Key Points
Changes in IPSS-R score between diagnosis and transplantation did not affect transplant outcome when no prior therapy was applied.
Downstaging IPSS-R by chemotherapy improved transplant outcome, however stable or worse IPSS-R after therapy correlated with worst outcome.
Visual Abstract
In patients with myelodysplastic syndrome (MDS), higher revised International Prognostic Scoring System (IPSS-R) scores at transplant are associated with worse transplant outcome and, thus, lowering IPSS-R scores by therapeutic intervention before transplantation may seem beneficial. However, there is no evidence, to date, to support this approach. In a retrospective analysis, a total of 1482 patients with MDS with sufficient data to calculate IPSS-R score at diagnosis and at time of transplantation were selected from the European Society for Blood and Marrow Transplantation transplant registry and analyzed for transplant outcome in a multivariable Cox model including IPSS-R score at diagnosis, treatment intervention, change in IPSS-R score before transplant, and several patient and transplant variables. Transplant outcome was unaffected by IPSS-R score change in untreated patients and moderately superior in patients treated with chemotherapy with improved IPSS-R score at transplant. Improved IPSS-R score after hypomethylating agents (HMAs) or other therapies showed no beneficial effect. However, when IPSS-R score progressed after chemotherapy, HMAs, or other therapies, transplant outcome was worse than without any prior treatment. Similar results were found when reduction or increase in bone marrow (BM) blasts between diagnosis and transplantation was considered. The results show a limited benefit of IPSS-R score downstaging or reduction of BM blasts after chemotherapy and no benefit for HMAs or other treatments and thus question the role of prior therapy in patients with MDS scheduled for transplantation. The model-based survival estimates should help inform decision-making for both doctors and patients.
Introduction
The question whether a patient with myelodysplastic syndrome (MDS) who is scheduled for allogeneic hematopoietic cell transplantation (allo-HCT) should receive an induction therapy has been debated for a long time.1 Several analyses have found that the blast count before transplant is a significant risk factor for transplant outcome2,3 and many transplant-specific risk scores for MDS also include blasts (bone marrow [BM] or blood) at transplant as risk factor.4-6 On one hand, induction with either chemotherapy or hypomethylating agents (HMAs) could prevent progression to acute myeloid leukemia (AML) while waiting for the allo-HCT and reduce the disease burden; on the other hand, induction therapy could also cause selection pressure and thereby lead to the emergence of more resistant clones which are less affected by the subsequent conditioning, and thus potentially increase the risk of relapse after transplantation. In addition, prior therapy has potential toxicity and may increase pretransplant and posttransplant mortality.
In a situation in which a therapeutic intervention has a list of potential advantages and disadvantages, the optimal way to answer such a question would be a randomized prospective study. We currently have to rely on retrospective analyses while awaiting the result of the ongoing ACROBAT prospective trial (ClinicalTrials.gov identifier: NCT04184505) testing upfront allo-HCT vs azacytidine followed by allo-HCT in higher-risk MDS and <10% BM blasts. However, the study will not test upfront allo-HCT in patients with ≥10% BM blasts because these receive all induction therapy (azacytidine vs chemotherapy) before transplant.
A number of single center and national registry studies have tried to analyze the effect of induction therapy before allo-HCT.7-12 Overall, there has been no clear signal that induction confers a benefit in patients undergoing allo-HCT. However, the cohorts were rather small and heterogenous, including either only patients with MDS or patients with MDS and secondary AML (sAML). This resulted in only a very limited possibility to analyze confounding factors such as blast counts, genetic profiles or response to prior treatment, thereby precluding a broad generalization of such results. Current recommendations propose induction therapy for patients with MDS with >10% blasts and sAML, partly based on studies in the 1980s and 1990s showing that patients with an excess of blasts had an inferior outcome after upfront transplantation.1
MDS is a highly heterogeneous disease, and several prognostic classifications exist. In 2012 a revised International Prognostic Scoring System (IPSS-R) was published, including blast counts, genetic abnormalities, and cytopenias.13 More recently the molecular IPPS (IPSS-M) was introduced,14 adding molecular parameters to the IPSS-R. However, molecular data are not uniformly available for all patients, in particular in registry data. Thus the IPSS-R is still used to assess the prognosis of newly diagnosed MDS13 and, although originally not developed for this purpose, also during the disease course in untreated patients with MDS.15 IPSS-R score either at diagnosis or at the time of transplantation has been shown to have prognostic relevance for posttransplant survival.5,16,17 However, when discussing treatment options before allo-HCT, an unknown factor is the effect of a change in IPSS-R score between diagnosis and transplant. Using a large data set of patients with MDS who underwent allo-HCT, we investigated the effects of a change in IPSS-R score between diagnosis and transplant and the role of prior therapy on posttransplant outcomes.
Patients and methods
This retrospective registry-based study was performed on behalf of the Chronic Malignancies Working Party (CMWP) of the European Society for Blood and Marrow Transplantation (EBMT). The EBMT is a nonprofit, scientific society representing >600 transplant centers, primarily in Europe. The EBMT collects on a voluntary basis data on recipient and donor characteristics, treatment, and follow-up of patients undergoing blood and BM transplantation.
Patients with a diagnosis of MDS with available data on pretransplant treatment (none, induction chemotherapy such as 7+3, HMAs, or any other) who underwent a first allo-HCT in the period 2005 to 2018 were selected. Available data on IPSS-R score components at diagnosis and at time of allo-HCT (ie, cytogenetics, BM blast count, hemoglobin level, and platelet and white blood cell counts) was required.
The objective of this study was to evaluate IPSS-R score at diagnosis and allo-HCT as well as the type of pretransplant treatment, when given, for the prediction of survival outcomes after allo-HCT. Cytogenetics, BM blast count, hemoglobin, platelet count, and white blood cell count were categorized according to IPSS-R score. IPSS-R score was computed at diagnosis and before transplant. The change in IPSS-R score between diagnosis and transplant was coded as progressive (higher IPSS-R score at transplant), improving (lower IPSS-R score at transplant), or same.
Statistics
Overall survival (OS) and progression free survival (PFS) were estimated using the Kaplan-Meier product limit estimation method, and differences in subgroups were assessed by the log-rank test. Median follow-up was determined using the reverse Kaplan-Meier method.
Multivariable Cox proportional hazards regression was applied to investigate the simultaneous impact of multiple covariates on the outcomes OS, PFS, relapse, and nonrelapse mortality (NRM). Relapse and progression are used synonymously in this analysis. For relapse and NRM, cause-specific hazards were modeled. All models include the same covariate constellation: IPSS-R score at diagnosis (low, intermediate, high, and very high vs very low), IPSS-R score change (progressive or improving vs same), prior therapy, interval between diagnosis and transplant (years), age at transplant (decades), conditioning intensity (reduced vs standard), and donor (unrelated vs related). In addition, an interaction between treatment (chemotherapy, HMAs, or other vs untreated) and IPSS-R score change was included, allowing for the estimation of different hazard ratios (HRs) of IPSS-R score change (progressive or improving vs same) in each of the pretreatment groups.
The multivariable models were also used to provide 5-year predicted outcomes of OS and PFS, relapse, and NRM for reference patients. Predicted outcomes are calculated for each combination of IPSS-R score at diagnosis and the IPSS-R score change at transplant for reference patients aged 60 years (median age at transplant), who received transplantation 9 months after diagnosis (median time between diagnosis and transplant), receiving reduced intensity conditioning and a stem cell graft from a related donor.
Continuous variables are summarized by median and interquartile range, and categorical variables as percentages within the group of patients with available data. All survival estimates, predictions, and HRs are reported with corresponding 95% confidence intervals (CI) in parentheses. All P values were 2-sided, and P < .05 was considered significant. Statistical analyses were performed in R version 3.6.0 (R Development Core Team, Vienna, Austria), using packages “survival,” “prodlim,” “cmprsk,” and “riskRegression.”
The study was approved by the CMWP and conducted in accordance with the Declaration of Helsinki, using Good Clinical Practice guidelines. EBMT centers commit to obtain informed consent according to the local regulations applicable at the time of transplantation in order to report pseudonymized data to the EBMT.
Results
Effect of IPSS-R score at diagnosis and transplant
A total of 1482 patients with MDS had sufficient information to calculate IPSS-R score both at diagnosis and at transplant and were available for this analysis. The median follow-up after allo-HCT in the whole cohort was 48.1 months (95% CI, 42.7-53.3) and the median time from MDS diagnosis to allo-HSCT was 9 months (interquartile range, 5.7-17.2). Baseline characteristics are shown in Table 1. At MDS diagnosis, 81 patients had a very low risk IPSS-R score, 317 had a low, 468 an intermediate, 390 a high, and 226 patients had a very high-risk score. Between diagnosis and allo-HCT, the majority of patients showed an improved IPSS-R score (n = 659), whereas 429 had an unchanged score and 394 patient had worse risk category at transplant. The distribution according to IPSS-R score at diagnosis is shown in Table 2.
Baseline characteristics of total cohort and subgroups according to treatment before transplantation
| . | Group . | Total . | Untreated . | Chemotherapy . | HMAs . | Other . | P . |
|---|---|---|---|---|---|---|---|
| N (%) . | n (%) . | n (%) . | n (%) . | n (%) . | |||
| Total | 1482 (100) | 423 (28.5) | 534 (36.0) | 328 (22.1) | 197 (13.3) | ||
| Patient sex | Male | 897 (60.5) | 256 (60.5) | 326 (61.0) | 193 (58.8) | 122 (61.9) | .893 |
| Female | 585 (39.5) | 167 (39.5) | 208 (39.0) | 135 (41.2) | 75 (38.1) | ||
| IPSS-R at diagnosis | Very low | 81 (5.5) | 39 (9.2) | 21 (3.9) | 11 (3.4) | 10 (5.1) | <.001 |
| Low | 317 (21.4) | 123 (29.1) | 108 (20.2) | 37 (11.3) | 49 (24.9) | ||
| Intermediate | 468 (31.6) | 149 (35.2) | 152 (28.5) | 112 (34.1) | 55 (27.9) | ||
| High | 390 (26.3) | 90 (21.3) | 153 (28.7) | 101 (30.8) | 46 (23.4) | ||
| Very high | 226 (15.2) | 22 (5.2) | 100 (18.7) | 67 (20.4) | 37 (18.8) | ||
| Age at HCT (y) | Median (IQR) | 59 (50.8-64) | 55.7 (45.1-62.4) | 58.2 (50.6-63.5) | 61.7 (56.4-66.3) | 59.7 (54-64.3) | <.001 |
| Interval from diagnosis to HCT (mo) | Median (IQR) | 9 (5.7-17.2) | 7.9 (4.8-16) | 7.7 (5.1-15.5) | 9.6 (6.8-14.9) | 15 (8.5-30.9) | <.001 |
| Stem cell source | BM | 159 (10.7) | 52 (12.3) | 51 (9.6) | 32 (9.8) | 24 (12.2) | .001 |
| PB | 1253 (84.6) | 357 (84.4) | 452 (84.8) | 289 (88.1) | 155 (78.7) | ||
| BM + PB | 9 (0.6) | 5 (1.2) | 1 (0.2) | 2 (0.6) | 1 (0.5) | ||
| CB | 60 (4.1) | 9 (2.1) | 29 (5.4) | 5 (1.5) | 17 (8.6) | ||
| Donor | Related | 537 (36.2) | 159 (37.6) | 197 (36.9) | 114 (34.8) | 67 (34.0) | .761 |
| Unrelated | 945 (63.8) | 264 (62.4) | 337 (63.1) | 214 (65.2) | 130 (66.0) | ||
| TBI | No | 1222 (82.7) | 361 (85.7) | 414 (77.7) | 288 (88.3) | 159 (80.7) | <.001 |
| Yes | 255 (17.3) | 60 (14.3) | 119 (22.3) | 38 (11.7) | 38 (19.3) | ||
| Conditioning | Standard | 493 (33.4) | 154 (36.4) | 182 (34.3) | 90 (27.5) | 67 (34.2) | .071 |
| Reduced | 984 (66.6) | 269 (63.6) | 349 (65.7) | 237 (72.5) | 129 (65.8) |
| . | Group . | Total . | Untreated . | Chemotherapy . | HMAs . | Other . | P . |
|---|---|---|---|---|---|---|---|
| N (%) . | n (%) . | n (%) . | n (%) . | n (%) . | |||
| Total | 1482 (100) | 423 (28.5) | 534 (36.0) | 328 (22.1) | 197 (13.3) | ||
| Patient sex | Male | 897 (60.5) | 256 (60.5) | 326 (61.0) | 193 (58.8) | 122 (61.9) | .893 |
| Female | 585 (39.5) | 167 (39.5) | 208 (39.0) | 135 (41.2) | 75 (38.1) | ||
| IPSS-R at diagnosis | Very low | 81 (5.5) | 39 (9.2) | 21 (3.9) | 11 (3.4) | 10 (5.1) | <.001 |
| Low | 317 (21.4) | 123 (29.1) | 108 (20.2) | 37 (11.3) | 49 (24.9) | ||
| Intermediate | 468 (31.6) | 149 (35.2) | 152 (28.5) | 112 (34.1) | 55 (27.9) | ||
| High | 390 (26.3) | 90 (21.3) | 153 (28.7) | 101 (30.8) | 46 (23.4) | ||
| Very high | 226 (15.2) | 22 (5.2) | 100 (18.7) | 67 (20.4) | 37 (18.8) | ||
| Age at HCT (y) | Median (IQR) | 59 (50.8-64) | 55.7 (45.1-62.4) | 58.2 (50.6-63.5) | 61.7 (56.4-66.3) | 59.7 (54-64.3) | <.001 |
| Interval from diagnosis to HCT (mo) | Median (IQR) | 9 (5.7-17.2) | 7.9 (4.8-16) | 7.7 (5.1-15.5) | 9.6 (6.8-14.9) | 15 (8.5-30.9) | <.001 |
| Stem cell source | BM | 159 (10.7) | 52 (12.3) | 51 (9.6) | 32 (9.8) | 24 (12.2) | .001 |
| PB | 1253 (84.6) | 357 (84.4) | 452 (84.8) | 289 (88.1) | 155 (78.7) | ||
| BM + PB | 9 (0.6) | 5 (1.2) | 1 (0.2) | 2 (0.6) | 1 (0.5) | ||
| CB | 60 (4.1) | 9 (2.1) | 29 (5.4) | 5 (1.5) | 17 (8.6) | ||
| Donor | Related | 537 (36.2) | 159 (37.6) | 197 (36.9) | 114 (34.8) | 67 (34.0) | .761 |
| Unrelated | 945 (63.8) | 264 (62.4) | 337 (63.1) | 214 (65.2) | 130 (66.0) | ||
| TBI | No | 1222 (82.7) | 361 (85.7) | 414 (77.7) | 288 (88.3) | 159 (80.7) | <.001 |
| Yes | 255 (17.3) | 60 (14.3) | 119 (22.3) | 38 (11.7) | 38 (19.3) | ||
| Conditioning | Standard | 493 (33.4) | 154 (36.4) | 182 (34.3) | 90 (27.5) | 67 (34.2) | .071 |
| Reduced | 984 (66.6) | 269 (63.6) | 349 (65.7) | 237 (72.5) | 129 (65.8) |
Patient/transplant characteristics in the whole cohort and stratified by pretreatment. P values were obtained using the χ2 test for categorical variables, and the Kruskal-Wallis test for continuous data. Data were missing on stem cell source (n = 1), TBI (n = 5), and conditioning (n = 5).
CB, cord blood; IQR, interquartile range; PB, peripheral blood; TBI, total body irradiation.
IPSS-R score at diagnosis and before transplant
| Group . | Total . | IPSS-R score at transplant . | ||||
|---|---|---|---|---|---|---|
| Very low . | Low . | Intermediate . | High . | Very high . | ||
| N (%) . | n (%) . | n (%) . | n (%) . | n (%) . | n (%) . | |
| Total IPSS-R score at diagnosis | 1482 (100) | 268 (18.1) | 409 (27.6) | 363 (24.5) | 303 (20.4) | 139 (9.4) |
| Very low | 81 (5.5) | 20 (7.5) | 30 (7.3) | 18 (5.0) | 10 (3.3) | 3 (2.2) |
| Low | 317 (21.4) | 33 (12.3) | 126 (30.8) | 86 (23.7) | 57 (18.8) | 15 (10.8) |
| Intermediate | 468 (31.6) | 86 (32.1) | 115 (28.1) | 143 (39.4) | 99 (32.7) | 25 (18.0) |
| High | 390 (26.3) | 72 (26.9) | 95 (23.2) | 77 (21.2) | 95 (31.4) | 51 (36.7) |
| Very high | 226 (15.2) | 57 (21.3) | 43 (10.5) | 39 (10.7) | 42 (13.9) | 45 (32.4) |
| Group . | Total . | IPSS-R score at transplant . | ||||
|---|---|---|---|---|---|---|
| Very low . | Low . | Intermediate . | High . | Very high . | ||
| N (%) . | n (%) . | n (%) . | n (%) . | n (%) . | n (%) . | |
| Total IPSS-R score at diagnosis | 1482 (100) | 268 (18.1) | 409 (27.6) | 363 (24.5) | 303 (20.4) | 139 (9.4) |
| Very low | 81 (5.5) | 20 (7.5) | 30 (7.3) | 18 (5.0) | 10 (3.3) | 3 (2.2) |
| Low | 317 (21.4) | 33 (12.3) | 126 (30.8) | 86 (23.7) | 57 (18.8) | 15 (10.8) |
| Intermediate | 468 (31.6) | 86 (32.1) | 115 (28.1) | 143 (39.4) | 99 (32.7) | 25 (18.0) |
| High | 390 (26.3) | 72 (26.9) | 95 (23.2) | 77 (21.2) | 95 (31.4) | 51 (36.7) |
| Very high | 226 (15.2) | 57 (21.3) | 43 (10.5) | 39 (10.7) | 42 (13.9) | 45 (32.4) |
Percentages are of IPSS-R score at diagnosis groups, within IPSS-R score at transplant groups.
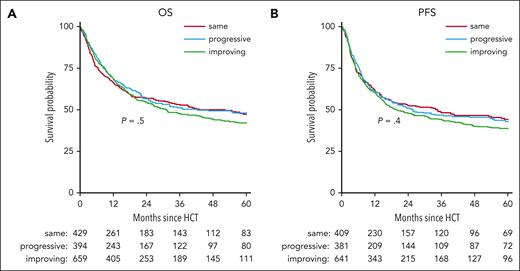
Although IPSS-R score, assessed either at diagnosis or at transplant, had a significant effect on OS and PFS after transplant in univariable analysis (supplemental Figure 1, available on the Blood website), a change in IPSS-R score analyzed in a univariable model did not show any effect on OS or PFS (Figure 1).
Kaplan-Meier curves of OS and PFS until 5 years after transplant, stratified by IPSS-R score change. (A) Estimated OS by 5 years is 47% (95% CI, 42-53), 48% (95% CI, 43-54), and 42% (95% CI, 38-47) in same, progressive, and improving, respectively. (B) Estimated 5-year PFS is 44% (95% CI, 39-50), 43% (95% CI, 37-49), and 39% (95% CI, 34-43), respectively. Corresponding log-rank P values are indicated in the plots.
Kaplan-Meier curves of OS and PFS until 5 years after transplant, stratified by IPSS-R score change. (A) Estimated OS by 5 years is 47% (95% CI, 42-53), 48% (95% CI, 43-54), and 42% (95% CI, 38-47) in same, progressive, and improving, respectively. (B) Estimated 5-year PFS is 44% (95% CI, 39-50), 43% (95% CI, 37-49), and 39% (95% CI, 34-43), respectively. Corresponding log-rank P values are indicated in the plots.
Because there were several imbalances regarding IPSS-R score at transplant and treatment between diagnosis and transplant as depicted in Table 1, a multivariable analysis for OS and PFS was performed (supplemental Table 1). However, there was a nonnegligible interaction between IPSS-R score change and treatment categories (none, chemotherapy, HMAs, and other) and this interaction was added to the model for all subsequent analyses.
Based on this refined model, predicted transplant outcomes at 5 years were calculated for reference patients, as defined above.
OS
In the multivariable analysis, OS was significantly affected by IPSS-R score at diagnosis, interval between diagnosis and transplant, and donor relationship (Table 3). There was a significant (P = .008) interaction between prior treatment and change in IPSS-R score between diagnosis and transplant. For patients receiving chemotherapy and showing an improved IPSS-R score, there was a significant benefit compared with those with an unchanged IPSS-R score (HR, 0.55; 95% CI, 0.41-0.76; P < .001). Conversely, a progressive IPSS-R score after HMAs (vs unchanged score) had a significant negative effect (HR, 1.98; 95% CI, 1.27-3.07; P = .002).
Multivariable analysis for OS
| Risk factor . | Group . | N . | D . | HR (95% CI) . | P . |
|---|---|---|---|---|---|
| Pretreatment × IPSS-R score change | |||||
| Chemotherapy | Same | 120 | 63 | ||
| Improving | 317 | 142 | 0.55 (0.41-0.76) | <.001 | |
| Progressive | 94 | 51 | 1.11 (0.76-1.61) | .6 | |
| HMAs | Same | 91 | 44 | ||
| Improving | 175 | 93 | 1.00 (0.69-1.43) | >.99 | |
| Progressive | 61 | 38 | 1.98 (1.27-3.07) | .002 | |
| Other | Same | 47 | 28 | ||
| Improving | 96 | 56 | 0.77 (0.48-1.21) | .3 | |
| Progressive | 53 | 33 | 1.45 (0.87-2.42) | .16 | |
| Untreated | Same | 170 | 70 | ||
| Improving | 67 | 35 | 1.05 (0.70-1.58) | .8 | |
| Progressive | 186 | 70 | 0.95 (0.68-1.34) | .8 | |
| Adjustment factors | |||||
| IPSS-R score at diagnosis | Very low | 81 | 27 | ||
| Low | 317 | 127 | 1.31 (0.86-2.00) | .2 | |
| Intermediate | 468 | 216 | 1.94 (1.27-2.96) | .002 | |
| High | 389 | 208 | 2.81 (1.81-4.35) | <.001 | |
| Very high | 222 | 145 | 4.12 (2.60-6.54) | <.001 | |
| Interval from diagnosis to HCT | 1477 | 723 | 1.05 (1.01-1.09) | .02 | |
| Age at HCT (dec) | 1477 | 723 | 1.12 (1.04-1.21) | .004 | |
| Conditioning | Standard | 493 | 223 | ||
| Reduced | 984 | 500 | 1.06 (0.9-1.26) | .5 | |
| Donor | Related | 533 | 245 | ||
| Unrelated | 944 | 478 | 1.19 (1.02-1.39) | .03 |
| Risk factor . | Group . | N . | D . | HR (95% CI) . | P . |
|---|---|---|---|---|---|
| Pretreatment × IPSS-R score change | |||||
| Chemotherapy | Same | 120 | 63 | ||
| Improving | 317 | 142 | 0.55 (0.41-0.76) | <.001 | |
| Progressive | 94 | 51 | 1.11 (0.76-1.61) | .6 | |
| HMAs | Same | 91 | 44 | ||
| Improving | 175 | 93 | 1.00 (0.69-1.43) | >.99 | |
| Progressive | 61 | 38 | 1.98 (1.27-3.07) | .002 | |
| Other | Same | 47 | 28 | ||
| Improving | 96 | 56 | 0.77 (0.48-1.21) | .3 | |
| Progressive | 53 | 33 | 1.45 (0.87-2.42) | .16 | |
| Untreated | Same | 170 | 70 | ||
| Improving | 67 | 35 | 1.05 (0.70-1.58) | .8 | |
| Progressive | 186 | 70 | 0.95 (0.68-1.34) | .8 | |
| Adjustment factors | |||||
| IPSS-R score at diagnosis | Very low | 81 | 27 | ||
| Low | 317 | 127 | 1.31 (0.86-2.00) | .2 | |
| Intermediate | 468 | 216 | 1.94 (1.27-2.96) | .002 | |
| High | 389 | 208 | 2.81 (1.81-4.35) | <.001 | |
| Very high | 222 | 145 | 4.12 (2.60-6.54) | <.001 | |
| Interval from diagnosis to HCT | 1477 | 723 | 1.05 (1.01-1.09) | .02 | |
| Age at HCT (dec) | 1477 | 723 | 1.12 (1.04-1.21) | .004 | |
| Conditioning | Standard | 493 | 223 | ||
| Reduced | 984 | 500 | 1.06 (0.9-1.26) | .5 | |
| Donor | Related | 533 | 245 | ||
| Unrelated | 944 | 478 | 1.19 (1.02-1.39) | .03 |
Multivariable Cox proportional hazards models for OS, with interactions between pretreatment and change in IPSS-R score. The total number of patients in each group and the number of events are reported in the N and D columns, respectively. The interval from diagnosis to HCT is in years, and age at HCT is in decades. Effect estimates are given with 95% CIs. Corresponding P values are calculated using the Wald test. All interaction effects between pretreatment and IPSS-R score change include both corresponding main effects.
dec, decades.
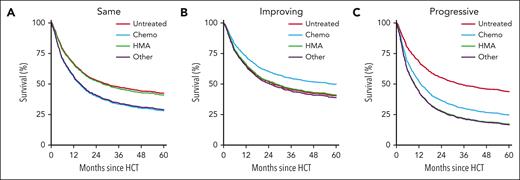
For reference patients with high-risk IPSS-R scores at diagnosis and no change until transplant, the predicted OS at 5 years was 42% (95% CI, 33-54) without prior therapy, 28% (95% CI, 19-42) after chemotherapy, 41% (95% CI, 30-56) after HMAs, and 29% (95% CI, 17-49) with other treatments (Figure 2; Table 4). When IPSS-R score improved between diagnosis and transplant, 5-year OS was estimated at 40% (95% CI, 29-56) without prior therapy, 50% (95% CI, 42-58) with chemotherapy, 41% (95% CI, 32-52) with HMAs, and 39% (95% CI, 29-52) with other treatments. In case of a worsening IPSS-R score, OS was predicted to be 44% (95% CI, 34-56) with no prior therapy, 25% (95% CI, 15-41) after chemotherapy, 17% (95% CI, 9-33) after HMAs, and 17% (95% CI, 8-35) with other therapies. The predictions for other IPSS-R risk categories at diagnosis are shown in Table 4.
Model-based figures of predicted OS until 5 years after transplant with high-risk IPSS-R score at diagnosis. Figures are based on the multivariable Cox model including the interaction between prior treatment and change in IPSS-R score (Table 3). Figures are shown for no change in IPSS-R score between diagnosis and transplant (A), improving IPSS-R score (B), and progressive IPSS-R score (C). Each of the curves refer to reference patients with high-risk IPSS-R score at diagnosis, aged 60 years, receiving a graft from a related donor after reduced intensity conditioning, and receiving a transplant 9 months after diagnosis. Chemo, chemotherapy.
Model-based figures of predicted OS until 5 years after transplant with high-risk IPSS-R score at diagnosis. Figures are based on the multivariable Cox model including the interaction between prior treatment and change in IPSS-R score (Table 3). Figures are shown for no change in IPSS-R score between diagnosis and transplant (A), improving IPSS-R score (B), and progressive IPSS-R score (C). Each of the curves refer to reference patients with high-risk IPSS-R score at diagnosis, aged 60 years, receiving a graft from a related donor after reduced intensity conditioning, and receiving a transplant 9 months after diagnosis. Chemo, chemotherapy.
Model-based estimates for 5-year OS for reference patients according to IPSS-R score at diagnosis, prior therapy and change in IPSS-R score
Pretreatment . | IPSS-R score change . | IPSS-R score at diagnosis . | ||||
|---|---|---|---|---|---|---|
| Very low . | Low . | Intermediate . | High . | Very high . | ||
| Untreated | Same | 74 (63-85) | 67 (59-76) | 55 (47-65) | 42 (33-54) | 28 (19-42) |
| Improving | 65 (55-78) | 54 (43-67) | 40 (29-56) | 26 (16-44) | ||
| Progressive | 75 (65-85) | 68 (60-77) | 57 (48-67) | 44 (34-56) | ||
| Chemotherapy | Same | 64 (51-80) | 55 (46-67) | 42 (32-55) | 28 (19-42) | 16 (8-29) |
| Improving | 72 (65-80) | 62 (55-69) | 50 (42-58) | 36 (28-46) | ||
| Progressive | 61 (48-78) | 52 (42-65) | 38 (28-52) | 25 (15-41) | ||
| HMAs | Same | 73 (62-86) | 66 (56-77) | 54 (44-66) | 41 (30-56) | 27 (17-43) |
| Improving | 66 (57-76) | 54 (46-64) | 41 (32-52) | 27 (19-38) | ||
| Progressive | 53 (39-74) | 44 (32-60) | 30 (19-46) | 17 (9-33) | ||
| Other | Same | 64 (50-83) | 56 (43-73) | 43 (30-60) | 29 (17-49) | 16 (8-35) |
| Improving | 64 (55-76) | 52 (42-64) | 39 (29-52) | 25 (16-39) | ||
| Progressive | 53 (38-74) | 43 (31-61) | 29 (18-48) | 17 (8-35) | ||
Pretreatment . | IPSS-R score change . | IPSS-R score at diagnosis . | ||||
|---|---|---|---|---|---|---|
| Very low . | Low . | Intermediate . | High . | Very high . | ||
| Untreated | Same | 74 (63-85) | 67 (59-76) | 55 (47-65) | 42 (33-54) | 28 (19-42) |
| Improving | 65 (55-78) | 54 (43-67) | 40 (29-56) | 26 (16-44) | ||
| Progressive | 75 (65-85) | 68 (60-77) | 57 (48-67) | 44 (34-56) | ||
| Chemotherapy | Same | 64 (51-80) | 55 (46-67) | 42 (32-55) | 28 (19-42) | 16 (8-29) |
| Improving | 72 (65-80) | 62 (55-69) | 50 (42-58) | 36 (28-46) | ||
| Progressive | 61 (48-78) | 52 (42-65) | 38 (28-52) | 25 (15-41) | ||
| HMAs | Same | 73 (62-86) | 66 (56-77) | 54 (44-66) | 41 (30-56) | 27 (17-43) |
| Improving | 66 (57-76) | 54 (46-64) | 41 (32-52) | 27 (19-38) | ||
| Progressive | 53 (39-74) | 44 (32-60) | 30 (19-46) | 17 (9-33) | ||
| Other | Same | 64 (50-83) | 56 (43-73) | 43 (30-60) | 29 (17-49) | 16 (8-35) |
| Improving | 64 (55-76) | 52 (42-64) | 39 (29-52) | 25 (16-39) | ||
| Progressive | 53 (38-74) | 43 (31-61) | 29 (18-48) | 17 (8-35) | ||
Predicted 5-year OS, expressed in percentages (with 95% CIs), based on the multivariable Cox model including the interaction between pretreatment and change in IPSS-R score.Estimates are given for each IPSS-R categories at diagnosis for reference patients with age 60 years, receiving a graft from a related donor after reduced intensity conditioning, and receiving transplantation 9 months after diagnosis. IPSS-R score change was coded as improving when IPSS-R risk was at least 1 category lower at transplant than at diagnosis, or as progressive when IPSS-R score at transplant was at least 1 category higher.
PFS
In the multivariable model, IPSS-R score at diagnosis significantly affected PFS. There was a significant interaction between treatment and change in IPSS-R score (P = .007). PFS was significantly better in patients with improved IPSS-R score (vs unchanged score) after chemotherapy (HR, 0.59; 95% CI, 0.43-0.8; P < .001) and worse with a progressive IPSS-R score after HMAs (HR, 2.12; 95% CI, 1.38-3.25; P < .001) or other therapies (HR, 1.82; 95% CI, 1.07-3.07; P = .03) compared with no change in IPSS-R score between diagnosis and transplant (Table 5).
Multivariable analysis for PFS
| Risk factor . | Group . | N . | D . | HR (95% CI) . | P . |
|---|---|---|---|---|---|
| Pretreatment × IPSS-R score change | |||||
| Chemotherapy | Same | 116 | 65 | ||
| Improving | 307 | 150 | 0.59 (0.43-0.80) | <.001 | |
| Progressive | 90 | 50 | 1.05 (0.72-1.52) | .8 | |
| HMAs | Same | 86 | 44 | ||
| Improving | 168 | 101 | 1.08 (0.76-1.55) | .7 | |
| Progressive | 59 | 41 | 2.12 (1.38-3.25) | <.001 | |
| Other | Same | 42 | 60 | ||
| Improving | 95 | 37 | 0.94 (0.58-1.52) | .8 | |
| Progressive | 53 | 24 | 1.82 (1.07-3.07) | .03 | |
| Untreated | Same | 165 | 75 | ||
| Improving | 67 | 37 | 1.02 (0.69-1.52) | .9 | |
| Progressive | 179 | 77 | 0.99 (0.72-1.37) | >.99 | |
| Adjustment factors | |||||
| IPSS-R score at diagnosis | Very low | 78 | 29 | ||
| Low | 309 | 143 | 1.32 (0.88-1.98) | .17 | |
| Intermediate | 453 | 226 | 1.75 (1.16-2.63) | .007 | |
| High | 373 | 215 | 2.46 (1.61-3.75) | <.001 | |
| Very high | 214 | 148 | 3.73 (2.39-5.82) | <.001 | |
| Interval from diagnosis to HCT | 1427 | 761 | 1.03 (0.99-1.08) | .1 | |
| Age at HCT (dec) | 1427 | 761 | 1.07 (1-1.16) | .06 | |
| Conditioning | Standard | 482 | 239 | ||
| Reduced | 945 | 522 | 1.11 (0.94-1.31) | .2 | |
| Donor | Related | 515 | 260 | ||
| Unrelated | 912 | 501 | 1.13 (0.97-1.31) | .12 |
| Risk factor . | Group . | N . | D . | HR (95% CI) . | P . |
|---|---|---|---|---|---|
| Pretreatment × IPSS-R score change | |||||
| Chemotherapy | Same | 116 | 65 | ||
| Improving | 307 | 150 | 0.59 (0.43-0.80) | <.001 | |
| Progressive | 90 | 50 | 1.05 (0.72-1.52) | .8 | |
| HMAs | Same | 86 | 44 | ||
| Improving | 168 | 101 | 1.08 (0.76-1.55) | .7 | |
| Progressive | 59 | 41 | 2.12 (1.38-3.25) | <.001 | |
| Other | Same | 42 | 60 | ||
| Improving | 95 | 37 | 0.94 (0.58-1.52) | .8 | |
| Progressive | 53 | 24 | 1.82 (1.07-3.07) | .03 | |
| Untreated | Same | 165 | 75 | ||
| Improving | 67 | 37 | 1.02 (0.69-1.52) | .9 | |
| Progressive | 179 | 77 | 0.99 (0.72-1.37) | >.99 | |
| Adjustment factors | |||||
| IPSS-R score at diagnosis | Very low | 78 | 29 | ||
| Low | 309 | 143 | 1.32 (0.88-1.98) | .17 | |
| Intermediate | 453 | 226 | 1.75 (1.16-2.63) | .007 | |
| High | 373 | 215 | 2.46 (1.61-3.75) | <.001 | |
| Very high | 214 | 148 | 3.73 (2.39-5.82) | <.001 | |
| Interval from diagnosis to HCT | 1427 | 761 | 1.03 (0.99-1.08) | .1 | |
| Age at HCT (dec) | 1427 | 761 | 1.07 (1-1.16) | .06 | |
| Conditioning | Standard | 482 | 239 | ||
| Reduced | 945 | 522 | 1.11 (0.94-1.31) | .2 | |
| Donor | Related | 515 | 260 | ||
| Unrelated | 912 | 501 | 1.13 (0.97-1.31) | .12 |
Multivariable Cox proportional hazards models for PFS, with interactions between pretreatment and change in IPSS-R score. The total number of patients in each group and the number of events are reported in the N and D columns, respectively. The interval from diagnosis to HCT is in years, and age at HCT is in decades. Effect estimates are given with 95% CIs. Corresponding P values are calculated using the Wald test. All interaction effects between pretreatment and IPSS-R score change include both corresponding main effects.
dec, decades.
For reference patients with high-risk IPSS-R score at diagnosis and no change until transplantation, the predicted PFS at 5 years was 40% (95% CI, 31-51) without prior treatment, 25% (95% CI, 16-39) after chemotherapy, 37% (95% CI, 26-52) after HMAs, and 31% (95% CI, 18-52) after other therapies (Figure 3; supplemental Table 2). In case the IPSS-R score improved between diagnosis and transplant, 5-year PFS was 39% (95% CI, 28-54) without prior therapy, 45% (95% CI, 37-54) with chemotherapy, 34% (95% CI, 26-45) with HMAs, and 33% (95% CI, 24-47) with other treatment. Conversely, for a worsened IPSS-R score the predicted 5-year PFS was 40% (95% CI, 30-52) without prior treatment, 24% (95% CI, 14-39) after chemotherapy, 12% (95% CI, 6-26) after HMAs, and 12% (95% CI, 5-28) with other therapies. Model-based prediction for other IPSS-R categories at diagnosis are shown in supplemental Table 2.
Model-based figures of predicted PFS until 5 years after transplant with high-risk IPSS-R score at diagnosis. Figures are based on the multivariable Cox model for PFS including the interaction between prior treatment and change in IPSS-R score (Table 5). Figures are shown for no change in IPSS-R between diagnosis and transplant (A), improving IPSS-R score (B), and progressive IPSS-R score (C). Each of the curves refer to reference patients with high-risk IPSS-R score at diagnosis, aged 60 years, receiving a graft from a related donor after reduced intensity conditioning, and receiving a transplant 9 months after diagnosis. Chemo, chemotherapy; RIC, reduced intensity conditioning.
Model-based figures of predicted PFS until 5 years after transplant with high-risk IPSS-R score at diagnosis. Figures are based on the multivariable Cox model for PFS including the interaction between prior treatment and change in IPSS-R score (Table 5). Figures are shown for no change in IPSS-R between diagnosis and transplant (A), improving IPSS-R score (B), and progressive IPSS-R score (C). Each of the curves refer to reference patients with high-risk IPSS-R score at diagnosis, aged 60 years, receiving a graft from a related donor after reduced intensity conditioning, and receiving a transplant 9 months after diagnosis. Chemo, chemotherapy; RIC, reduced intensity conditioning.
Cumulative RI
In the multivariable model, high and very-high risk IPSS-R score at diagnosis and reduced intensity conditioning were significant risk factors for increased relapse risk. There was no significant overall interaction between prior treatment and change in IPSS-R score, but a progressive IPSS-R score after treatment significantly increased incidence of relapse (RI) for HMAs (HR, 1.90; 95% CI, 1.02-3.47; P = .04) or other therapies (HR, 2.33; 95% CI, 1.09-4.98; P = .03; Table 6). The predicted RI at 5 years for the reference patient with high-risk IPSS-R score at diagnosis with unchanged IPSS-R score was 20% (95% CI, 12-29) without prior treatment, 47% (95% CI, 32-61) after chemotherapy, 45% (95% CI, 30-58) after HMAs, and 42% (95% CI, 23-60) after other treatments. With an improvement of IPSS-R score between diagnosis and allo-HCT, RI at 5 years was estimated to be 11% (95% CI, 4-22) without prior treatment, 39% (95% CI, 31-47) with chemotherapy, 50% (95% CI, 39-60) with HMAs, and 43% (95% CI, 30-56) with other treatments. A worsened IPSS-R score led to a RI of 34% (95% CI, 23-45) with no prior therapy, 52% (95% CI, 33-68) with chemotherapy, 56% (95% CI, 37-71) with HMAs, and 64% (95% CI, 42-79) with other treatments. Estimates for other IPSS categories at diagnosis are depicted in supplemental Table 3.
Multivariable analysis for RI
| Risk factor . | Group . | N . | D . | HR (95% CI) . | P . |
|---|---|---|---|---|---|
| Pretreatment × IPSS-R score change | |||||
| Chemotherapy | Same | 116 | 31 | ||
| Improving | 307 | 94 | 0.66 (0.43-1.01) | .05 | |
| Progressive | 90 | 24 | 1.16 (0.67-1.99) | .6 | |
| HMAs | Same | 86 | 25 | ||
| Improving | 168 | 65 | 1.17 (0.73-1.86) | .5 | |
| Progressive | 59 | 19 | 1.90 (1.03-3.47) | .04 | |
| Other | Same | 42 | 11 | ||
| Improving | 95 | 33 | 1.00 (0.50-2.00) | >.99 | |
| Progressive | 53 | 19 | 2.33 (1.09-4.98) | .03 | |
| Untreated | Same | 165 | 20 | ||
| Improving | 67 | 6 | 0.58 (0.23- 1.45) | .2 | |
| Progressive | 179 | 32 | 1.75 (0.99- 3.07) | .05 | |
| Adjustment factors | |||||
| IPSS-R score at diagnosis | Very low | 78 | 14 | ||
| Low | 309 | 56 | 1.06 (0.59-1.92) | .8 | |
| Intermediate | 453 | 105 | 1.62 (0.90-2.93) | .11 | |
| High | 373 | 108 | 2.46 (1.34-4.54) | .004 | |
| Very high | 214 | 96 | 4.51 (2.39-8.51) | <.001 | |
| Interval from diagnosis to HCT | 1427 | 379 | 1.02 (0.96-1.08) | .5 | |
| Age at HCT (dec) | 1427 | 379 | 0.97 (0.87-1.08) | .5 | |
| Conditioning | Standard | 482 | 113 | ||
| Reduced | 945 | 266 | 1.36 (1.07-1.74) | .01 | |
| Donor | Related | 515 | 151 | ||
| Unrelated | 912 | 228 | 0.84 (0.69-1.04) | .11 |
| Risk factor . | Group . | N . | D . | HR (95% CI) . | P . |
|---|---|---|---|---|---|
| Pretreatment × IPSS-R score change | |||||
| Chemotherapy | Same | 116 | 31 | ||
| Improving | 307 | 94 | 0.66 (0.43-1.01) | .05 | |
| Progressive | 90 | 24 | 1.16 (0.67-1.99) | .6 | |
| HMAs | Same | 86 | 25 | ||
| Improving | 168 | 65 | 1.17 (0.73-1.86) | .5 | |
| Progressive | 59 | 19 | 1.90 (1.03-3.47) | .04 | |
| Other | Same | 42 | 11 | ||
| Improving | 95 | 33 | 1.00 (0.50-2.00) | >.99 | |
| Progressive | 53 | 19 | 2.33 (1.09-4.98) | .03 | |
| Untreated | Same | 165 | 20 | ||
| Improving | 67 | 6 | 0.58 (0.23- 1.45) | .2 | |
| Progressive | 179 | 32 | 1.75 (0.99- 3.07) | .05 | |
| Adjustment factors | |||||
| IPSS-R score at diagnosis | Very low | 78 | 14 | ||
| Low | 309 | 56 | 1.06 (0.59-1.92) | .8 | |
| Intermediate | 453 | 105 | 1.62 (0.90-2.93) | .11 | |
| High | 373 | 108 | 2.46 (1.34-4.54) | .004 | |
| Very high | 214 | 96 | 4.51 (2.39-8.51) | <.001 | |
| Interval from diagnosis to HCT | 1427 | 379 | 1.02 (0.96-1.08) | .5 | |
| Age at HCT (dec) | 1427 | 379 | 0.97 (0.87-1.08) | .5 | |
| Conditioning | Standard | 482 | 113 | ||
| Reduced | 945 | 266 | 1.36 (1.07-1.74) | .01 | |
| Donor | Related | 515 | 151 | ||
| Unrelated | 912 | 228 | 0.84 (0.69-1.04) | .11 |
Multivariable Cox cause-specific hazards models for relapse incidence, with interactions between pretreatment and change in IPSS-R score. The total number of patients in each group and the number of events are reported in the N and D columns, respectively. The interval from diagnosis to HCT is in years, and age at HCT is in decades. Effect estimates are given with 95% CIs. Corresponding P values are calculated using the Wald test. All interaction effects between pretreatment and IPSS-R score change include both corresponding main effects.
dec, decades.
NRM
In multivariable analysis, NRM was significantly affected by IPSS-R score at diagnosis, age, and use of an unrelated donor. There was a significant (P < .001) interaction for prior therapy and IPSS-R score at diagnosis, and NRM was significantly reduced with an improved IPSS-R score after chemotherapy (HR, 0.49; 95% CI, 0.32-0.77; P = .002) whereas NRM increased with a worsened IPSS-R score after HMAs (HR, 2.41; 95% CI, 1.30-4.48; P = .005; Table 7).
Multivariable analysis for NRM
| Risk factor . | Group . | N . | D . | HR (95% CI) . | P . |
|---|---|---|---|---|---|
| Pretreatment × IPSS-R score change | |||||
| Chemotherapy | Same | 116 | 34 | ||
| Improving | 307 | 56 | 0.49 (0.32-0.77) | .002 | |
| Progressive | 90 | 26 | 0.98 (0.58-1.64) | .9 | |
| HMAs | Same | 86 | 19 | ||
| Improving | 168 | 36 | 0.96 (0.55-1.69) | .9 | |
| Progressive | 59 | 22 | 2.41 (1.30-4.48) | .005 | |
| Other | Same | 42 | 13 | ||
| Improving | 95 | 27 | 0.85 (0.44-1.65) | .6 | |
| Progressive | 53 | 18 | 1.43 (0.69-2.96) | .3 | |
| Untreated | Same | 165 | 55 | ||
| Improving | 67 | 31 | 1.22 (0.78-1.90) | .4 | |
| Progressive | 179 | 45 | 0.71 (0.48-1.07) | .1 | |
| Adjustment factors | |||||
| IPSS-R score at diagnosis | Very low | 78 | 15 | ||
| Low | 309 | 87 | 1.57 (0.90-2.73) | .11 | |
| Intermediate | 453 | 121 | 1.85 (1.05-3.26) | .03 | |
| High | 373 | 107 | 2.47 (1.38-4.42) | .002 | |
| Very high | 214 | 52 | 2.87 (1.53-5.41) | .001 | |
| Interval from diagnosis to HCT | 1427 | 382 | 1.05 (0.99-1.1) | .1 | |
| Age at HCT (dec) | 1427 | 382 | 1.18 (1.06-1.32) | .002 | |
| Conditioning | Standard | 482 | 126 | ||
| Reduced | 945 | 256 | 0.94 (0.74-1.18) | .6 | |
| Donor | Related | 515 | 109 | ||
| Unrelated | 912 | 273 | 1.51 (1.21-1.89) | <.001 |
| Risk factor . | Group . | N . | D . | HR (95% CI) . | P . |
|---|---|---|---|---|---|
| Pretreatment × IPSS-R score change | |||||
| Chemotherapy | Same | 116 | 34 | ||
| Improving | 307 | 56 | 0.49 (0.32-0.77) | .002 | |
| Progressive | 90 | 26 | 0.98 (0.58-1.64) | .9 | |
| HMAs | Same | 86 | 19 | ||
| Improving | 168 | 36 | 0.96 (0.55-1.69) | .9 | |
| Progressive | 59 | 22 | 2.41 (1.30-4.48) | .005 | |
| Other | Same | 42 | 13 | ||
| Improving | 95 | 27 | 0.85 (0.44-1.65) | .6 | |
| Progressive | 53 | 18 | 1.43 (0.69-2.96) | .3 | |
| Untreated | Same | 165 | 55 | ||
| Improving | 67 | 31 | 1.22 (0.78-1.90) | .4 | |
| Progressive | 179 | 45 | 0.71 (0.48-1.07) | .1 | |
| Adjustment factors | |||||
| IPSS-R score at diagnosis | Very low | 78 | 15 | ||
| Low | 309 | 87 | 1.57 (0.90-2.73) | .11 | |
| Intermediate | 453 | 121 | 1.85 (1.05-3.26) | .03 | |
| High | 373 | 107 | 2.47 (1.38-4.42) | .002 | |
| Very high | 214 | 52 | 2.87 (1.53-5.41) | .001 | |
| Interval from diagnosis to HCT | 1427 | 382 | 1.05 (0.99-1.1) | .1 | |
| Age at HCT (dec) | 1427 | 382 | 1.18 (1.06-1.32) | .002 | |
| Conditioning | Standard | 482 | 126 | ||
| Reduced | 945 | 256 | 0.94 (0.74-1.18) | .6 | |
| Donor | Related | 515 | 109 | ||
| Unrelated | 912 | 273 | 1.51 (1.21-1.89) | <.001 |
Multivariable Cox cause-specific hazards models for NRM, with interactions between pretreatment and change in IPSS-R score. The total number of patients in each group and the number of events are reported in the N and D columns, respectively. The interval from diagnosis to HCT is in years, and age at HCT is in decades. Effect estimates are given with 95% CIs. Corresponding P values are calculated using the Wald test. All interaction effects between pretreatment and IPSS-R score change include both corresponding main effects.
dec, decades.
For reference patients with high-risk IPSS-R score at diagnosis and no change in IPSS-R score at allo-HCT, the predicted NRM at 5 years was 38% (95% CI, 28-48) with no prior therapy, 28% (95% CI, 18-40) after chemotherapy, 20% (95% CI, 12-30) after HMAs, and 28% (95% CI, 14-44) after other treatments. When IPSS-R score improved, 5-year NRM was estimated at 46% (95% CI, 32-59) with no prior treatment, 18% (95% CI, 13-23) with chemotherapy, 19% (95% CI, 12-26) with HMAs, and 25% (95% CI, 16-35) with other therapies. When IPSS-R score at allo-HCT was worse than at diagnosis, 5-year NRM was 26% (95% CI, 18-35) without prior treatment, 26% (95% CI, 15-39) with chemotherapy, 33% (95% CI, 19-47) with HMAs, and 27% (95% CI, 14-41) with other treatments. Estimates for all IPSS-R categories at diagnosis are shown in supplemental Table 4.
Change in BM blasts between diagnosis and transplant
Additional analyses were performed focusing on the blast count at diagnosis and the role of blast reduction before allo-HCT. Patients were categorized in 2 groups according to the percentage of blast count at diagnosis, being either ≤5% or >5%.
For patients with up to 5% blasts, we considered the scenario of an increase to >5% vs remaining at ≤5%. In a multivariable model for OS, an increase in blast count after chemotherapy was a significant risk factor, whereas this was not found in patients with no prior therapy and those receiving HMAs or other (supplemental Table 5). However, the use of HMAs or other treatment was a significant risk factor compared with not receiving treatment, as well as age at allo-HCT and IPSS-R score at diagnosis. For reference patients with IPSS-R intermediate risk at diagnosis and blasts of ≤5% at transplant, the predicted 5-year OS was 65%, whereas it was estimated at 57% with blasts of >5%, both without prior treatment (Table 8). All patients with prior therapy had inferior OS estimates. For PFS, very similar results were obtained: significant risk factors were increase in blasts after chemotherapy, treatment with chemotherapy, HMAs or other, and IPSS-R score at diagnosis (supplemental Table 6).
Five-year estimates for OS for patients with less than or equal to 5% blasts at diagnosis
| Pretreatment . | Blast change . | IPSS-R score at diagnosis . | ||||
|---|---|---|---|---|---|---|
| Very low . | Low . | Intermediate . | High . | Very high . | ||
| Untreated | Same | 75 (65-86) | 70 (61-79) | 64 (54-75) | 44 (32-60) | 45 (28-73) |
| Higher at HCT | 70 (57-86) | 64 (53-78) | 57 (45-73) | 36 (23-57) | 37 (19-71) | |
| Chemotherapy | Same | 67 (55-83) | 61 (51-73) | 54 (43-67) | 32 (20-52) | 33 (17-64) |
| Higher at HCT | 50 (32-76) | 41 (27-64) | 33 (20-57) | 13 (5-38) | 14 (4-55) | |
| HMAs | Same | 58 (43-80) | 51 (37-69) | 43 (30-62) | 22 (10-45) | 23 (9-58) |
| Higher at HCT | 67 (50-89) | 60 (44-83) | 53 (36-78) | 32 (16-63) | 33 (14-78) | |
| Other | Same | 58 (43-79) | 51 (38-68) | 43 (30-62) | 21 (11-44) | 23 (9-57) |
| Higher at HCT | 66 (45-95) | 59 (38-90) | 52 (31-87) | 30 (11-81) | 31 (11-88) | |
| Pretreatment . | Blast change . | IPSS-R score at diagnosis . | ||||
|---|---|---|---|---|---|---|
| Very low . | Low . | Intermediate . | High . | Very high . | ||
| Untreated | Same | 75 (65-86) | 70 (61-79) | 64 (54-75) | 44 (32-60) | 45 (28-73) |
| Higher at HCT | 70 (57-86) | 64 (53-78) | 57 (45-73) | 36 (23-57) | 37 (19-71) | |
| Chemotherapy | Same | 67 (55-83) | 61 (51-73) | 54 (43-67) | 32 (20-52) | 33 (17-64) |
| Higher at HCT | 50 (32-76) | 41 (27-64) | 33 (20-57) | 13 (5-38) | 14 (4-55) | |
| HMAs | Same | 58 (43-80) | 51 (37-69) | 43 (30-62) | 22 (10-45) | 23 (9-58) |
| Higher at HCT | 67 (50-89) | 60 (44-83) | 53 (36-78) | 32 (16-63) | 33 (14-78) | |
| Other | Same | 58 (43-79) | 51 (38-68) | 43 (30-62) | 21 (11-44) | 23 (9-57) |
| Higher at HCT | 66 (45-95) | 59 (38-90) | 52 (31-87) | 30 (11-81) | 31 (11-88) | |
Predicted 5-year OS, expressed in percentages (with 95% CIs), based on the multivariable Cox model including the interaction between pretreatment and change in blast count.
Estimates are given for each IPSS-R category at diagnosis for reference patients with age 60 years, receiving a graft from a related donor after reduced intensity conditioning, and receiving transplantation 9 months after diagnosis.
Blast change was coded “same” when ≤5% at transplant, and “higher at HCT” when >5%.
Patients with a blast count of >5% at diagnosis were analyzed according to whether or not they showed a reduction to ≤5% at allo-HCT. OS was positively influenced by a decreased blast count after chemotherapy but not after HMAs or other therapy. In a very small subgroup of patients with decreased blasts after no prior therapy, OS was worse (supplemental Table 7). Additional significant risk factors were IPSS-R score and interval between diagnosis and allo-HCT. For reference patients with high-risk IPSS-R score at diagnosis and blasts of >5% at diagnosis, the estimated 5-year OS was 49% with reduction in blasts after chemotherapy vs 33% without response (Table 9). Untreated patients with unchanged blasts at allo-HCT had 5-year OS of 46%. For PFS, a multivariable analysis of patients with >5% blasts at diagnosis showed a significant positive effect for reduced blasts after chemotherapy and a negative effect of reduced blasts after no treatment and IPSS-R score at diagnosis (supplemental Table 8).
Five-year estimates for OS for patients with greater than 5% blasts at diagnosis
| Pretreatment . | Blast change . | IPSS-R score at diagnosis . | |||
|---|---|---|---|---|---|
| Low . | Intermediate . | High . | Very high . | ||
| Untreated | Same | 67 (55-82) | 56 (46-68) | 46 (36-60) | 31 (20-47) |
| Lower at HCT | 46 (28-76) | 33 (18-59) | 23 (10-51) | 10 (3-37) | |
| Chemotherapy | Same | 56 (41-77) | 44 (32-60) | 33 (22-50) | 18 (10-36) |
| Lower at HCT | 69 (58-82) | 59 (50-68) | 49 (41-60) | 34 (25-45) | |
| HMAs | Same | 58 (44-77) | 46 (35-60) | 36 (25-50) | 20 (12-35) |
| Lower at HCT | 64 (51-80) | 53 (43-65) | 43 (33-56) | 27 (18-40) | |
| Other | Same | 57 (39-81) | 44 (30-65) | 34 (20-56) | 19 (9-41) |
| Lower at HCT | 59 (45-78) | 47 (35-61) | 36 (25-52) | 21 (12-37) | |
| Pretreatment . | Blast change . | IPSS-R score at diagnosis . | |||
|---|---|---|---|---|---|
| Low . | Intermediate . | High . | Very high . | ||
| Untreated | Same | 67 (55-82) | 56 (46-68) | 46 (36-60) | 31 (20-47) |
| Lower at HCT | 46 (28-76) | 33 (18-59) | 23 (10-51) | 10 (3-37) | |
| Chemotherapy | Same | 56 (41-77) | 44 (32-60) | 33 (22-50) | 18 (10-36) |
| Lower at HCT | 69 (58-82) | 59 (50-68) | 49 (41-60) | 34 (25-45) | |
| HMAs | Same | 58 (44-77) | 46 (35-60) | 36 (25-50) | 20 (12-35) |
| Lower at HCT | 64 (51-80) | 53 (43-65) | 43 (33-56) | 27 (18-40) | |
| Other | Same | 57 (39-81) | 44 (30-65) | 34 (20-56) | 19 (9-41) |
| Lower at HCT | 59 (45-78) | 47 (35-61) | 36 (25-52) | 21 (12-37) | |
Predicted 5-year OS, expressed in percentages (with 95% CIs), based on the multivariable Cox model including the interaction between pretreatment and change in blast count.
Estimates are given for each IPSS-R category at diagnosis for reference patients with age 60 years, receiving a graft from a related donor after reduced intensity conditioning, and receiving transplantation 9 months after diagnosis.
Blast change was coded “same” when >5% at transplant, and “lower at HCT” when ≤5%.
Discussion
Leveraging the EBMT registry database, we analyzed a large cohort of patients with MDS with available data to calculate IPSS-R score, both at diagnosis and at time of allo-HCT, as well as information on pretransplant treatment.
How do we integrate the results, which, to our knowledge, for the first time, incorporate the dynamics of disease risk between diagnosis and allo-HCT and include the role of prior therapy, into current MDS allo-HCT algorithms?
Indeed, the recommendation to initiate induction therapy in patients with increased blasts before transplantation seems questionable. The 2013 European LeukemiaNet guidelines18 give a weak recommendation for induction therapy in patients with ≥10% blasts, however, add a warning that it may be associated with relevant NRM. In 2017, an expert panel concluded that patients with MDS with >10% blasts should be offered induction therapy before proceeding to allo-HCT.19 This was based on retrospective studies showing that a higher blast percentage was a negative factor for posttransplant survival.2,3,20 However, the recommendation was limited to cases with normal karyotype, whereas for high-risk cytogenetics the authors only claim that induction “might be useful.” In addition, a retrospective study21 was quoted, showing no benefit in allo-HCT outcome for patients with high-risk genetics in complete remission (CR) after induction compared with patients who are receiving transplantation “upfront.” The recent European Society for Oncology guideline22 mentions chemotherapy or HMAs as options for bridging to allo-HCT; however, the question whether bridging should be applied at all, remains open and is currently being explored for patients with <10% BM blasts in the ACROBAT study run by the GIMEMA (Gruppo Italiano Malattie Ematologiche dell'Adulto) as introduced above. Nevertheless, the European Society for Oncology guideline also quotes the aforementioned expert opinion to use induction therapy in cases with ≥10% blasts.19 The American National Comprehensive Cancer Network guidelines23 recommend to consider induction with HMAs for patients with ≥5% blasts in order to reduce blast counts before transplantation in an attempt to reduce posttransplant relapse. However, the authors stress that this recommendation is not evidence-based and HMA treatment should not replace or delay transplantation.
The notion to attempt reducing blast counts before transplantation seems to be mainly based on patients who received induction therapy. In this case, it is plausible that patients with a response (and lower blasts) have a better allo-HCT outcome than those with resistant disease (and higher blasts) and our results support this latter finding.
However, the real question is whether the attempt of blast reduction should be made at all in view of uncertain risks and benefits.
A number of retrospective studies have addressed the role of induction therapy before transplantation. Nakai et al assessed 283 patients with MDS or sAML from the Japanese transplant registry,8 with 188 patients receiving induction therapy and 95 undergoing upfront allo-HCT. Patients with a response to induction had a similar transplant outcome as those who received upfront allo-HCT, whereas nonresponders showed a markedly inferior survival. The Italian Group for Blood and Marrow Transplantation (GITMO) analyzed 457 patients with MDS or oligoblastic AML,9 of whom 209 had received induction therapy, with 47% achieving CR, and 248 who underwent upfront transplantation. Whereas untreated and patients in CR had a comparable survival after allo-HCT, OS was worse for those failing to respond to induction. The French SFGM cooperative group published a registry-based study of 128 patients, 40 pretreated with azacytidine, and 88 who underwent upfront transplantation.10 Posttransplant outcome was similar in univariate and multivariate analysis, however, patients not responding to induction had a significantly worse survival. Very similar results were reported by a single-center retrospective study from Detroit,11 in which 172 patients with MDS receiving an allogeneic transplant were split in 3 groups: HMA-treated with response, HMA-treated without response, and no therapy. PFS after transplant was similar in HMA responders and patients with no pre-HSCT treatment but significantly worse in HMA nonresponders. A recent meta-analysis, including 7 small retrospective studies, showed no difference in OS after transplant between patients receiving HMAs before transplant compared with best supportive care.12 Although all these studies uniformly show no benefit of a response to induction with either chemotherapy or HMAs, it must be remarked that they all suffer from heterogeneous patient cohorts and, thus, imbalances in other prognostically relevant parameters may have confounded the results. Ideally, a randomized prospective study with stratification for relevant parameters such as age or IPSS-R score at diagnosis would be required. One such attempt is now ongoing, testing upfront allo-HCT in higher-risk patients with MDS with <10% blasts (ClinicalTrials.gov identifier: NCT04184505). In the meantime, we offer a retrospective registry analysis with a large number of patients and availability of data on a broad spectrum of potential factors with prognostic relevance, analyzing treatment effects while adjusting for patient-, disease- and transplant-related risk factors.
An inevitable limitation of any retrospective transplant registry study is potential for selection bias. However, because we did not investigate the question whether to transplant or not, but rather whether to treat before transplantation, the question of selection bias would be a different one: is it possible that patients receiving induction therapy with or without response are selected in a different way to undergo transplantation than patients without prior treatment? In treated patients, the risk of not reaching transplantation because of morbidity and mortality of induction should be similar in responding or nonresponding patients. Still, there may be a reluctance to proceed to transplant with patients not responding to therapy, and patients who are nevertheless offered transplantation might be positively selected based on younger age or fitness. Thus, the patients in our analysis may represent a best-case scenario of nonresponders to prior therapy, but still show the worst survival after transplant.
In contrast, what may have influenced the decision to undergo upfront allo-HCT without induction? Were these patients older or unfit, who should be spared from additional toxicity, or was there an earlier donor availability? In addition, the choice of therapy may have been influenced by patient factors such as age or fitness. Although we cannot exclude such selection effects, our data show no difference in age or time from diagnosis to transplant between treated and untreated patients and many parameters were included in the multivariable analysis and thus controlled for.
Recently, a new prognostic score was introduced including not only cytogenetic but also molecular markers and thus termed IPSS-M.14 A recent multicenter retrospective analysis showed IPSS-M score at diagnosis to be related to posttransplant survival.24 Although any effort is currently taken to capture these additional parameters in the EBMT database, such information is lacking for the majority of patients who received transplantation in the last 10 years. Thus, the IPSS-R score is currently the best available MDS risk score to be used with registry data while awaiting further results using the IPSS-M once more molecular data are included in registry data. Whether the advent of novel treatment options and the use of the IPSS-M will change our view on treatment between diagnosis and transplantation or on transplantation per se will need to be investigated in the future.
Several outcomes of this study will help to inform the decision-making process regarding MDS treatment before transplantation. First, posttransplant outcome was largely dependent on IPSS-R score at diagnosis, and in patients with no prior treatment IPSS-R score progression before transplant did not show a negative influence. Secondly, when patients received pretransplant chemotherapy and responded, an improved IPSS-R score was associated with a moderate but significant improvement of OS, PFS, and NRM. However, with a similar or worse IPSS-R score after chemotherapy, posttransplant OS and PFS were markedly inferior than that of untreated patients. Third, when HMAs were used, an improved or unchanged IPSS-R score before allo-HCT did not affect OS, PFS, RI, and NRM, however, transplant outcomes were significantly worse when IPSS-R score had worsened.
In summary, our retrospective analysis questions the benefit of prior therapy before undergoing allo-HCT in patients with MDS, and provides estimates for transplant outcome for several scenarios. Until prospective studies are completed, this analysis of a large, well-documented series is the best available evidence and should be taken into account for patients with MDS considered for allo-HCT.
Acknowledgments
The authors thank the patients and the participating centers for contributing data to the EBMT registry.
Authorship
Contribution: C.S., M.R., D.-J.E., and L.C.d.W. designed the study; C.S., D.-J.E., M.v.G., L.C.d.W., F.O., M.R., I.Y.-A., D.P.M., K.R., and C.G. wrote the manuscript; L.K. prepared the data extraction; D.-J.E. and L.C.d.W. performed the statistical analyses; U.S., J.M., J.P., D.B., J.L.B., N.K., K.S., P.C., J.H.B., J.J.C., H.S., J.F., J.A.S., T.G.-D., J.C., U.S., A.P., P.H., K.R., and J.D.-S. included patients and provided data; and all authors reviewed and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Amit Patel died in October 2021.
Correspondence: Christof Scheid, Department I of Medicine, University of Cologne, Kerpener Str 62, 50937 Cologne, Germany; email: c.scheid@uni-koeln.de.
References
Author notes
Requests for sharing registry data used in this analysis should be addressed to the corresponding author, Christof Scheid (c.scheid@uni-koeln.de).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal