In this issue of Blood, Lum et al report that HLA-mismatched hematopoietic stem cell transplantation (HSCT) with ex vivo depletion of CD3+TCRαβ/CD19+ (TCRαβ) has higher overall survival (OS) compared with graft-versus-host disease (GVHD) prophylaxis using posttransplant cyclophosphamide (PTCY) for patients with inborn errors of immunity (IEI).1
T-cell depletion methods through the years have attempted to balance minimizing GVHD while promoting posttransplant immune reconstitution in the context of mismatched unrelated or haploidentical grafts. In the 1980s, T cells were removed from marrow utilizing soybean lectin agglutination/sheep red blood cell rosetting. This approach was restricted to specialty laboratories, and although lower rates of GVHD were noted, this came with high rates of graft failure and delayed immune reconstitution.2,3 In the 1990s, commercially available selection for CD34+ stem cells from peripheral blood was used to overcome issues with graft failure; however, this approach led to ongoing high transplant-related mortality (TRM) due to significantly delayed immune reconstitution.2,4 Moving forward in the 2000s, efforts focused on removing only CD3+ T cells and keeping other cells in the product to enhance immunity in the recipient. High rates of posttransplant lymphoproliferative disease with this approach led to the concept of simultaneously removing CD19 cells to prevent this, but the CD3+/CD19+ depletion approach still led to major delays in immune reconstitution and higher mortality.2
Over the last decade approaches to manipulate the graft have included removal of the TCRαβ+ T and CD19+ cells as well as alternatively removing naive CD45RA+ T cells.2,5 The goal of removing TCRαβ+ CD3+ cells is to prevent GVHD while allowing TCRγδ+ T cells to remain in the product and aid with immune reconstitution5 and potentially graft-versus-leukemia effect in patients who received transplants for hematological malignancies.2 This approach retains key cells in the graft including natural killer cells, dendritic and myeloid cells, and monocytes, enhancing engraftment and immune reconstitution.6,7 High CD34+ stem cell doses are included with this approach to improve engraftment. While these sophisticated approaches were being developed, PTCY began to revolutionize the field as a readily available option in low-resource settings with good outcomes.2,8 Cyclophosphamide given on days 3 and 4 posttransplant leads to in vivo depletion of alloreactive T cells, a high engraftment rate, and a reasonably fast immune reconstitution rate with acceptable levels of GVHD.2 Results of PTCY in pediatric malignancies have shown low TRM and severe acute GVHD and favorable survival rates,8 making it an attractive option.
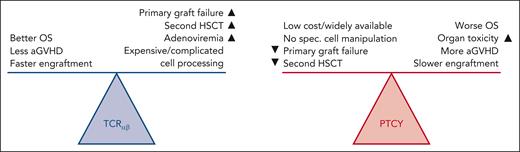
But which of these 2 approaches is better? Hints regarding the answer to that question are found in Lum et al, which is a retrospective, multicenter study, conducted through the Inborn Errors Working Party/European Society for Blood and Marrow Transplantation registry. They compared outcomes of these 2 GVHD prophylaxis strategies for HSCT in children with IEI. The study spans a decade (2010-2019) and includes a large cohort of 306 patients. In vivo or in vitro T-cell depletion is critical for patients lacking HLA-matched donors, a common challenge in HSCT for IEI. The primary outcome of the study was the 3-year OS rate, which was higher in the TCRαβ group (78%) compared with the PTCY group (66%; P = .013). In addition, patients in the PTCY group experienced higher rates of severe acute GvHD, veno-occlusive disease, acute kidney injury, and pulmonary complications. On the other hand, the TCRαβ group experienced higher rates of adenoviremia and primary graft failure or need for a second HSCT (although most rejected grafts were salvaged). These findings highlight the distinct profiles of risks and benefits associated with each T-cell depletion strategy. The study also demonstrated that TCRαβ allowed for faster neutrophil and platelet engraftment. Although PTCY was associated with a higher rate of acute GvHD, there was no difference in chronic GvHD rates compared with TCRαβ.1 It should be noted that although TCRαβ appeared to lead to better OS, a serious caveat was that patients who received PTCY had higher levels of comorbidities pre-HSCT (see figure).1 This concern was addressed by a subanalysis of patients with comorbidity scores of 0 to 1, which still favored TCRαβ (OS 88% vs 73%, P = .009). The largest limitation of the study was that TCRαβ and PTCY procedures were generally performed in different centers and countries, introducing the possibility of center practice and socioeconomic bias.
Advantages and disadvantages of TCRαβ and PTCY GVHD prophylaxis strategies in patients with IEI. Figure courtesy of Tim Luetkens, University of Maryland.
Advantages and disadvantages of TCRαβ and PTCY GVHD prophylaxis strategies in patients with IEI. Figure courtesy of Tim Luetkens, University of Maryland.
In the not-too-distant past, haploidential or significantly HLA-mismatched HSCT was typically offered to patients with IEI only at highly specialized centers, and outcomes were suboptimal. As T-cell-depletion technologies have evolved, data are coming from many centers, large and small, and outcomes are overall much better. Although the light at the end of the tunnel is still not clear (66%-78% survival is not good enough in nonmalignant HCT), it is at least starting to shine. Lum et al made an extraordinary effort to gather the data presented here, assembling the largest cohort of pediatric patients with IEI treated with these approaches to date. This study emphasizes the role of pre-HSCT morbidity scores, conditioning regimens, and active infections as significant predictors of survival,9,10 suggesting that optimizing these factors prior to transplantation (in some cased offering transplant earlier in a patient’s course) could lead to better patient outcomes.
Lum et al provide a clue, but not a definitive answer to the question of best GVHD prophylaxis for patients with IEI undergoing mismatched HSCT. Prospective and randomized comparisons of the approaches would be ideal, but such studies are expensive and challenging to design in rare diseases. But if outcomes for TCRαβ are better, we either need to try to make this approach more readily available or improve outcomes for PTCY. Newer approaches including decreased PTCY doses or using safer pharmacokinetics-optimized conditioning regimens may allow that. Ultimately, best outcomes with patients with IEI will only come as we treat patients with no or controlled infection who have not experienced significant organ damage using approaches that consistently establish engraftment with minimal GVHD and rapid immune reconstitution. We hope this study plants seeds that will aid in developing such approaches.
Conflict-of-interest disclosure: M.A.P. served on one-time advisory boards for Autolus, Pfizer, Cargo, Novartis, Gentibio, Bluebird, and Vertex; served on study steering committees for Novartis and Autolus; received research support from Miltenyi, and Adaptivene; and served on advisory boards for Autolus, Pfizer, Cargo, Novartis, Gentibio, Bluebird, and Vertex. E.M. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal