | ||||||
| PTCY-HSCT (n = 139) | 71% (63%-78%) | 66% (57%-74%) | 60% (52%-69%) | 57% (48%-66%) | 46% (38%-55%) | 41% (32%-50%) | 30% (22%-38%) | 15% (9%-21%) | 11% (6%-17%) | ||||||
| Year of HSCT | .367 | .768 | .360 | .989 | .238 | .859 | |||||||||
| 2011 to 2015 (n = 114) | 81% (73%-88%) | 75% (67%-83%) | 65% (55%-74%) | 58% (49%-68%) | 54% (45%-64%) | 49% (39%-59%) | 22% (15%-30%) | 7% (2%-12%) | 10% (5%-16%) | ||||||
| 2016 to 2019 (n = 192) | 74% (68%-81%) | 70% (63%-77%) | 59% (52%-67%) | 57% (49%-65%) | 48% (41%-56%) | 46% (39%-54%) | 22% (16%-28%) | 11% (7%-16%) | 8% (4%-12%) | ||||||
| Age at HSCT | .665 | .691 | .547 | .921 | .590 | .382 | |||||||||
| <5 y (n = 235) | 77% (71%-82%) | 72% (66%-78%) | 61% (55%-68%) | 57% (50%-64%) | 52% (45%-58%) | 49% (42%-56%) | 22% (17%-28%) | 9% (5%-13%) | 9% (5%-12%) | ||||||
| >5 y (n = 71) | 77% (68%-87%) | 74% (63%-84%) | 62% (50%-75%) | 60% (47%-73%) | 46% (34%-59%) | 42% (30%-55%) | 23% (13%-32%) | 11% (4%-19%) | 11% (3%-18%) | ||||||
| Diagnosis | .084 | .197 | .258 | .589 | .831 | .692 | |||||||||
| Non-SCID (n = 183) | 81% (75%-87%) | 75% (68%-82%) | 64% (56%-71%) | 61% (54%-69%) | 53% (45%-60%) | 50% (42%-57%) | 24% (18%-30%) | 9% (5%-14%) | 9% (5%-13%) | ||||||
| SCID (n=123) | 70% (62%-78%) | 68% (59%-76%) | 58% (49%-67%) | 52% (42%-62%) | 48% (38%-57%) | 44% (35%-54%) | 20% (13%-27%) | 10% (5%-15%) | 9% (4%-14%) | ||||||
| Pre-HSCT infection | .002 | .108 | .020 | .394 | .121 | .337 | |||||||||
| No (n = 66) | 92% (86%-99%) | 87% (78%-96%) | 72% (60%-84%) | 67% (54%-80%) | 66% (54%-78%) | 58% (45%-72%) | 19% (9%-28%) | 5% (0%-10%) | 6% (0%-12%) | ||||||
| Yes (n = 240) | 73% (67%-78%) | 68% (62%-74%) | 59% (52%-65%) | 55% (48%-62%) | 47% (40%-53%) | 44% (38%-51%) | 23% (18%-29%) | 11% (7%-15%) | 10% (6%-14%) | ||||||
| Pre-HSCT organ damage | .002 | .090 | .018 | .283 | .031 | .615 | |||||||||
| No (n = 180) | 84% (78%-89%) | 80% (74%-86%) | 68% (61%-76%) | 63% (55%-71%) | 57% (49%-65%) | 53% (45%-61%) | 20% (14%-26%) | 7% (3%-11%) | 9% (4%-13%) | ||||||
| Yes (n = 121) | 67% (59%-76%) | 63% (54%-72%) | 54% (45%-63%) | 53% (44%-62%) | 43% (34%-52%) | 41% (32%-50%) | 26% (18%-34%) | 14% (8%-21%) | 10% (4%-16%) | ||||||
| Pre-HSCT autoimmunity | .517 | .230 | .088 | .127 | .221 | .333 | |||||||||
| No (n = 197) | 79% (74%-85%) | 75% (69%-81%) | 65% (58%-72%) | 61% (53%-68%) | 54% (47%-62%) | 51% (43%-58%) | 20% (14%-25%) | 8% (4%-12%) | 10% (6%-14%) | ||||||
| Yes (n = 82) | 77% (67%-86%) | 72% (61%-82%) | 59% (47%-70%) | 55% (44%-67%) | 47% (36%-59%) | 45% (34%-57%) | 27% (17%-37%) | 12% (5%-20%) | 6% (0%-11%) | ||||||
| Pre-HSCT malignancy | .657 | .342 | .740 | .702 | .453 | .483 | |||||||||
| No (n = 285) | 76% (72%-81%) | 72% (66%-77%) | 61% (55%-67%) | 57% (50%-63%) | 50% (44%-57%) | 47% (41%-53%) | 23% (18%-27%) | 9% (6%-13%) | 9% (5%-12%) | ||||||
| Yes (n = 21) | 81% (64%-98%) | 76% (57%-94%) | 68% (48%-89%) | 68% (48%-89%) | 53% (30%-75%) | 53% (30%-75%) | 19% (2%-36%) | 14% (0%-29%) | 15% (0%-31%) | ||||||
| Pre-HSCT morbidity score|| | <.001 | .031 | .010 | .755 | .042 | .853 | |||||||||
| 0 to 1 (n = 159) | 87% (82%-92%) | 83% (76%-89%) | 69% (61%-77%) | 63% (54%-72%) | 57% (49%-65%) | 52% (44%-61%) | 21% (15%-28%) | 6% (3%-10%) | 9% (4%-14%) | ||||||
| ≥2 (n = 147) | 66% (58%-73%) | 61% (53%-69%) | 54% (46%-62%) | 52% (44%-61%) | 44% (36%-52%) | 42% (34%-51%) | 24% (17%-31%) | 13% (8%-19%) | 9% (4%-14%) | ||||||
| Conditioning | <.001 | <.001 | <.001 | .123 | .001 | .646 | |||||||||
| Busulfan-based (n = 112) | 79% (71%-86%) | 78% (70%-85%) | 68% (58%-77%) | 68% (58%-77%) | 54% (44%-64%) | 51% (41%-61%) | 25% (17%-33%) | 14% (7%-20%) | 7% (2%-11%) | ||||||
| Treosulfan-based (n = 115) | 87% (81%-93%) | 83% (76%-90%) | 68% (58%-77%) | 63% (53%-73%) | 57% (48%-67%) | 55% (45%-65%) | 16% (9%-23%) | 2% (0%-4%) | 11% (5%-17%) | ||||||
| Other/none (n = 79) | 60% (49%-71%) | 49% (37%-61%) | 45% (34%-56%) | 38% (27%-50%) | 37% (26%-48%) | 32% (21%-43%) | 28% (18%-38%) | 16% (8%-24%) | 10% (3%-17%) | ||||||
| . | OS (95% CI) . | EFS∗ (95% CI) . | GEFS† (95% CI) . | Grade II-IV aGvHD (95% CI) . | Grade III-IV aGvHD (95% CI) . | cGvHD (95% CI) . | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1-y . | 3-y . | P value‡ . | 1-y . | 3-y . | P value‡ . | 1-y . | 3-y . | P value‡ . | Day 100 . | P value§ . | Day 100 . | P value§ . | 1-year . | P value§ . | |
| Method | .013 | .804 | .080 | .009 | .007 | .173 | |||||||||
| TCRαβ-HSCT (n = 167) | 82% (76%-88%) | 78% (71%-84%) | 62% (54%-70%) | 58% (50%-66%) | 54% (46%-62%) | 53% (44%-61%) | 16% (10%-22%) | 6% (2%-9%) | 7% (3%-11%) | ||||||
| PTCY-HSCT (n = 139) | 71% (63%-78%) | 66% (57%-74%) | 60% (52%-69%) | 57% (48%-66%) | 46% (38%-55%) | 41% (32%-50%) | 30% (22%-38%) | 15% (9%-21%) | 11% (6%-17%) | ||||||
| Year of HSCT | .367 | .768 | .360 | .989 | .238 | .859 | |||||||||
| 2011 to 2015 (n = 114) | 81% (73%-88%) | 75% (67%-83%) | 65% (55%-74%) | 58% (49%-68%) | 54% (45%-64%) | 49% (39%-59%) | 22% (15%-30%) | 7% (2%-12%) | 10% (5%-16%) | ||||||
| 2016 to 2019 (n = 192) | 74% (68%-81%) | 70% (63%-77%) | 59% (52%-67%) | 57% (49%-65%) | 48% (41%-56%) | 46% (39%-54%) | 22% (16%-28%) | 11% (7%-16%) | 8% (4%-12%) | ||||||
| Age at HSCT | .665 | .691 | .547 | .921 | .590 | .382 | |||||||||
| <5 y (n = 235) | 77% (71%-82%) | 72% (66%-78%) | 61% (55%-68%) | 57% (50%-64%) | 52% (45%-58%) | 49% (42%-56%) | 22% (17%-28%) | 9% (5%-13%) | 9% (5%-12%) | ||||||
| >5 y (n = 71) | 77% (68%-87%) | 74% (63%-84%) | 62% (50%-75%) | 60% (47%-73%) | 46% (34%-59%) | 42% (30%-55%) | 23% (13%-32%) | 11% (4%-19%) | 11% (3%-18%) | ||||||
| Diagnosis | .084 | .197 | .258 | .589 | .831 | .692 | |||||||||
| Non-SCID (n = 183) | 81% (75%-87%) | 75% (68%-82%) | 64% (56%-71%) | 61% (54%-69%) | 53% (45%-60%) | 50% (42%-57%) | 24% (18%-30%) | 9% (5%-14%) | 9% (5%-13%) | ||||||
| SCID (n=123) | 70% (62%-78%) | 68% (59%-76%) | 58% (49%-67%) | 52% (42%-62%) | 48% (38%-57%) | 44% (35%-54%) | 20% (13%-27%) | 10% (5%-15%) | 9% (4%-14%) | ||||||
| Pre-HSCT infection | .002 | .108 | .020 | .394 | .121 | .337 | |||||||||
| No (n = 66) | 92% (86%-99%) | 87% (78%-96%) | 72% (60%-84%) | 67% (54%-80%) | 66% (54%-78%) | 58% (45%-72%) | 19% (9%-28%) | 5% (0%-10%) | 6% (0%-12%) | ||||||
| Yes (n = 240) | 73% (67%-78%) | 68% (62%-74%) | 59% (52%-65%) | 55% (48%-62%) | 47% (40%-53%) | 44% (38%-51%) | 23% (18%-29%) | 11% (7%-15%) | 10% (6%-14%) | ||||||
| Pre-HSCT organ damage | .002 | .090 | .018 | .283 | .031 | .615 | |||||||||
| No (n = 180) | 84% (78%-89%) | 80% (74%-86%) | 68% (61%-76%) | 63% (55%-71%) | 57% (49%-65%) | 53% (45%-61%) | 20% (14%-26%) | 7% (3%-11%) | 9% (4%-13%) | ||||||
| Yes (n = 121) | 67% (59%-76%) | 63% (54%-72%) | 54% (45%-63%) | 53% (44%-62%) | 43% (34%-52%) | 41% (32%-50%) | 26% (18%-34%) | 14% (8%-21%) | 10% (4%-16%) | ||||||
| Pre-HSCT autoimmunity | .517 | .230 | .088 | .127 | .221 | .333 | |||||||||
| No (n = 197) | 79% (74%-85%) | 75% (69%-81%) | 65% (58%-72%) | 61% (53%-68%) | 54% (47%-62%) | 51% (43%-58%) | 20% (14%-25%) | 8% (4%-12%) | 10% (6%-14%) | ||||||
| Yes (n = 82) | 77% (67%-86%) | 72% (61%-82%) | 59% (47%-70%) | 55% (44%-67%) | 47% (36%-59%) | 45% (34%-57%) | 27% (17%-37%) | 12% (5%-20%) | 6% (0%-11%) | ||||||
| Pre-HSCT malignancy | .657 | .342 | .740 | .702 | .453 | .483 | |||||||||
| No (n = 285) | 76% (72%-81%) | 72% (66%-77%) | 61% (55%-67%) | 57% (50%-63%) | 50% (44%-57%) | 47% (41%-53%) | 23% (18%-27%) | 9% (6%-13%) | 9% (5%-12%) | ||||||
| Yes (n = 21) | 81% (64%-98%) | 76% (57%-94%) | 68% (48%-89%) | 68% (48%-89%) | 53% (30%-75%) | 53% (30%-75%) | 19% (2%-36%) | 14% (0%-29%) | 15% (0%-31%) | ||||||
| Pre-HSCT morbidity score|| | <.001 | .031 | .010 | .755 | .042 | .853 | |||||||||
| 0 to 1 (n = 159) | 87% (82%-92%) | 83% (76%-89%) | 69% (61%-77%) | 63% (54%-72%) | 57% (49%-65%) | 52% (44%-61%) | 21% (15%-28%) | 6% (3%-10%) | 9% (4%-14%) | ||||||
| ≥2 (n = 147) | 66% (58%-73%) | 61% (53%-69%) | 54% (46%-62%) | 52% (44%-61%) | 44% (36%-52%) | 42% (34%-51%) | 24% (17%-31%) | 13% (8%-19%) | 9% (4%-14%) | ||||||
| Conditioning | <.001 | <.001 | <.001 | .123 | .001 | .646 | |||||||||
| Busulfan-based (n = 112) | 79% (71%-86%) | 78% (70%-85%) | 68% (58%-77%) | 68% (58%-77%) | 54% (44%-64%) | 51% (41%-61%) | 25% (17%-33%) | 14% (7%-20%) | 7% (2%-11%) | ||||||
| Treosulfan-based (n = 115) | 87% (81%-93%) | 83% (76%-90%) | 68% (58%-77%) | 63% (53%-73%) | 57% (48%-67%) | 55% (45%-65%) | 16% (9%-23%) | 2% (0%-4%) | 11% (5%-17%) | ||||||
| Other/none (n = 79) | 60% (49%-71%) | 49% (37%-61%) | 45% (34%-56%) | 38% (27%-50%) | 37% (26%-48%) | 32% (21%-43%) | 28% (18%-38%) | 16% (8%-24%) | 10% (3%-17%) | ||||||
Boldface indicates significant P values.
EFS is defined as survival without graft failure, secondary procedures (DLI or CD34 boost), and a second transplant.
GEFS is defined as survival without grade III-IV aGvHD, cGvHD, graft failure, secondary procedures (DLI or CD34 boost), and a second transplant.
Calculated via the log-rank test.
Calculated via Gray test.
The pre-HSCT morbidity score is calculated using the following variables: pretransplant infections, pretransplant organ damage, pretransplant malignancies, pretransplant lung disease, pretransplant gut disease, pretransplant renal problems, pretransplant neurological complications. A positive indication for any these variables contributes 1 point to a patient’s pre-HCT morbidity score.
When stratifying OS according to TCD method and pre-HSCT morbidity score: in recipients with a pre-HSCT morbidity score of 0-1, the 3-year OS was 88% (81%-95%) in TCRαβ and 73% (61%-86%) in PTCY (P = .009); in recipients with a pre-HSCT morbidity score ≥2, the 3-year OS was 62% (50%-74%) in TCRαβ and 60% (49%-71%) in PTCY (P = .751).
When stratifying OS according to TCD method and conditioning: in recipients who received busulfan-based conditioning (n = 112), the 3-year OS was 91% (79%-100%) for TCRαβ (n = 22) and 74% (65%-83%) for PTCY (n = 90, P = .105); in recipients who received treosulfan-based conditioning regimen (n=115), the 3-year OS was 84% (76%-91%) for TCRαβ (n=99) and 81% (61%-100%)for PTCY (n=16, P=.546). The 3-year OS was 58% (43%-73%) for TCRαβ (n=46) and 38% (20%-56%) for PTCY (n=33, P=.108) in patients who received other/no conditioning (n=79).
OS was not significantly influenced by the year of transplant. For TCRαβ, 3-year OS was 83% (75%-92%) for 2011 to 2015 (n = 79) and 72% (61%-82%) for 2016 to 2019 (n = 88, P=.113). For PTCY, 3-year OS was 57% (39%-74%) for 2011 to 2015 (n = 35) and 69% (60%-78%) for 2016 to 2019 (n = 104, P = .226).
The 3-year EFS was 58% (50%-66%) after TCRαβ and 57% (48%-66%) after PTCY (P = .804). Upon UVA, non-busulfan/treosulfan conditioning (P < .001) and pre-HSCT morbidity score (P = .031) were significant predictors of inferior EFS. Upon MVA, non-busulfan/treosulfan (HR 1.97, 1.25-3.10, P = .004) was the only independent predictor of inferior EFS (Figure 2D).
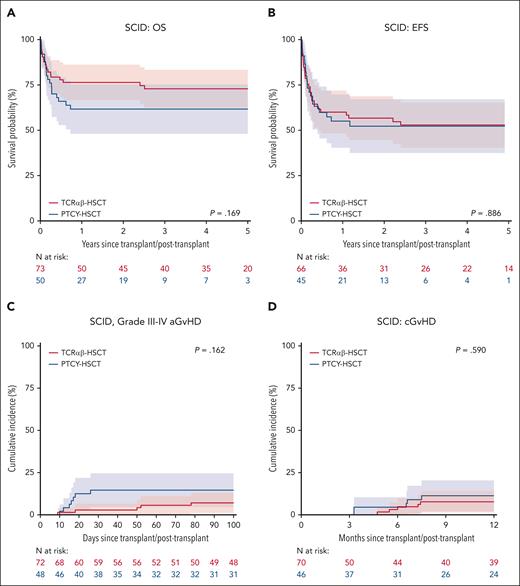
Subgroup analysis for SCID: OS (A), EFS (B), grade III-IV aGvHD (C), and chronic GvHD (D), stratified by TCD methods.
Subgroup analysis for SCID: OS (A), EFS (B), grade III-IV aGvHD (C), and chronic GvHD (D), stratified by TCD methods.
GvHD and GvHD-free, event-free survival
Day 100 CIN of grade II-IV aGvHD after PTCY and TCRαβ was 30% (22%-38%) and 16% (10%-22%), respectively (P = .009). Day 100 CIN of grade III-IV aGvHD was 15% (9%-21%) after PTCY and 6% (2%-9%) after TCRαβ (P = .007, Figure 1E). On UVA, organ damage (P = .031), pre-HSCT morbidity (P = .042), and conditioning (P = .001) were also significant predictors for grade III-IV aGvHD (Table 3). There was no significant difference in the CIN of cGvHD between TCRαβ (1-year CIN: 7%, 3%-11%) and PTCY (1-year CIN: 11%, 6%-17%) (P = .173, Figure 1F).
The 3-year GEFS was 53% (44%-61%) after TCRαβ and 41% (32%-50%) after PTCY (P = .080). Upon UVA, infection (P = .020), organ damage (P = .018), pre-HSCT morbidity score (P = .010), and non-busulfan/treosulfan conditioning (P < .001) were significant predictors for worse GEFS. Upon MVA, other conditioning (HR, 1.92; 1.25-2.93, P = .003) was the only independent predictor of inferior GEFS.
Immune reconstitution and donor chimerism
Immune reconstitution data at 1 month, 3, 6, and 12 months were collected, but there were high rates of missing data (supplemental Table 4). Mean CD3+ T-cell count at 3 months was 828.6 ± standard error 121.0 cells per μL (n = 69/139) after PTCY and 491.8 ± 64.7 cells/μL after TCRαβ (n = 110/167, supplemental Figure 3). Data for donor chimerism at latest follow-up were also limited and are summarized in supplemental Table 5.
Transplant outcomes in severe combined immunodeficiency
Of 123 patients with SCID, 73 (59.3%) had TCRαβ and 50 (40.7%) received PTCY (supplemental Table 6-7). The 3-year OS, EFS, and GEFS were comparable between TCRαβ and PTCY. The OS was 73% (62%-83%) after TCRαβ and 62% (48%-75%) after PTCY (P = .169, Figure 2A). Infection (P = .015), pretransplant organ damage (P = .015), pre-HSCT autoimmunity (P = .031), pre-HSCT morbidity score (P<.001), and non-busulfan/treosulfan conditioning (P = .003) were significantly associated with inferior OS in SCID (supplemental Table 6). On MVA, only pre-HSCT morbidity score ≥2 (HR 4.18, HR 1.45-12.09, P = .008) was independently associated with inferior OS.
The 3-year EFS was 53% (40%-65%) after TCRαβ and 52% (37%-67%) after PTCY (P = .886, Figure 2B). On UVA, EFS was influenced by pretransplant infection (P = .047), the number of the pre-HSCT morbidity score (P = .001), and non-Busulfan/Treosulfan conditioning (P = .019), but none was an independent predictor on MVA. The 1-year CIN of overall graft failure was 11% (3%-19%) for TCRαβ and 5% (0%-12%) for PTCY (P = .610). The 3-year GEFS was 47% (35%-59%) after TCRαβ and 40% (26%-55%) after PTCY (P = .384); pre-HSCT morbidity score ≥2 (HR 2.59, HR 1.08-6.251, P = .034) was the only independent predictor of inferior GEFS on MVA.
When comparing TCRαβ and PTCY in the SCID cohort, the CI of grade II-IV aGvHD was significantly higher after PTCY (31%, 18%-44%) compared with TCRαβ (13%, 5%-20%, P = .024). There was no statistically significant difference in the CIN of grade III-IV aGvHD (TCRαβ, 7%, 1%-13% vs PTCY, 15%, 5%-25%, P = .162, Figure 2C) and cGvHD (TCRαβ, 8%, 1%-14% vs PTCY, 11%, 2%-20%, P = .590, Figure 2D) between both groups.
Transplant outcomes in non-SCID IEIs
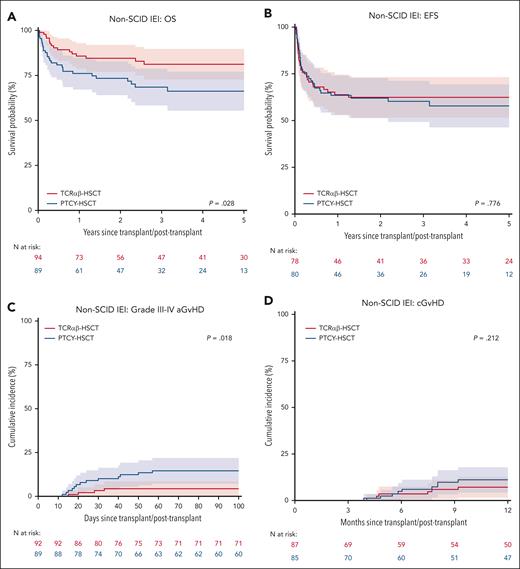
Of 183 patients without SCID, 94 (51.4%) had TCRαβ and 89 (48.6%) received PTCY (supplemental Table 8-9). The 3-year OS was 81% (73%-90%) after TCRαβ and 68% (58%-79%) after PTCY (P = .028, Figure 3A). On UVA, OS was also influenced by organ damage (P = .037), pre-HSCT morbidity score (P = .044), and conditioning (P = .001). On MVA, non-busulfan/treosulfan conditioning (HR 3.29, 1.76-6.14, P < .001) was associated with a significantly lower OS. When comparing TCD methods, HR for an event in the Cox model was 1.87 (0.99-3.56, P = .055) for PTCY as compared with TCRαβ.
Subgroup analysis for non-SCID IEI: OS (A), EFS (B), grade III-IV aGvHD (C), and chronic GvHD (D), stratified by TCD methods.
Subgroup analysis for non-SCID IEI: OS (A), EFS (B), grade III-IV aGvHD (C), and chronic GvHD (D), stratified by TCD methods.
EFS was comparable between TCRαβ (3-year EFS: 62%, 52%-73%) and PTCY (60%, 49%-71%, P = .249, Figure 3B). Conditioning was the only significant predictor for EFS; rates were similar for busulfan-based conditioning (69%, 58%-80%) and treosulfan-based conditioning (66%, 54%-78%), but lower after non-busulfan/treosulfan conditioning (38%, 20%-55%; upon MVA, HR 1.93, 1.02-3.65, P = .044). The 1-year CIN of overall graft failure was 18% (10%-26%) for TCRαβ and 14% (6%-22%) for PTCY (P = .452). The 3-year GEFS was 57%, (46%-68%) after TCRαβ and 42% (31%-53%) after PTCY (P = .099). Non-busulfan/treosulfan conditioning (HR 1.90, 1.05-3.43, P = .033) was the only significant predictor of GEFS.
In non-SCID IEIs, the day 100 CIN of grade II-IV aGvHD was 29% (20%-39%) after TCRαβ and 18% (11%-26%) after PTCY (P = .140). The day 100 CIN of grade III-IV acute GvHD was significantly higher after PTCY at 15% (7%-22%) compared with TCRαβ at 4% (0%-9%, P = .018, Figure 3C). There was no significant difference in the CIN of cGvHD between PTCY (1-year CIN: 11%, 4%-18%) and TCRαβ (1-year CIN: 7%, 2%-13%, P = .212, Figure 3D).
Discussion
To our knowledge, this is the largest retrospective study of HSCT outcomes for patients with IEIs who received an ex vivo or in vivo T-cell–depleted HLA-mismatched donor transplant using either TCRαβ or PTCY. These approaches are increasingly being used worldwide, and this is the first study to compare their outcomes in these disease entities. The OS with both outcomes compares favorably with historical data, particularly for patients with non-SCID IEIs, who were reported by the EBMT-IEWP to have a 3-year OS of only 46% after mismatched related donor HSCT in the period from 2000 to 2005.20
The OS was significantly better after TCRαβ compared with PTCY. However, there were a few differences between the 2 groups of patients. Infection, organ damage, and pre-HSCT morbidity score were all higher in the PTCY group, and on MVA, pre-HSCT morbidity score was the only independent predictor of OS. This is in keeping with other studies that show a higher pre-HSCT morbidity score has a negative impact on outcome.6,21,22 Certain geographical differences may have introduced an additional bias with regards to center practice, with some countries performing (almost) exclusively TCRαβ (ie, Italy, United Kingdom, Australia), whereas other countries predominantly use PTCY (ie, Brazil, France, India, Saudi Arabia; supplemental Table 2).
Acute GvHD was more frequent after PTCY, a finding explained by the higher amount of alloreactive T cells transferred to the recipients. However, pre-HSCT organ damage, which was also a predictor for severe aGvHD, was also more prevalent in the PTCY group. Importantly, comparably low rates of cGvHD were observed in both groups, and GEFS was not significantly influenced by the TCD methods. The GvHD rates observed in this study were comparable with those observed with matched donors in other recent IEWP studies.4,5 Nevertheless, it remains the objective to improve GvHD rates for PTCY, whether with the implementation of newer agents into the GvHD prophylaxis or with the optimization of T-cell–directed serotherapy.23,24 More importantly, patients should be considered for transplant at an earlier stage of the disease, with fewer comorbidities present.
Both neutrophil and platelet recovery was faster after TCRαβ, likely because of the higher number of CD34+ cells infused than in the PTCY cohort. There was no significant difference in overall graft failure between both groups, but TCRαβ had a significantly higher incidence of primary graft failure and second-conditioned HSCT compared with PTCY. This needs to be interpreted with caution because of the differences in conditioning chemotherapy and serotherapy. Busulfan-based conditioning was predominantly used in PTCY, in contrast to TCRαβ, who mostly received treosulfan-based conditioning. Because of the retrospective nature of this study, insufficient data were available to document pharmacokinetic monitoring of busulfan. Both busulfan-based and treosulfan-based conditioning led to better outcomes in this study than other conditioning regimens. Importantly, the 3-year EFS was equivalent between both approaches. Other differences in outcome such as higher rates of liver, kidney, and pulmonary toxicity in the PTCY cohort may be related to the differences in conditioning but could also have been influenced by pre-HSCT comorbidities.
As expected after T-depleted HSCT, the rate of infection with CMV was high, but overt CMV disease was low with both methods. The introduction of letermovir into HSCT standard practice may result in lower CMV reactivation rates in the future.25 The lower rate of adenoviremia in PTCY could be explained by earlier CD4+ recovery in PTCY, but immune reconstitution data were too incomplete to draw definite conclusions.26 Our data also support the use of pretransplant rituximab in TCRαβ transplants (41.3% received rituximab), where it significantly reduced EBV viremia, but this was not observed in the PTCY group (28.1% received rituximab). The role of pretransplant rituximab in preventing EBV viremia has been reported in adults and children undergoing HSCT using different donors, especially when profound T-cell depletion is applied.27-30 Additional cellular therapy has been increasingly used in clinical practice to boost immune recovery and provide virus-specific immunity after HSCT, including single or multiple virus-specific T cells and memory (CD45RO+) T-lymphocyte addback.31-35 Model-based serotherapy dosing has a potential role in achieving optimal posttransplant exposure to enhance early immune reconstitution, lower viral infection and GvHD rates for both TCRαβ and PTCY.36,37
In SCID, TCRαβ and PTCY have comparable OS, EFS, and GEFS. An improved outcome from HSCT if SCID is diagnosed at birth has been reported in other studies.22 This study lacked a sufficient number of newborn, diagnosed patients with SCID for comparison of the 2 approaches in this specific group. In the non-SCID IEI cohort, there was better OS after TCRαβ on UVA, whereas EFS and GEFS were not significantly different between both strategies. Only non-busulfan/treosulfan conditioning was a significant predictor for inferior OS, EFS, and GEFS on MVA.
Although we could not detect any effect of center size on outcome (supplemental data 10), we believe that successfully applying both techniques requires a certain degree of center experience. Neven et al15 reported an OS of 77.7%, grade III-IV aGvHD of 8%, and cGvHD of 29.6% with 0% extensive cGvHD using PTCY in 27 patients with IEIs.15 Two other reports by Kurzay et al16 (n = 13) and Klein et al18 (n = 25) both reported an OS of 92% and no patient had grade III-IV aGvHD; 0% cGvHD was reported by Kurzay et al16, whereas 14% had cGvHD with 5% severe in Klein’s report.15,16,38 In the largest single-center experience of using PTCY for first haploidentical donor HSCT in 55 patients and salvage second HSCT in 18 patients, Fernandes et al reported an OS of 66%, grade III-IV aGvHD of 14% for the entire cohort, and cGvHD in the patients with first HSCT of 6%.39 In 38 patients affected by IEIs and given TCRαβ haplo-HSCT reported by the Rome group, OS was 92.1% (95% CI, 77.5-97.4). The OS for patients with SCID (n = 21) and patients with non-SCID IEI (n = 17) was 90.5% (95% CI, 67.0-97.5) and 94.1% (95% CI, 65.0-99.1), respectively.17 Lum et al reported an OS of 87% using TCRαβ for young children (<5 years of age, n = 34), but OS using TCRαβ was inferior (55%) in older children (n = 13). Severe grade III-IV aGvHD was observed in 2% (1 patient who did not receive serotherapy) of TCRαβ compared with 7% of matched unrelated donor transplant (MUD) PBSC HSCT and none had cGvHD in the entire cohort.40 In a more recent report on TCRαβ in SCID, OS was 91% after TCRαβ (n = 16).41 Altogether, these previously published data indicate that in centers with active programs and a large experience with a specific platform, the results can be significantly better than those reported in registry-based analyses.
There are several limitations to our study, most importantly, a significant heterogeneity in the study group with respect to pre-HSCT clinical status, similar to many retrospective studies. As data on pretransplant clinical status, including infections and organ damage, were collected as binary outcomes, comprehensive analysis of impact of severity organ damage and infections could not be performed. Although the conditioning regimen in this study has been classified as busulfan-based, treosulfan-based and other conditioning, the combination of conditioning regimen and GvHD prophylaxis schemes varied within each group. Significant missing data hindered comparisons of long-term graft function and immune reconstitution between TCD methods.
In conclusion, this retrospective multicenter study demonstrates that both TCRαβ and PTCY led to good results in patients with a wide range of IEIs, with pre-HSCT infections, organ damage, and morbidity being predictors of inferior outcome. Further optimization of both approaches is required to improve transplant outcomes, especially concerning GvHD and transplant-related toxicities after PTCY and viral infections after TCRαβ. This study supports the notion that all patients with IEIs with an indication can safely receive HSCT even in the absence of an HLA-matched donor. The optimization of patient selection and timing of transplant before organ damage and accumulation of comorbidities are important to improve outcomes.
Acknowledgments
The authors thank all the following individuals and contributing institutions: Cecile Renard, Institut d`Hematologie et d`Oncologie Pediatrique, Lyon, France; Charlotte Niemeyer, University of Freiburg, Freiburg, Germany; Franca Fagioli, Onco-Ematologia Pediatrica, Torino, Italy; Elena Soncini, Universitá degli Studi di Brescia, Brescia, Italy; Fulvio Porta, Universitá degli Studi di Brescia, Brescia, Italy; Gergely Kriván, Central Hospital of Southern Pest, Budapest, Hungary; Jacek Winiarski, Karolinska University Hospital, Stockholm, Sweden; Krzysztof Kałwak, Clinical Department of Paediatric BMT, Wroclaw Medical University, Wroclaw, Poland; Peter Bader, Universitaetsklinikum Frankfurt Goethe-Universitaet, Frankfurt, Germany; Peter J. Shaw, The Children`s Hospital at Westmead, Sydney, Australia; Tayfun Güngör, University Children`s Hospital, Zurich, Switzerland; Tobias Gedde-Dahl, Oslo University Hospital, Rikshospitalet, Oslo, Norway; Wolfgang Holter, St. Anna Kinderspital, Vienna, Austria; and Yana Novis, Hospital Sirio-Libanes, São Paulo, Brazil.
Authorship
Contribution: S.H.L., M.H.A., M.A., F.L., A.L., A.R.G., B.N., and M.S. conceived the study and wrote the manuscript; T.S. managed the data; P.G. performed the statistical analysis; and all authors reviewed and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Su Han Lum, Newcastle University, Clinical Resource Building, Floor 4, Block 2 Great North Children’s Hospital, Queen Victoria Road, Newcastle Upon Tyne NE1 4LP, United Kingdom; email: nshl5@newcastle.ac.uk.
References
Author notes
S.H.L., M.H.A., B.N., and M.S. contributed equally to this study.
Original data are available on request from the corresponding author, Su Han Lum (nshl5@newcastle.ac.uk).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal