In this issue of Blood, Scheid and colleagues present a retrospective analysis from the European Society for Blood and Marrow Transplantation (EBMT) database examining whether downstaging of the Revised International Prognostic Scoring Sytem (IPSS-R) between diagnosis and time of hematopoietic cell transplantation (HCT) is associated with improved post-HCT outcomes.1
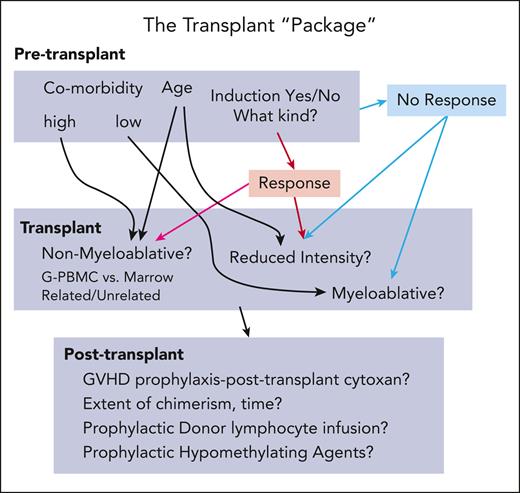
What is the best way to treat patients with myelodysplastic syndromes (MDSs) prior to allogeneic HCT? This is one of the key unanswered questions in the treatment of patients with MDSs. Prior retrospective studies have demonstrated that disease status at time of HCT is one of the most important predictors of posttransplant outcomes. They have also shown that relapse is the primary cause of failure following HCT.2 Naturally, it would follow that efforts to reduce disease burden prior to HCT could lower relapse risk and improve post-HCT outcomes. This was referred to by my mentor, Joachim Deeg, as the “transplant package” (see figure). The road map to a successful outcome using allogeneic HCT in patients with MDS is a complicated pathway with many twists and turns.
The transplant “package.” The complicated road to a successful outcome using allogeneic HCT to treat patients with MDS. G-PBMC, granulocyte colony-stimulating factor–mobilized peripheral blood mononuclear cells; GVHD, graft-versus-host disease.
The transplant “package.” The complicated road to a successful outcome using allogeneic HCT to treat patients with MDS. G-PBMC, granulocyte colony-stimulating factor–mobilized peripheral blood mononuclear cells; GVHD, graft-versus-host disease.
Several retrospective studies evaluated the impact of pre-HCT induction chemotherapy in patients with MDS. Most of these studies have shown that there was no overall benefit of administering pre-HCT cytoreductive therapy with induction chemotherapy; however, some did show that if patients were able to obtain a remission, then post-HCT outcomes were improved compared with patients who did not achieve remission or who did not receive induction chemotherapy.3-5 It is impossible to know for certain which patients would or would not respond to the induction chemotherapy prior to giving it. Furthermore, decreasing the bone marrow myeloblast count alone may not change the fundamental underlying biology of MDS. Following the approval of azacitidine and decitabine, there was an increased interest in using these agents for pre-HCT cytoreduction. Perhaps they would be safer and equally effective, and thus even more patients would be able to successfully undergo HCT. Indeed, most retrospective studies did show similar outcomes between induction chemotherapy and hypomethylating therapy given as pre-HCT cytoreduction in patients with MDS.6-8 All of these studies were limited by significant selection bias, as patients were selected to receive either induction chemotherapy or hypomethylating agents likely based on confounding factors. HCT databases do not capture patients with MDS who were considered for HCT but did not undergo this intervention either due to disease progression or toxicity of treatment. One clue of how impactful this omission might be was provided by the VidazaAllo study.9 In this prospective donor vs no donor HCT study, all patients were recommended to receive at least 4 cycles of azacitidine treatment followed by either HCT if an HLA-matched donor was identified or continuation of azacitidine if there was no HLA-matched donor identified. The investigators wisely captured the number of patients who did not undergo treatment assignment due to complications during the 4-month run-in phase with azacitidine. Thirty-three percent (n = 54) of patients treated in the 4-month run-in phase were prematurely withdrawn from the study prior to treatment allocation primarily due to disease progression (48%) and death primarily from infectious causes (22%). It is not clear that patients who progressed on azacitidine or who were not able to tolerate azacitidine would have benefited from HCT. Nevertheless, this has led some to question the wisdom of giving any pre-HCT cytoreduction.
The analysis performed by Scheid and colleagues compared change in IPSS-R from time of diagnosis to time of transplant to determine if “downstaging” MDS with pre-HCT therapy is an effective strategy. There was a total of 1482 patients with MDS in the EBMT database for whom there was sufficient data to calculate both the IPSS-R at diagnosis and at time of HCT. In the entire population of 1482 MDS patients, there was no improvement in overall survival (OS) or progression-free survival (PFS) observed with downstaging of the IPSS-R based upon a univariable model. Multivariable analysis included IPSS-R at diagnosis, treatment, and several other transplant- and patient-specific variables. However, the manner in which downstaging of IPSS-R was obtained appears to be relevant, as a subset analysis did show that if patients with MDS received induction chemotherapy and the IPSS-R decreased, there was improvement in OS. In contrast, there was no benefit in OS or PFS if the IPSS-R decreased following hypomethylating agents. The OS and PFS were markedly worse if the IPSS-R did not change with any intervention. Another single-center study with fewer patients also found no benefit of IPSS-R downstaging.10 The major limitations of these analyses are the lack of data on patients who did not make it to HCT and the potential selection bias of the pre-HCT reduction strategy. Although efforts were made to adjust for disease severity and patient-related factors, these cannot be completely corrected for in a retrospective analysis. Did the patients who were selected to receive hypomethylating therapy have worse comorbidities and better disease markers? Did the patients who were selected to receive induction chemotherapy have fewer comorbidities and worse disease markers? Why were some patients selected for no intervention? Additionally, what happened to the patients who would have been considered for HCT but did not ultimately undergo HCT? There is no way to know a priori whether an individual patient would respond to any selected intervention. Given the historical nature of this data set, there was limited information regarding mutational data and minimal identifiable disease. This pivotal information may in the future allow us to better select patients who should go immediately to HCT vs those who should receive additional therapy.
The authors are congratulated for completing this monumental undertaking with complete assessments of the IPSS-R at both time of diagnosis and at time of HCT. Although there are many limitations of this analysis, to date there has not been a prospective randomized trial to answer this key clinical question. Until there is, we are left with wondering if perhaps change is not always good. If that is the case, then the road map (see figure) for allogeneic HCT in MDS has become a bit more straightforward with fewer twists and turns than originally thought. After all, the fastest connection between 2 points has always been a straight line.
Conflict-of-interest disclosure: B.L.S. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal