Key Points
MRD-negativity at 12 months reduced the risk of progression; the treatment effect on MRD was correlated with the treatment effect on PFS.
MRD-negativity is reasonably likely to eventually demonstrate a treatment effect on PFS.
Visual Abstract
Estimating progression-free survival (PFS) and overall survival superiority during clinical trials of multiple myeloma (MM) has become increasingly challenging as novel therapeutics have improved patient outcomes. Thus, it is imperative to identify earlier end point surrogates that are predictive of long-term clinical benefit. Minimal residual disease (MRD)-negativity is a common intermediate end point that has shown prognostic value for clinical benefit in MM. This meta-analysis was based on the US Food and Drug Administration guidance for considerations for a meta-analysis of MRD as a clinical end point and evaluates MRD-negativity as an early end point reasonably likely to predict long-term clinical benefit. Eligible studies were phase 2 or 3 randomized controlled clinical trials measuring MRD-negativity as an end point in patients with MM, with follow-up of ≥6 months following an a priori–defined time point of 12 ± 3 months after randomization. Eight newly diagnosed MM studies evaluating 4907 patients were included. Trial-level associations between MRD-negativity and PFS were R2WLSiv, 0.67 (95% confidence interval [CI], 0.43-0.91) and R2copula 0.84 (0.64 to >0.99) at the 12-month time point. The individual-level association between 12-month MRD-negativity and PFS resulted in a global odds ratio (OR) of 4.02 (95% CI, 2.57-5.46). For relapse/refractory MM, there were 4 studies included, and the individual-level association between 12-month MRD-negativity and PFS resulted in a global OR of 7.67 (4.24-11.10). A clinical trial demonstrating a treatment effect on MRD is reasonably likely to eventually demonstrate a treatment effect on PFS, suggesting that MRD may be an early clinical end point reasonably likely to predict clinical benefit in MM, that may be used to support accelerated approval and thereby, expedite the availability of new drugs to patients with MM.
Introduction
Recent therapeutic advancements from clinical trials have resulted in substantial improvements in the survival of patients with newly diagnosed multiple myeloma (NDMM),1 a malignant plasma cell disorder affecting almost 180 000 individuals annually worldwide.2,3 This improved survival has correspondingly increased the prevalence of patients with multiple myeloma (MM), reflected in over 450 000 patients living with the disease globally.2-5 However, no curative therapy has yet been defined. Indeed, according to the National Cancer Institute (NCI)–Surveillance, Epidemiology, and End Results Program database, patients diagnosed with MM are confronted with a 5-year relative survival rate (measured from 2013-2019) of 59.8%,6 highlighting the strong need for more effective and less toxic treatments. Importantly, improved survival rates have also complicated the development of such therapeutics because demonstrating clinical benefit (ie, the application of current regulatory end points) through improved life expectancy now requires lengthier clinical trials with larger sample sizes and longer follow-up periods.7
Improving overall survival (OS) remains the ultimate goal for therapeutic agents. However, the extended time period required to measure OS in clinical trials necessitated the adoption of progression-free survival (PFS) as a clinical end point that may be predictive of OS, can be obtained in a shorter time period, and provides direct clinical benefit to patients (eg, allowing patients to forego changes to therapy and limiting the anxiety caused by progressive disease).7 The endorsement of PFS as a regulatory end point has allowed more rapid drug development, facilitating the approval of 13 new drugs for the treatment of MM in the United States over the last decade, resulting in the improvement of survival rates and quality of life for patients.8 Yet, advancements in the clinical efficacy of these therapeutic agents have once again created a demand for increasingly lengthy trials, thereby delaying the availability of improved therapeutic options to patients with great unmet medical needs.7 Thus, it is prudent to identify and make use of intermediate clinical end points as surrogates to predict direct clinical benefit (ie, PFS and OS), which can be measured earlier than progression or death and will expedite access to advantageous therapeutics for patients with MM.
Minimal residual disease (MRD) negativity has been found to correlate well with improved survival in patients with NDMM, which motivates the investigation of whether a treatment’s effect on MRD-negativity may potentially be correlated with the treatment’s effect on both PFS and OS.9-15
The use of MRD-negativity (determined by a validated bone marrow–based assay able to rule out at least 1 myeloma cell in 100 000 tested cells; assay sensitivity of 10–5) as a response category and as early evidence of clinical activity is supported by The International Myeloma Working Group (IMWG)16 and the National Comprehensive Cancer Network17 clinical guidelines. Additionally, MRD has been established by the United States Food and Drug Administration (FDA) as a key prognostic indicator and end point in several other hematologic malignancies, including acute lymphocytic leukemia, chronic lymphocytic leukemia, acute promyelocytic leukemia, and chronic myeloid leukemia.18
Over the past decade, numerous published meta-analyses have evaluated the prognostic value of MRD for PFS or OS in clinical studies of treatments for MM, and these meta-analyses have indicated that MRD-negativity has strong prognostic value for clinical benefit as measured by PFS or OS.9,15,19-22
Here, we were motivated to perform a comprehensive meta-analysis designed to evaluate MRD as an intermediate clinical end point for MM (the EVIDENCE meta-analysis). Specifically, we wanted to examine the potential role of MRD-negativity as an intermediate clinical end point reasonably likely to predict long-term clinical benefit in patients with MM. Sponsors for published, eligible randomized controlled trials (RCTs) reporting on MRD-negativity (based on an assay with a sensitivity of 10–5 or better) as an end point in the assessment of MM were invited to participate in this analysis. The analysis incorporates the FDA guidance for considerations for a meta-analysis to be used for validation of MRD as a clinical end point and potential basis for accelerated approval.18 As part of this study, we first developed a formal Statistical Analysis Plan (SAP) together with the FDA. Once the SAP was approved by the FDA, we performed the statistical analysis, The results from this analysis are supportive of the regulatory consideration of MRD as an early clinical end point reasonably likely to predict clinical benefit in MM that may be used to support accelerated approval and thereby expedite the availability of new drugs to patients with MM.
Methods
A systematic literature review was conducted to identify RCTs reporting MRD-negativity as an end point in the assessment of NDMM. Relevant studies were identified by searching PubMed, clinical trial registries (including ClinicalTrials.gov, the International Standard Randomised Controlled Trial Number registry, the European Union Clinical Trial Register, and the Australian New Zealand Clinical Trials Registry), cooperative groups’ websites, research organization meeting websites, and other sources such as personal communications. Identified studies were restricted to RCTs with human subjects in which relevant documentation was written in English. Full text or title and abstract review was performed to determine adherence to eligibility criteria, and bibliographies of eligible articles were examined for identification of additional studies. The final list of studies was reviewed and approved by the study principal investigator. This systematic literature review adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines for meta-analyses.
Eligibility criteria
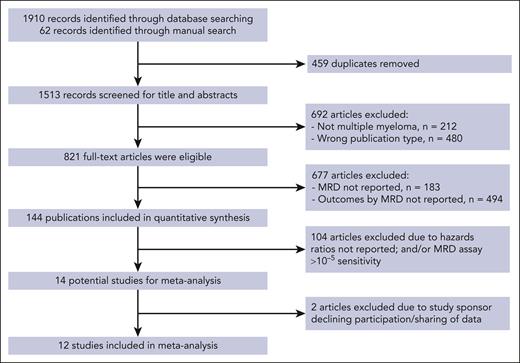
For this study, in accordance with the SAP approved by the FDA, we included phase 2 or 3 RCTs that enrolled patients with transplant-eligible or transplant-ineligible NDMM (TE NDMM and TIE NDMM, respectively) or relapsed/refractory multiple myeloma (RRMM) and specified MRD-negativity as a primary, secondary, or exploratory end point. Maintenance studies and those in which the primary end point was safety, toxicity, quality of life, or feasibility were excluded from consideration. Eligible studies must have performed MRD assays with a sensitivity of 10–5 or better (ie, <10–5) by multiparameter-flow cytometry and/or next-generation sequencing under guidelines from IMWG, National Comprehensive Cancer Network, the FDA, and NCI, as well as institutional standards of care for the treatment of patients with MM. To allow proper evaluation between MRD testing and clinical outcomes, eligible trials must have had a median follow-up of at least 6 months following an a priori–defined time point of 12 ± 3 months after randomization for the assessment of MRD-negativity (Figure 1).
PRISMA flowchart of the systematic literature review search strategy and article selection. The literature review was conducted in adherence to PRISMA guidelines. Medline and EMBASE databases were searched for articles published in English up to 8 June 2019; there was no date limit on the indexed database searches. Details of the search strategy were performed as follows: medical subject heading (MeSH) terms for MM were “MM” and “neoplasm, residual.” Non-MeSH search terms were “Kahler disease” (or “Kahler’s disease” or “myelomatosis” or “plasma cell myeloma”) and/or “MRD.” Selected congress abstracts published between 2016 and 2019, including additional literature, were manually reviewed. Bibliographies of systematic literature review articles on MM published between 2014 and 2019 were reviewed manually to identify additional potentially relevant publications. Additional sources were used for validation, including studies identified in public assessment reports published by the European Medicines Agency and the FDA. PICOS (Population, interventions, comparisons, outcomes, and study design) criteria were used to define eligibility. Patients could have received any type of therapy except allogeneic stem cell transplantation. Studies with PFS or OS data that could not be extracted or reconstructed were excluded. Studies with patients who did not have a primary diagnosis of MM were also excluded, as were those with MRD measured only in peripheral blood or assessed only by positron emission tomography–computed tomography scanning. Two independent investigators selected the articles for potential inclusion. RCTs and observational studies that reported PFS or OS rates stratified by MRD status in patients with MM following therapy were eligible for inclusion (supplemental Table 1). The methodological quality of the studies was assessed using the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) reporting recommendations. A manual search was conducted to identify any updated publications on selected studies. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
PRISMA flowchart of the systematic literature review search strategy and article selection. The literature review was conducted in adherence to PRISMA guidelines. Medline and EMBASE databases were searched for articles published in English up to 8 June 2019; there was no date limit on the indexed database searches. Details of the search strategy were performed as follows: medical subject heading (MeSH) terms for MM were “MM” and “neoplasm, residual.” Non-MeSH search terms were “Kahler disease” (or “Kahler’s disease” or “myelomatosis” or “plasma cell myeloma”) and/or “MRD.” Selected congress abstracts published between 2016 and 2019, including additional literature, were manually reviewed. Bibliographies of systematic literature review articles on MM published between 2014 and 2019 were reviewed manually to identify additional potentially relevant publications. Additional sources were used for validation, including studies identified in public assessment reports published by the European Medicines Agency and the FDA. PICOS (Population, interventions, comparisons, outcomes, and study design) criteria were used to define eligibility. Patients could have received any type of therapy except allogeneic stem cell transplantation. Studies with PFS or OS data that could not be extracted or reconstructed were excluded. Studies with patients who did not have a primary diagnosis of MM were also excluded, as were those with MRD measured only in peripheral blood or assessed only by positron emission tomography–computed tomography scanning. Two independent investigators selected the articles for potential inclusion. RCTs and observational studies that reported PFS or OS rates stratified by MRD status in patients with MM following therapy were eligible for inclusion (supplemental Table 1). The methodological quality of the studies was assessed using the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) reporting recommendations. A manual search was conducted to identify any updated publications on selected studies. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Data sources
Studies meeting eligibility criteria and included in this study’s analyses are described in Table 1. Secure transfer of information was requested from clinical trial sponsors for eligible studies, with data provided for MRD evaluation technique, and follow-up data on disease and outcomes.
PFS and OS data for studies in patients with NDMM included in the meta-analysis
| Study/sponsor . | Treatment, n (%) . | N . | Median follow-up,∗ mo . | Median PFS, mo . | Median OS, mo . | MRD assay† . |
|---|---|---|---|---|---|---|
| TE NDMM | ||||||
| Randomized phase 3 trial for previously untreated MM to evaluate 2 regimens of bortezomib–based induction therapy and lenalidomide consolidation followed by lenalidomide maintenance treatment (MM5)/University Hospital Heidelberg21https://www.clinicaltrialsregister.eu/ctr-search/trial/2010-019173-16/DE | A1‡: 149 (24.8%) A2‡: 151 (25.1%) B1‡: 150 (25%) B2‡: 151 (25.1%) | 601 | 57.1 (IQR, 44.4-64) | 40.5 (95% CI, 36.5-43.2) | NR | MFC |
| Phase 2, randomized, open-label study comparing D-RVd vs RVd in subjects with NDMM eligible for high-dose chemotherapy and autologous stem cell transplantation (GRIFFIN-MMY2004)/Janssen R&D23 NCT02874742 | D-RVd: 120 (53.8%) RVd: 103 (46.2%) | 223 | 25.4 (IQR, 22.1-28.3) | NR (95% CI, 34.1-NR) | NR | NGS |
| Study of daratumumab in combination with bortezomib (Velcade), VTD in the first line treatment of TE subjects with NDMM (CASSIOPEIA-MMY3006)/Intergroupe Francophone du Myelome24,25 NCT02541383 | D-VTd: 543 (50%) VTd: 542 (50%) | 1085 | 18.2 (IQR, 13.7-24) | NR | NR | NGS |
| TIE NDMM | ||||||
| A randomized, open-label phase 3 study of KMP vs VMP in TIE patients with NDMM (CLARION)/Amgen15 NCT01818752 | KMP: 428 (50.2%) VMP: 425 (49.8%) | 853 | 22.3 (IQR, 19.1-27.6) | 22.2 (95% CI, 21-24.2) | NR | MFC |
| A phase 3, randomized, controlled, open-label study of Velcade VMP compared with daratumumab in combination with VMP (D-VMP) in subjects with previously untreated MM who are ineligible for high-dose therapy (ALCYONE-MMY3007) Janssen R&D26,27 NCT02195479 | D-VMP: 350 (49.6%) VMP: 356 (50.4%) | 706 | 39.9 (IQR, 37.4-42.9) | 24 (95% CI, 21.6-27.4) | NR | NGS |
| A phase 3, randomized, double-blind, multicenter study comparing oral MLN9708 plus Rd vs placebo plus Rd in adult patients with NDMM (TOURMALINE-MM2)/Takeda18 NCT01850524 | IRd: 351 (49.8%) Rd: 354 (50.2%) | 705 | 54.6 (IQR, 22-60.7) | 27.9 (95% CI, 23.9-35.8) | NR | MFC |
| A phase 3 study comparing DRd vs Rd in subjects with previously untreated MM who are ineligible for high-dose therapy (MAIA-MMY3008)/Janssen R&D16,17 NCT02252172 | DRd: 368 (49.9%) Rd: 369 (50.1%) | 737 | 62.4 (IQR, 57.9-66.8) | 44.8 (95% CI, 40.9-52.4) | 73.7 (95% CI, 69.7-NA) | NGS |
| A phase 3, multicenter, randomized, controlled, open-label study of Velcade VMP compared to daratumumab in combination with VMP (D-VMP), in subjects with previously untreated MM who are ineligible for high-dose therapy (Asia-Pacific region-OCTANS-MMY3011)/Janssen R&D NCT03217812 | D-VMP: 146 (66.4%) VMP: 74 (33.6%) | 220 | 22.9 (IQR, 19-30) | 28.2 (95% CI, 24.4-NR) | 41.6 (95% CI, 41.6-NA) | MFC |
| Study/sponsor . | Treatment, n (%) . | N . | Median follow-up,∗ mo . | Median PFS, mo . | Median OS, mo . | MRD assay† . |
|---|---|---|---|---|---|---|
| TE NDMM | ||||||
| Randomized phase 3 trial for previously untreated MM to evaluate 2 regimens of bortezomib–based induction therapy and lenalidomide consolidation followed by lenalidomide maintenance treatment (MM5)/University Hospital Heidelberg21https://www.clinicaltrialsregister.eu/ctr-search/trial/2010-019173-16/DE | A1‡: 149 (24.8%) A2‡: 151 (25.1%) B1‡: 150 (25%) B2‡: 151 (25.1%) | 601 | 57.1 (IQR, 44.4-64) | 40.5 (95% CI, 36.5-43.2) | NR | MFC |
| Phase 2, randomized, open-label study comparing D-RVd vs RVd in subjects with NDMM eligible for high-dose chemotherapy and autologous stem cell transplantation (GRIFFIN-MMY2004)/Janssen R&D23 NCT02874742 | D-RVd: 120 (53.8%) RVd: 103 (46.2%) | 223 | 25.4 (IQR, 22.1-28.3) | NR (95% CI, 34.1-NR) | NR | NGS |
| Study of daratumumab in combination with bortezomib (Velcade), VTD in the first line treatment of TE subjects with NDMM (CASSIOPEIA-MMY3006)/Intergroupe Francophone du Myelome24,25 NCT02541383 | D-VTd: 543 (50%) VTd: 542 (50%) | 1085 | 18.2 (IQR, 13.7-24) | NR | NR | NGS |
| TIE NDMM | ||||||
| A randomized, open-label phase 3 study of KMP vs VMP in TIE patients with NDMM (CLARION)/Amgen15 NCT01818752 | KMP: 428 (50.2%) VMP: 425 (49.8%) | 853 | 22.3 (IQR, 19.1-27.6) | 22.2 (95% CI, 21-24.2) | NR | MFC |
| A phase 3, randomized, controlled, open-label study of Velcade VMP compared with daratumumab in combination with VMP (D-VMP) in subjects with previously untreated MM who are ineligible for high-dose therapy (ALCYONE-MMY3007) Janssen R&D26,27 NCT02195479 | D-VMP: 350 (49.6%) VMP: 356 (50.4%) | 706 | 39.9 (IQR, 37.4-42.9) | 24 (95% CI, 21.6-27.4) | NR | NGS |
| A phase 3, randomized, double-blind, multicenter study comparing oral MLN9708 plus Rd vs placebo plus Rd in adult patients with NDMM (TOURMALINE-MM2)/Takeda18 NCT01850524 | IRd: 351 (49.8%) Rd: 354 (50.2%) | 705 | 54.6 (IQR, 22-60.7) | 27.9 (95% CI, 23.9-35.8) | NR | MFC |
| A phase 3 study comparing DRd vs Rd in subjects with previously untreated MM who are ineligible for high-dose therapy (MAIA-MMY3008)/Janssen R&D16,17 NCT02252172 | DRd: 368 (49.9%) Rd: 369 (50.1%) | 737 | 62.4 (IQR, 57.9-66.8) | 44.8 (95% CI, 40.9-52.4) | 73.7 (95% CI, 69.7-NA) | NGS |
| A phase 3, multicenter, randomized, controlled, open-label study of Velcade VMP compared to daratumumab in combination with VMP (D-VMP), in subjects with previously untreated MM who are ineligible for high-dose therapy (Asia-Pacific region-OCTANS-MMY3011)/Janssen R&D NCT03217812 | D-VMP: 146 (66.4%) VMP: 74 (33.6%) | 220 | 22.9 (IQR, 19-30) | 28.2 (95% CI, 24.4-NR) | 41.6 (95% CI, 41.6-NA) | MFC |
DRd, daratumumab, lenalidomide, and dexamethasone; D-RVd, daratumumab, lenalidomide, bortezomib, and dexamethasone; D-VMP, daratumumab, bortezomib, melphalan, and prednisone; D-VTd, daratumumab, bortezomib, thalidomide, and dexamethasone; IRd, isatuximab, lenalidomide, and dexamethasone; Janssen R&D, Janssen Research and Development, LLC; KMP, carfilzomib, melphalan, and prednisone; MFC, multiparameter-flow cytometry; NA, not available; NGS, next-generation sequencing; NR, not reached; Rd, lenalidomide and dexamethasone; RVd, lenalidomide, bortezomib, and dexamethasone; VMP, bortezomib, melphalan, and prednisone.
Follow-up is calculated for the end point of PFS using the reverse Kaplan-Meier estimate.
MFC or NGS.
A1, bortezomib, doxorubicin, dexamethasone, high-dose melphalan, autologous blood stem cell transplantation, and lenalidomide consolidation followed by lenalidomide maintenance therapy for 2 years. B1, bortezomib, doxorubicin, dexamethasone, high-dose melphalan, autologous blood stem cell transplantation, and lenalidomide consolidation followed by lenalidomide maintenance until achievement of CR. A2, bortezomib, cyclophosphamide, dexamethasone, high-dose melphalan, autologous blood stem cell transplantation, and lenalidomide consolidation followed by lenalidomide maintenance therapy for 2 years. B2, bortezomib, cyclophosphamide, and dexamethasone, high-dose melphalan, autologous blood stem cell transplantation, and lenalidomide consolidation followed by lenalidomide maintenance until achievement of CR.
Outcomes for analysis
The primary objective of this study was to evaluate whether MRD-negativity while in at least a complete response (CR; as defined by IMWG criteria)16 at the a priori–defined time point is a reasonably likely end point to predict clinical benefit as measured by PFS in patients with TE NDMM, in patients with TIE NDMM, in a combined “all-NDMM” population, and in the RRMM population. The primary end point of MRD-negativity at 12 ± 3 months was defined as follows: if a patient achieved at least a CR at or before the time window, the MRD assessment closest to 12 months within the window was selected for the primary end point (if the patient had multiple assessments); if the patient did not have an MRD assessment during the window but progressed or died during the window, the patient was considered MRD-positive; lastly, if the patient did not have an MRD assessment and remained alive and in remission during the window, the primary end point was considered missing. Only trials in which the MRD end point could be ascertained in ≥80% of patients were included in the primary analysis. For all trials fulfilling the >80% criterion, patients with missing MRD information were considered MRD-positive, and the intent-to-treatment principle was followed.
Key secondary objectives of this study were to evaluate MRD-negativity as an end point reasonably likely to predict clinical benefit in NDMM and RRMM for OS, in addition to the attainment of MRD-negativity at least once was also considered as end points reasonably likely to predict PFS. For all analyses, OS was defined as the time from randomization to death from any cause, and PFS was defined as the time randomization to progression of disease or death. Censoring rules for all trials followed the censoring rules outlined in each study’s protocol.
Data analysis
To evaluate MRD-negativity as a reasonably likely end point for the prediction of clinical benefit (PFS or OS) in clinical trials of treatments for MM, a correlation approach was undertaken at both the individual and trial level. This approach evaluated the veracity of the following statements:
MRD-negativity is prognostic for clinical benefit.
A treatment effect on MRD-negativity in a clinical trial is predictive of a treatment effect on PFS or OS in the same clinical trial.
Within-trial treatment effects for MRD-negativity and survival were estimated using logistic regression and the Cox proportional hazard regression model, respectively. The trial-level correlation was estimated using either a Plackett copula model28 or weighted least squares (WLS), in which the weights were derived from either the sample size of each trial or the estimated inverse variance (WLSiv) of the log odds ratios (ORs) of the treatment effect on MRD for each trial. The WLS approach estimates the treatment effect on the 2 outcomes using 2 marginal models, while the copula approach accounts for patient-level correlation. For the individual-level association, the Placket copula estimated the global OR for comparing OS and PFS for patients with and without MRD. In addition to the copula, the association between MRD at 12 months and PFS and OS was assessed using a 12-month landmark. A random-effects meta-analysis estimated the average effect of MRD on the 2 long-term outcomes. Various sensitivity analyses were conducted, including leave-one-trial out re-estimation of the trial-level correlation. Analyses were conducted using R (v4.2.2) and SAS.
Results
Analysis sets and demographic and baseline characteristics
In NDMM, a total of 5130 patients were randomized from 8 studies, 1 of which provided 2 randomized comparisons (Table 1). Median (range) sample size was 306 (220-1085). There were 3 studies assessing patients with TE NDMM and 5 assessing patients with TIE NDMM. Eight of the 9 total comparisons, representing 4907 patients, fulfilled the criteria for the 12-month MRD-negative end point and were included in the primary individual-level and trial-level analysis for the all-NDMM population. One comparison was excluded from this primary analysis due to >20% of patients being assigned a value of “missing” for the primary end point definition based on MRD. The median (interquartile range [IQR]) follow-up for PFS was 29 months (19-58), and the median (IQR) follow-up for OS was 37 months (22-59).
In RRMM, 4 randomized studies fulfilled the study inclusion criteria, with 1835 patients in total. The median (IQR) follow-up for the included studies was 37.7 months (22-54.2) for PFS and 38.7 months (26.3-43.8) for OS.
Individual-level associations in NDMM
The global OR demonstrated strong individual-level associations between 12-month MRD-negativity and PFS, as well as between 12-month MRD-negativity and OS, for both the all-NDMM population (PFS, 4.72; [95% confidence interval [CI], 3.53-5.90], OS, 4.02; [95% CI, 2.57-5.46]), the TIE subgroup (PFS, 6.15; [95% CI, 4.27-8.03], OS, 4.08; [95% CI, 2.44-5.72]), and the TE subgroup (PFS, 2.45; [95% CI, 1.40-3.51], OS, 3.78; [95% CI, 0.78-6.78]) These individual-level associations remain strong in sensitivity analyses of other groupings of clinical trial results (supplemental Table 1, available on the Blood website) and indicate that being MRD negative at 12 months is highly prognostic of better long-term outcomes.
An alternative analysis investigated the association between MRD-negativity at 12 months and PFS by creating a 12-month post-randomization landmark. Among patients who were alive, progression-free, and under follow-up at 12 months, those who were MRD-negative at 12 months had reduced risk for progression or death in 1 out of 2 TE studies and 5 out of 5 TIE studies. Using a random-effects meta-analysis, the average estimated hazard ratio in the NDMM population was 0.40 (95% CI, 0.24-0.68). For the individual trials included in the analysis, the association with OS was less strong (supplemental Figures 1-2). This further supports the value of MRD-negativity as a prognostic marker for better long-term outcomes.
Trial-level associations in NDMM
Correlations between the treatment effect on MRD-negativity and the treatment effect on PFS at the trial level were R2WLSiv (95% CI) of 0.67 (0.43-0.91) for the all-NDMM population and 0.83 (0.71-0.96) for TIE NDMM subgroups. Using the copula model, R2copula (95% CI) was 0.84 (0.64 to >0.99) for the all-NDMM population and 0.85 (0.62 to >0.99) for the TIE subgroup. The 3 TE NDMM studies were too limited to estimate trial-level corrections. Sensitivity analyses additionally support a correlation between MRD-negativity and PFS across various groupings of included trials (Table 2). Similar results for the WLS R2 were observed when the weights were derived from the sample size of each trial.
Trial-level R2 estimates for PFS: sensitivity analyses (all-NDMM population)
| Sensitivity analysis . | Total follow-up, mo (IQR) . | R2copula (95% CI) . | R2WLSiv (95% CI) . |
|---|---|---|---|
| Adding study 2.1 to all NDMM | 28.6 (19-56.4) | 0.82 (0.62-1.03) | 0.68 (0.45-0.91) |
| All NDMM without study 1.1A and 1.1B | 26.9 (18-53.4) | 0.72 (0.35->0.99) | 0.53 (0.22-0.83) |
| All NDMM without study 1.2 | 28.2 (18.8-55.6) | 0.87 (0.69->0.99) | 0.69 (0.47-0.92) |
| All NDMM without study 1.3 | 43.8 (24.3-61.1) | 0.91 (0.79->0.99) | 0.88 (0.78-0.98) |
| All NDMM without study 1.4 | 38 (18.9-59.5) | 0.82 (0.58->0.99) | 0.56 (0.27-0.86) |
| All NDMM without study 1.5 | 27.4 (18.1-58.9) | 0.84 (0.63->0.99) | 0.62 (0.36-0.89) |
| All NDMM without study 1.6 | 26.5 (18-44.4) | 0.88 (0.72->0.99) | 0.82 (0.67-0.96) |
| All NDMM without study 1.7 | 30.5 (18.9-58.3) | 0.78 (0.47->0.99) | 0.64 (0.39-0.9) |
| Sensitivity analysis . | Total follow-up, mo (IQR) . | R2copula (95% CI) . | R2WLSiv (95% CI) . |
|---|---|---|---|
| Adding study 2.1 to all NDMM | 28.6 (19-56.4) | 0.82 (0.62-1.03) | 0.68 (0.45-0.91) |
| All NDMM without study 1.1A and 1.1B | 26.9 (18-53.4) | 0.72 (0.35->0.99) | 0.53 (0.22-0.83) |
| All NDMM without study 1.2 | 28.2 (18.8-55.6) | 0.87 (0.69->0.99) | 0.69 (0.47-0.92) |
| All NDMM without study 1.3 | 43.8 (24.3-61.1) | 0.91 (0.79->0.99) | 0.88 (0.78-0.98) |
| All NDMM without study 1.4 | 38 (18.9-59.5) | 0.82 (0.58->0.99) | 0.56 (0.27-0.86) |
| All NDMM without study 1.5 | 27.4 (18.1-58.9) | 0.84 (0.63->0.99) | 0.62 (0.36-0.89) |
| All NDMM without study 1.6 | 26.5 (18-44.4) | 0.88 (0.72->0.99) | 0.82 (0.67-0.96) |
| All NDMM without study 1.7 | 30.5 (18.9-58.3) | 0.78 (0.47->0.99) | 0.64 (0.39-0.9) |
The 2004 comparison was not included in the primary analysis due to >20% of patients being assigned a value of missing for the primary end point definition based on MRD. All-NDMM (ie, TE and TIE combined) includes studies 1.1A, 1.1B, 1.2, 1.3, 1.4, 1.5, 1.6, and 1.7. TE NDMM includes studies 1.1A, 1.1B, and 1.3.
Correlation coefficients were lower in comparison of the treatment effect on MRD-negativity and the treatment effect on OS, with R2WLSiv (95% CI) of 0.21 (<0.01 to 0.53) for the all-NDMM population and 0.79 (0.63-0.95) for TIE NDMM subgroup (supplemental Figure 3). Using the copula model, R2copula (95% CI) was 0.32 (<0.01 to 0.86) for the all-NDMM population and 0.63 (0.12 to >0.99) for TIE NDMM subgroup.
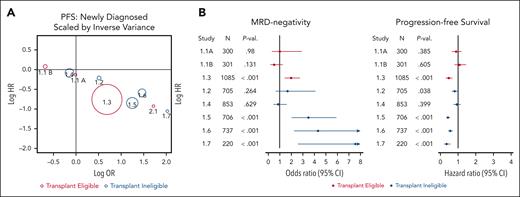
Treatment effects on MRD-negativity were statistically significant in 4 of the 8 comparisons, among which the treatment effect was also statistically significant on PFS in 4 studies and on OS in 3 studies (Table 3). Of the 4 treatment comparisons that did not have a statistically significant treatment effect on MRD-negativity, the treatment effect was also not significant on PFS in 3 out of 4 studies and on OS in 4 studies. Side-by-side Forest plots of the treatment effects on MRD-negativity and on PFS showed that studies with a strong treatment effect on MRD (producing MRD-negativity) tended to also have a strong treatment effect on PFS (Figure 2); the association was not clear for OS (supplemental Figure 4).
Concordance of significance for MRD with PFS and OS (all-NDMM population)
| study . | Treatment effect on MRD∗ (2-sided test) P value . | Treatment effect on PFS∗ (2-sided test) P value . | Treatment effect on OS∗ (2-sided test) P value . |
|---|---|---|---|
| TE NDMM | |||
| 1.1A | .98 | .385 | .686 |
| 1.1B | .131 | .605 | .901 |
| 1.3 | <.001 | <.001 | .008 |
| TIE NDMM | |||
| 1.4 | .629 | .399 | .232 |
| 1.2 | .264 | .038 | .806 |
| 1.5 | <.001 | <.001 | <.001 |
| 1.6 | <.001 | <.001 | <.001 |
| 1.7 | <.001 | <.001 | .377 |
| study . | Treatment effect on MRD∗ (2-sided test) P value . | Treatment effect on PFS∗ (2-sided test) P value . | Treatment effect on OS∗ (2-sided test) P value . |
|---|---|---|---|
| TE NDMM | |||
| 1.1A | .98 | .385 | .686 |
| 1.1B | .131 | .605 | .901 |
| 1.3 | <.001 | <.001 | .008 |
| TIE NDMM | |||
| 1.4 | .629 | .399 | .232 |
| 1.2 | .264 | .038 | .806 |
| 1.5 | <.001 | <.001 | <.001 |
| 1.6 | <.001 | <.001 | <.001 |
| 1.7 | <.001 | <.001 | .377 |
Does not include stratification factors used in randomization.
Correlations with MRD negativity. (A) Correlation between treatment effect on 12-month MRD-negativity and treatment effect on PFS scaled by sample size: all-NDMM population. All-NDMM combines TE and TIE patients. Study 2.1 was not included in the primary analysis due to >20% of patients being assigned a value of missing for the primary end point definition based on MRD. This study was included in sensitivity analyses. (B) Forest plot of treatment effect on MRD and PFS.
Correlations with MRD negativity. (A) Correlation between treatment effect on 12-month MRD-negativity and treatment effect on PFS scaled by sample size: all-NDMM population. All-NDMM combines TE and TIE patients. Study 2.1 was not included in the primary analysis due to >20% of patients being assigned a value of missing for the primary end point definition based on MRD. This study was included in sensitivity analyses. (B) Forest plot of treatment effect on MRD and PFS.
Evaluation of any MRD in NDMM
The attainment of MRD-negativity at least once was evaluated as an alternative measure of clinical benefit, in terms of their correlation with PFS and OS across studies. Data from 9 studies showed that attainment of MRD-negativity at any time during the study (at least once) and PFS were R2WLSiv (95% CI), 0.54 (0.23-0.84) and R2copula (95% CI), 0.76 (0.49-0.99); and its correlation with OS was R2WLSiv (95% CI), 0.07 (<0.01-0.28) and R2copula (95% CI), 0.11 (<0.01-0.49). These data, combined with those of the primary analysis, support MRD as an end point reasonably likely to predict clinical benefit in studies of patients with NDMM.
Relapse/refractory MM
Among the 4 studies included in the primary analysis, the global OR between MRD and PFS was 7.67 (95% CI, 4.24-11.10) using the copula model, and the OR between MRD and OS was 6.03 (3.12-6.23). These estimates indicate a strong association between MRD and both PFS and OS. Trial-level correlations could not be estimated with only 4 studies. Supplemental Figure 5 provides a plot of the treatment estimate on MRD and the treatment estimate on PFS.
Discussion
Initially, this effort was launched in 2009 as a US inter-agency collaboration between investigators at NCI (O.L. and M.S.-S.), National Heart, Lung, and Blood Institute (G.M.), and the FDA (G.M.)14; subsequently, additional collaborators were invited to join. The EVIDENCE meta-analysis was designed to further evaluate the prognostic value of bone marrow MRD-negativity and assess its use for the prediction of long-term clinical benefit, as measured by PFS, in patients with MM. The ultimate goal was to examine the potential role of MRD-negativity as an intermediate clinical end point reasonably likely to predict long-term clinical benefit in patients with MM. Therefore, the analysis incorporates the FDA guidance for considerations for a meta-analysis to be used for validation of MRD as a clinical end point and potential basis for accelerated approval.18 Data were compiled and analyzed from all available (N = 8) studies for NDMM that used MRD-negativity (sensitivity 10–5 or better) as a measure of efficacy. According to our meta-analysis, the OR relating MRD-negativity at 12 months to either prolonged PFS or OS was approximately 4 and was statistically significant. These results indicate a strong association between MRD-negativity and PFS and a moderate association between MRD-negativity and OS, suggesting that MRD-negativity measured using a prespecified time point may be an objective measure of anti-myeloma clinical activity that is highly prognostic of long-term outcomes. Additionally, clinical trial designs easily allow for extended follow-up of patients assessed for MRD, allowing subsequent intratrial evaluation of PFS and OS to confirm clinical benefit of investigational therapies. Furthermore, in the primary analysis for RRMM, the global OR between MRD and PFS was 7.67 using the copula model, and the OR between MRD and OS was 6.03, showing there is a strong association between MRD and both PFS and OS. Establishing associations between intermediate end points and OS, in general, are difficult in diseases with highly efficacious treatments because of subsequent effective therapies that patients receive after trial treatment and off-study “crossover” of control arms whereby patients may derive benefit from the experimental agent but not be captured in the shorter-term end points (ie, PFS, overall response rate, CR, or MRD-negativity) assessment.
Our findings align with those of previous meta-analyses, which have described strong evidence for the prognostic value of MRD-negativity in clinical trials of new therapeutic agents in patients with NDMM.9,15,19-21 The methods used to measure MRD have advanced in recent decades, resulting in sensitivity such that several studies have demonstrated MRD-positivity, that is, disease burden that was not detected by current, conventional evaluation techniques for assessment of CR.29-31 This suggests that MRD-negativity describes a deeper level of response driven by the availability of new technologies and has led to the IMWG including MRD-negativity as a response criterion for patients with MM.16 The EVIDENCE meta-analysis is consistent with multiple prior studies that have shown that depth of response correlates with clinical benefit, namely PFS.9,11-13,19
While some patient subgroups (transplant-eligible NDMM and RRMM) were limited by the number of available studies, the EVIDENCE meta-analysis shows a moderate-to-high trial-level correlation between the overall NDMM and the transplant-ineligible NDMM subgroup. As more studies incorporate MRD testing into their study protocols, future meta-analyses will be able to better estimate the trial-level correlation across all patient subgroups (transplant-ineligible NDMM, transplant-eligible NDMM, and RRMM).
Although substantial improvements in the treatment of patients with MM have been made over the last decade, further advancement is limited by the extended time periods currently required to properly estimate and demonstrate clinical benefit as measured by PFS or OS. Extended clinical trial durations may deny patients access to effective subsequent therapeutic options for many years. To speed up the development process and provide access to new therapeutic options for these patients, an objective and reliably measured intermediate end point that is well-correlated with an anti-myeloma treatment effect and long-term outcomes is needed. This meta-analysis has provided evidence that MRD-negativity (defined as 10–5 or better) may well be that surrogate marker that is predictive for long-term outcomes at an earlier time point.
As with all studies, additional analyses will provide further insight into and evidence for the prognostic value of this metric across all patients with MM. The fact that this meta-analysis is limited to 8 comparisons of heterogenous patient populations restricts extensive extrapolation, and additional analyses of broader patient populations, such as those with relapsing/refractory MM, will be necessary. It should be noted that measurement of MRD-negativity, including that of studies included in this analysis, has previously involved challenges of capture rate, resulting in an inability to assess MRD status in a subset of patients (up to ∼20% using early assays). However, modern technology (such as ClonoSEQ) results in a 90% to 95% capture rate among patients, allowing trial populations to be adequately assessed for this end point to achieve statistical significance.32 It may additionally prove beneficial to analyze potential differences in the correlation of MRD and outcomes of clinical benefit across categories of therapeutic agents, such as standard therapies vs chimeric antigen receptor T-cell therapy. Furthermore, it should be stated that any surrogate marker has the inherent limitation where toxicity can lead to excess deaths despite superior PFS. Therefore, it is important to confirm superior PFS and rule out inferior OS for full regulatory approval. Lastly, as shown in this analysis, there is a strong relationship between clinical outcomes and the attainment of MRD-negativity obtained at least once. Over time, an increasing number of studies have started to include repeated MRD testing to confirm sustained MRD-negativity (eg, annually). As expected, sustained MRD-negativity has an even stronger correlation with clinical outcomes.
Conclusions
There exists a strong demand for an early clinical end point that is reasonably likely to predict long-term clinical benefit in patients with MM. The EVIDENCE meta-analysis was designed based on the FDA guidance for considerations for a meta-analysis of MRD as a clinical end point and potential basis for accelerated approval,18 and it assessed the prognostic value of bone marrow MRD-negativity and prediction of the treatment effects for PFS and OS in clinical trials of patients with MM. The results support consideration of MRD as an early clinical end point reasonably likely to predict clinical benefit in MM that may be used to support accelerated approval and thereby expedite approval and adoption of novel therapeutic agents and advantageous therapeutic regimens for the treatment of patients with MM.
Acknowledgments
O.L. is supported by the Sylvester Comprehensive Cancer Center National Cancer Institute (NCI) Core Grant (P30 CA 240139) and the Riney Family Multiple Myeloma Research Program Fund, Tow Foundation, and Myeloma Solutions Fund. Medical writing support was provided by Alex Pilote of Lumanity Communications Inc, which was funded by Janssen Scientific Affairs, LLC. S.M.D. is supported by the MSKCC Comprehensive Cancer Center NCI Core Grant (P30 CA 008748).
Authorship
Contribution: O.L. was involved in conceptualization, methodology, validation, data curation, investigation, resources, visualization, writing, supervision, project administration, and funding acquisition; all authors fully reviewed and commented on all previous versions of the manuscript and approved the final manuscript; T.J.P. was involved in conceptualization, data curation, investigation, methodology, supervision, and writing; T.M. was involved in conceptualization, investigation, methodology, visualization, supervision, and writing; C.H. was involved in conceptualization, methodology, investigation, visualization, writing; O.F.B. was involved in conceptualization, data curation, investigation, writing; A.B.D., H.E., and H.G. were involved in conceptualization, methodology, investigation, visualization, and writing; S.K. contributed to data acquisition and critically revised the manuscript; C.L., U.-H.M., I.M., C.O., J.A.R., M.T., J.R.H., J.M.A., N.W., R.Z., M.S.-S., G.M., and D.K. were involved in conceptualization, methodology, investigation, visualization, and writing; and S.M.D. was responsible for statistical analyses, conceptualization, methodology, investigation, visualization, and writing.
Conflict-of-interest disclosure: O.L. received research funding from National Cancer Institute (NCI)/National Institutes of Health (NIH), United States Food and Drug Administration (FDA), Leukemia & Lymphoma Society, Rising Tide Foundation, Memorial Sloan Kettering Cancer Center, Multiple Myeloma Research Foundation (MMRF), International Myeloma Foundation, Paula and Rodger Riney Foundation, Tow Foundation, Perelman Family Foundation, Myeloma Solutions Fund, Amgen, Celgene, Janssen, Takeda, Glenmark, Seattle Genetics, and Karyopharm; has received honoraria for scientific talks/participated in advisory boards for Adaptive, Amgen, Binding Site, Bristol Myers Squibb (BMS), Celgene, Cellectis, Glenmark, Janssen, Juno, and Pfizer; and served on independent data monitoring committees (IDMC) for international randomized trials by Takeda, Merck, Janssen, and Theradex. T.J.P., T.M., and C.H. are employees of Janssen Research and Development and report stock and other ownership interests in Johnson & Johnson. O.F.B. is an employee of AbbVie and reports stock and other ownership interests in AbbVie. A.B.D. is an employee of Takeda Pharmaceuticals. H.E. has a consulting or advisory role for BMS/Celgene, Janssen, Amgen, Takeda, Sanofi, GlaxoSmithKline (GSK), and Novartis, and reports receiving honoraria from BMS/Celgene, Janssen, Amgen, Takeda, Sanofi, GSK, and Novartis and travel support from BMS/Celgene, Janssen, Amgen, Takeda, Sanofi, GSK, and Novartis. H.G. received grants and/or provision of Investigational Medicinal Products from Amgen, Array Biopharma/Pfizer, BMS/Celgene, Chugai, Dietmar-Hopp-Foundation, Janssen, Johns Hopkins University, Mundipharma GmbH, Sanofi; research support from Amgen, BMS, Celgene, GlycoMimetics Inc, GSK, Heidelberg Pharma, Hoffmann-La Roche, Karyopharm, Janssen, Incyte Corporation, Millenium Pharmaceuticals Inc, Molecular Partners, Merck Sharp and Dohme, MorphoSys AG, Pfizer, Sanofi, Takeda, Novartis; serves on the advisory boards of Adaptive Biotechnology, Amgen, BMS, Janssen, Sanofi; honoraria from Amgen, BMS, Chugai, GSK, Janssen, Novartis, Sanofi, Pfizer; and received support for attending meetings and/or travel Amgen, BMS, GSK, Janssen, Novartis, Sanofi, Pfizer. S.K. has a consulting or advisory role for BMS/Celgene, Janssen, Amgen, Takeda, Sanofi, GSK, and Novartis, reports receiving honoraria from BMS/Celgene, Janssen, Amgen, Takeda, Sanofi, GSK, and Novartis, and reports travel support from BMS/Celgene, Janssen, Amgen, Takeda, Sanofi, GSK, and Novartis. C.L., I.M., C.O., and M.T. are employees of Amgen. J.A.R. is an employee of AbbVie. J.R.H. received research funding from Adaptive Biotechnologies, BioLinRx, Pfizer, Sanofi, GSK, Regeneron, Janssen, and Takeda. J.M.A. is on patient advocacy committees for Pfizer, BMS, Janssen, Takeda Oncology, and Sanofi. D.K. acknowledges research funding from NCI/NIH, FDA, MMRF, DoD-PROMETHEUS (Murtha Cancer Center Research Program), Amgen, Celgene, Janssen, and Karyopharm; has received honoraria for advisory boards and presentations for AlphaSights, Aptitude Health, Arcellx, BMS, Bridger Consulting Group, Curio Science, Karyopharm, MJH Life Sciences, MMRF, Plexus Communications, and Sanofi; and served on IDMC for Aperture Medical Technology and Arcellx. R.Z. is an employee of Sanofi. S.M.D. acknowledges research funding from Janssen and served on IDMC for Miltenyi Biotec. The remaining authors declare no competing financial interests.
Correspondence: Ola Landgren, Division of Myeloma, Department of Medicine, Sylvester Myeloma Research Institute, Sylvester Comprehensive Cancer Center, University of Miami, 1120 NW 14th St, Clinical Research Bldg, Room 650D, Miami, FL 33136; email: col15@miami.edu.
References
Author notes
M.S.-S., G.M., and S.M.D. are joint senior authors.
Data are available on request from the corresponding author, Ola Landgren (col15@miami.edu).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal