Although CD20×CD3 bispecific antibodies are effective against systemic B-cell lymphomas, their efficacy in central nervous system (CNS) lymphoma is unknown. Here, we report the CD20×CD3 bispecific glofitamab penetrates the blood-brain barrier, stimulates immune-cell infiltration of CNS tumors, and induces clinical responses in patients with secondary CNS.
TO THE EDITOR:
Remarkable progress has been made in the treatment of systemic B-cell lymphomas with the introduction and recent US Food and Drug Administration approval of CD20×CD3 bispecific antibodies (BsAbs). CD20 BsAbs function through their ability to activate and redirect T cells to the tumor,1-4 and are associated with high response rates of 50% to 80% in patients with relapsed/refractory B-cell lymphomas.5-9 Although CD20 BsAbs are effective in systemic B-cell lymphomas, it is unclear whether these large immunoglobulin G–based macromolecules (150-200 kDa) can cross the blood-brain barrier (BBB) or induce clinical responses in lymphomas involving the central nervous system (CNS). These are timely and relevant questions as the 5-year survival of patients with primary or secondary CNS lymphoma is only 30% to 40%,10,11 underscoring the importance of identifying improved treatment options for these patients. Here, we compiled the first reported case series of patients with CNS lymphoma treated with CD20 BsAb-based therapy to assess the ability of CD20 BsAbs to cross the BBB and induce clinical responses in patients.
The clinical characteristics of the 4 patients included in the case series are provided in Table 1. All 4 patients had biopsy-proven CD20+ diffuse large B-cell lymphoma with secondary CNS involvement. Patients were heavily pretreated with a median of 4 prior lines of therapy, and all patients relapsed after prior CD19-directed chimeric antigen receptor T-cell therapy. Two patients presented with lymphoma confined to the CNS at the time of CD20 BsAb treatment, and 2 patients had concomitant systemic and CNS disease. Sites of CNS involvement included the CNS parenchyma (n = 3) and leptomeninges (n = 1).
Clinical characteristics of patients with secondary CNS lymphoma
| Characteristic . | Patient 1 . | Patient 2 . | Patient 3 . | Patient 4 . |
|---|---|---|---|---|
| Age, y | 82 | 56 | 40 | 41 |
| Sex | M | F | F | F |
| No. of lines of prior therapy | 4 | 4 | 4 | 5 |
| Prior therapies | 1. R-miniCHOP 2. Lenalidomide 3. Focal RT 4. Lisocabtagene maraleucel | 1. R-CHOP 2. High-dose MTX 3. R-GDP 4. Axicabtagene ciloleucel | 1. R-HyperCVAD 2. WBRT 3. AVM0703 4. Lisocabtagene maraleucel | 1. R-CHOP 2. HyperCVAD 3. WBRT 4. R-GemOx 5. Lisocabtagene maraleucel |
| Sites of disease | CNS parenchyma | Bone, leptomeninges, and CSF | CNS parenchyma | CNS parenchyma and cranial nerves |
| Concomitant therapies | None | Obinutuzumab pretreatment and intrathecal MTX | Obinutuzumab pretreatment, focal RT, corticosteroids, and acalabrutinib | Focal RT, corticosteroids, and acalabrutinib |
| Timing of CSF collection | 9 d after C4D1 | 16 h after C2D1 | 18 d after C3D1 | 18 d after C2D1 |
| CRS or ICANS | Gr 2 CRS after C1D1 | Gr 1 CRS after C1D8 | No | No |
| No. of glofitamab cycles | ≥8 | ≥6 | ≥7 | 2 |
| Glofitamab response | PR | CR | PR | PD |
| CSF cellular composition | NA | Preglofitamab: 29 leukocytes/μL 12 lymphocytes/μL 42% lymphoma cells 3 d postglofitamab: 366 leukocytes/μL 48 lymphocytes/μL 3% lymphoma cells | NA | NA |
| Characteristic . | Patient 1 . | Patient 2 . | Patient 3 . | Patient 4 . |
|---|---|---|---|---|
| Age, y | 82 | 56 | 40 | 41 |
| Sex | M | F | F | F |
| No. of lines of prior therapy | 4 | 4 | 4 | 5 |
| Prior therapies | 1. R-miniCHOP 2. Lenalidomide 3. Focal RT 4. Lisocabtagene maraleucel | 1. R-CHOP 2. High-dose MTX 3. R-GDP 4. Axicabtagene ciloleucel | 1. R-HyperCVAD 2. WBRT 3. AVM0703 4. Lisocabtagene maraleucel | 1. R-CHOP 2. HyperCVAD 3. WBRT 4. R-GemOx 5. Lisocabtagene maraleucel |
| Sites of disease | CNS parenchyma | Bone, leptomeninges, and CSF | CNS parenchyma | CNS parenchyma and cranial nerves |
| Concomitant therapies | None | Obinutuzumab pretreatment and intrathecal MTX | Obinutuzumab pretreatment, focal RT, corticosteroids, and acalabrutinib | Focal RT, corticosteroids, and acalabrutinib |
| Timing of CSF collection | 9 d after C4D1 | 16 h after C2D1 | 18 d after C3D1 | 18 d after C2D1 |
| CRS or ICANS | Gr 2 CRS after C1D1 | Gr 1 CRS after C1D8 | No | No |
| No. of glofitamab cycles | ≥8 | ≥6 | ≥7 | 2 |
| Glofitamab response | PR | CR | PR | PD |
| CSF cellular composition | NA | Preglofitamab: 29 leukocytes/μL 12 lymphocytes/μL 42% lymphoma cells 3 d postglofitamab: 366 leukocytes/μL 48 lymphocytes/μL 3% lymphoma cells | NA | NA |
CSF, cerebrospinal fluid; CR, complete response; CRS, cytokine release syndrome; CxDx, cycle and day of glofitamab treatment; F, female; Gr, grade; ICANS, immune effector cell–associated neurotoxicity syndrome; M, male; MTX, methotrexate; NA, not applicable; PD, progressive disease; PR, partial response; R-CHOP, rituximab, cyclophosphamide, hydroxydaunorubicin, Oncovin (vincristine), and prednisone; R-GDP, rituximab, gemcitabine, dexamethasone, and cisplatin; R-GemOx, rituximab, gemcitabine, and oxaliplatin; R-HyperCVAD, rituximab, cyclophosphamide, vincristine, Adriamycin (doxorubicin), and dexamethasone; R-miniCHOP, rituximab, reduced dose cyclophosphamide, hydroxydaunorubicin, Oncovin (vincristine), and prednisone; RT, radiotherapy; WBRT, whole brain radiotherapy.
Patients were all treated with the CD20 BsAb glofitamab as standard of care after providing informed consent for treatment. Glofitamab treatment was administered using the standard step-up dosing regimen, and treatment was given off-trial despite the fact that no such patients with CNS lymphoma have been enrolled in glofitamab clinical studies.7 Two patients received concomitant acalabrutinib and focal brain radiotherapy. Two patients received obinutuzumab pretreatment in accordance with the glofitamab package insert, whereas obinutuzumab pretreatment was omitted in 2 patients because of rapidly progressing disease. The median number of glofitamab cycles was 6.5 (range, 2-8). Transient grade 1 to 2 cytokine release syndrome occurred in 2 patients, and no patient exhibited immune effector cell-associated neurotoxicity syndrome or other neurologic adverse effects from treatment.
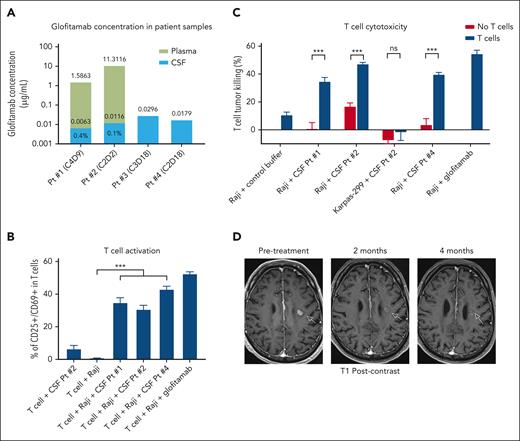
On-treatment cerebrospinal fluid (CSF) samples were collected from each patient to determine the extent to which glofitamab penetrated the BBB, as assessed by an enzyme-linked immunosorbent assay–based assay validated for human serum.8 Suitability of the assay for analysis of CSF samples was demonstrated. CSF samples were collected by lumbar puncture under direct fluoroscopy. Timing of CSF collections in relation to glofitamab dosing is provided in Table 1. Glofitamab was detected in the CSF of all 4 patients, with concentrations ranging from 0.00632 to 0.0296 μg/mL (Figure 1A). Two patients with synchronous plasma and CSF collections exhibited glofitamab CSF concentrations of 0.1% and 0.4% of that observed in the plasma.
Glofitamab penetrates the BBB and induces responses in patients with secondary CNS lymphoma. (A) Bar graph demonstrating the concentration of glofitamab in patient (Pt)–derived samples from the CSF and plasma. The relative proportion of glofitamab in the CSF compared with the plasma is listed as a percentage within the stacked bars for patients with synchronous CSF and plasma collections. (B) Induction of T-cell activation by the CSF of glofitamab-treated patient samples, as assessed by CD25 and CD69 upregulation. Peripheral blood T cells from a healthy donor were incubated for 24 hours with patient-derived CSF or buffer control solution in the presence of the CD20+ lymphoma cell line, Raji, at a 5:1 effector/target ratio. T cells incubated with glofitamab (0.1 μg/mL) and Raji served as a positive control. ∗∗∗P < .001. (C) T-cell cytotoxicity of CD20+ lymphoma cells induced by the CSF from glofitamab-treated patients. Peripheral blood T cells from a healthy donor were incubated for 24 hours with patient-derived CSF or buffer control solution in the presence of the CD20+ lymphoma cell line, Raji, at a 5:1 effector/target ratio. The extent of cytotoxicity was assessed by live/dead flow cytometric staining and was compared with conditions lacking T cells. Karpas-299, a CD20-negative lymphoma cell line, was used as a negative control. Raji incubated with T cells and glofitamab (0.1 μg/mL) served as a positive control. ∗∗∗P < .001. (D) Serial brain magnetic resonance imaging (MRI) scans from patient 1 with CNS lymphoma before (left panel), after 2 months (middle panel), and after 4 months (right panel) of glofitamab monotherapy. Imaging shows a near-complete response to treatment with a significant decrease in size and contrast enhancement within the CNS lesion (arrows) on T1-weighted postgadolinium contrast axial images. CxDx, cycle and day of glofitamab treatment; ns, not significant.
Glofitamab penetrates the BBB and induces responses in patients with secondary CNS lymphoma. (A) Bar graph demonstrating the concentration of glofitamab in patient (Pt)–derived samples from the CSF and plasma. The relative proportion of glofitamab in the CSF compared with the plasma is listed as a percentage within the stacked bars for patients with synchronous CSF and plasma collections. (B) Induction of T-cell activation by the CSF of glofitamab-treated patient samples, as assessed by CD25 and CD69 upregulation. Peripheral blood T cells from a healthy donor were incubated for 24 hours with patient-derived CSF or buffer control solution in the presence of the CD20+ lymphoma cell line, Raji, at a 5:1 effector/target ratio. T cells incubated with glofitamab (0.1 μg/mL) and Raji served as a positive control. ∗∗∗P < .001. (C) T-cell cytotoxicity of CD20+ lymphoma cells induced by the CSF from glofitamab-treated patients. Peripheral blood T cells from a healthy donor were incubated for 24 hours with patient-derived CSF or buffer control solution in the presence of the CD20+ lymphoma cell line, Raji, at a 5:1 effector/target ratio. The extent of cytotoxicity was assessed by live/dead flow cytometric staining and was compared with conditions lacking T cells. Karpas-299, a CD20-negative lymphoma cell line, was used as a negative control. Raji incubated with T cells and glofitamab (0.1 μg/mL) served as a positive control. ∗∗∗P < .001. (D) Serial brain magnetic resonance imaging (MRI) scans from patient 1 with CNS lymphoma before (left panel), after 2 months (middle panel), and after 4 months (right panel) of glofitamab monotherapy. Imaging shows a near-complete response to treatment with a significant decrease in size and contrast enhancement within the CNS lesion (arrows) on T1-weighted postgadolinium contrast axial images. CxDx, cycle and day of glofitamab treatment; ns, not significant.
We next assessed whether the concentration of glofitamab in patient-derived CSF samples was sufficient to induce T-cell activation and cytotoxicity against CD20+ lymphoma cells ex vivo. Healthy donor peripheral blood T cells and CD20+ lymphoma cell lines were incubated with patient-derived CSF samples at a 5:1 effector/target ratio. All 3 patient-derived CSF samples that were assessed induced significant T-cell activation, as determined by CD25 and CD69 upregulation, and significantly increased cytotoxicity of lymphoma cells compared with T cells incubated with lymphoma cells and control buffer (Figure 1B-C). No increase in T-cell–mediated cytotoxicity was observed when CSF samples were incubated with T cells and a CD20-negative lymphoma cell line (Figure 1C). These data indicate glofitamab achieved a sufficient concentration in the CSF to drive T-cell activation and cytotoxicity against CD20+ lymphoma cells ex vivo.
We collected CSF samples before and after glofitamab treatment in patient 2, who had lymphoma involving the CSF, which enabled a dynamic assessment of immune cell infiltration to the tumor site during therapy. In the week before glofitamab treatment, there were 29 leukocytes/μL of CSF. Remarkably, 3 days after the initial 2.5 mg glofitamab dose, the number of CSF leukocytes increased >12-fold to 366/μL, and there was a robust increase in the absolute number of lymphocytes from 12 to 48 cells/μL of CSF. The on-treatment CSF sample also demonstrated a substantial relative reduction in lymphoma cells compared with the pretreatment sample (3% vs 42% of all cells), which suggested glofitamab robustly redirected immune cells to the tumor site in this patient.
In terms of clinical efficacy, patient 1 received glofitamab monotherapy for isolated CNS parenchymal disease and demonstrated objective radiographic and clinical improvement with treatment. The patient had near resolution of his/her neurologic deficits (personality changes, memory loss, and postural imbalance) within days of the first glofitamab dose. Restaging brain magnetic resonance imaging (MRI) obtained after 2 and 4 months of treatment demonstrated ongoing reduction in the size of the primary left frontal lobe lesion from 16 to 9 mm with near complete resolution of contrast enhancement (Figure 1D). The patient remains on glofitamab beyond 8 cycles and continues to improve clinically. Patient 2 presented with systemic and leptomeningeal lymphoma (as described above), and was treated with glofitamab and obinutuzumab pretreatment. The patient achieved a complete metabolic response systemically and had complete clearance of lymphoma from the CSF that is ongoing beyond 6 cycles. Although this suggests clinical benefit from glofitamab, the patient also received 4 administrations of intrathecal chemotherapy following the first dose of glofitamab. Thus, we cannot entirely exclude the possibility that the intrathecal chemotherapy or the obinutuzumab pretreatment also contributed to the patient’s response. The robust increase of immune cells in the CSF shortly after glofitamab treatment in this patient, however, strongly supports the notion that glofitamab contributed significantly to the clearance of lymphoma from the CSF. Patient 3 was treated with acalabrutinib, radiotherapy, and glofitamab with obinutuzumab pretreatment. A brain MRI after 3 months revealed a decrease in the primary left temporal lobe lesion from 45 × 44 to 31 × 27 mm. This patient exhibited clinical improvement and remains on glofitamab beyond 7 cycles. However, because patient 3 received concomitant acalabrutinib, radiotherapy, and obinutuzumab, we are unable to definitively assess the contribution of glofitamab to the patient’s clinical response. Finally, patient 4 was refractory to treatment with glofitamab, acalabrutinib, and radiotherapy after presenting with bulky and rapidly progressing CNS disease.
On the basis of these clinical and correlative data, we conclude that glofitamab partially penetrates the BBB, and is capable of safely inducing clinical and radiographic responses in patients with secondary CNS lymphoma. As the average CSF concentration of glofitamab was only 0.1% to 0.4% of that in the peripheral blood, these data indicate only low levels of glofitamab are needed in the CSF to elicit responses in CNS lymphoma. This is consistent with results from the CD20 monoclonal antibody, rituximab, which has objective single-agent activity in CNS lymphoma despite only 0.1% of the drug crossing the BBB.12,13 Because preclinical data indicate CD20 BsAbs are orders of magnitude more potent than rituximab,2,3,14 CD20 BsAbs may require significantly lower CSF concentrations to induce responses in CNS lymphoma than rituximab, which may importantly translate into superior clinical activity. Although these preliminary results are encouraging, the safety and efficacy of CD20 BsAbs in SCNSL will need to be confirmed in dedicated prospective clinical trials before these treatments are routinely used in the clinic, as SCNSL patients are a vulnerable population at risk for relevant side effects from treatment including potentially serious neurologic toxicities. Additionally, as there is clearly an exposure-response relationship for CD20 BsAbs in systemic B-cell lymphomas,6 future clinical trials exploring CD20 BsAbs in CNS lymphoma should consider testing the safety and efficacy of higher doses of BsAbs or administering BsAbs intrathecally to increase CSF drug exposure and receptor occupancy, as has been done with rituximab.15-17
The sample collection/analysis study was approved by the City of Hope institutional review board. Human specimens were collected from patients registered at City of Hope National Medical Center (COHNMC) who had consented to an institutional review board (IRB)–approved protocol (IRB number 18067); healthy donor specimens were collected from participants who consented to COH IRB number 06229. Patient sample acquisition was approved by the IRB at the COHNMC, in accordance with an assurance filed with and approved by the Department of Health and Human Services and met all requirements of the Declaration of Helsinki.
Acknowledgments
The authors thank Olivier Kuster from Roche for performing the assays to determine the concentration of glofitamab in the patient samples, and the participants of their repository protocols for support of their research.
Research reported in this publication included work performed in the Hematopoietic Tissue Biorepository Core, supported by the National Institutes of Health, National Cancer Institute under grant P30CA033572.
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authorship
Contribution: J.K.G. designed the study, performed data collection, and wrote the manuscript; G.S., S.P., B.L., A.K., J.H.B., G.M., L.G., A.V.D., A.F.H., L.W.K., and L.E.B. reviewed and edited the manuscript; L.G. performed ex vivo functional studies and reviewed and edited the manuscript; J.Y.S. provided pathology review of cerebrospinal fluid specimens and reviewed and edited the manuscript; B.T.C. interpreted the magnetic resonance imaging findings and reviewed and edited the manuscript; and S.V. designed the assays to determine the concentration of glofitamab in the patient samples, evaluated the glofitamab concentration results, and reviewed and edited the manuscript.
Conflict-of-interest disclosure: J.K.G. receives research support from Merck, Janssen, and Secura Bio. L.E.B. receives research support from Merck, Amgen, Cellular Biomedicine Group, Inc, and AstraZeneca; and provides consultancy as part of advisory board for ADC Therapeutics, AstraZeneca, AbbVie, Nurix, Kite Pharma, Bristol Myers Squibb, Janssen, Regeneron, Genentech, and Roche. G.S. provides consultancy as part of an advisory board for AstraZeneca, BeiGene, and Kite Pharma; and serves on a speaker’s bureau for BeiGene and Kite Pharma. S.V. is an employee of Roche. The remaining authors declare no competing financial interests.
Correspondence: James K. Godfrey, Department of Hematology and Hematopoietic Cell Transplantation, City of Hope Comprehensive Cancer Center, 1500 E Duarte Rd, Duarte, CA 91010; email: jagodfrey@coh.org.
References
Author notes
Data are available on request from the corresponding author, James K. Godfrey (jagodfrey@coh.org).
The online version of this article contains a data supplement.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal