Key Points
Ibrutinib added to initial rituximab immunotherapy was not associated with clinically significant response rates in untreated PTLD.
Increasing the proportion of patients with a response to initial immunotherapy, who can avoid cytotoxic chemotherapy, remains a priority.
Visual Abstract
Posttransplant lymphoproliferative disorder (PTLD) is a rare complication of solid organ transplantation, and cytotoxic chemotherapy is associated with treatment-related morbidity and mortality. Current treatment takes a sequential, risk-stratified approach, and patients with low-risk disease after initial immunotherapy can avoid escalation to immunochemotherapy. TIDaL is a prospective, single-arm phase 2 trial investigating the activity and tolerability of ibrutinib combined with risk-stratified therapy for first-line treatment of PTLD. Eligible patients were adults with newly diagnosed CD20+ B-cell PTLD after solid organ transplant and performance status 0 to 2. Initial treatment comprised 49 days of ibrutinib 560 mg once daily, with 4 doses of weekly rituximab. Treatment response on interim scan and baseline International Prognostic Index were used to allocate patients to either a low-risk arm (who continued ibrutinib, alongside 4 further doses of 3-weekly rituximab) or high-risk (escalation to rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone [R-CHOP] immunochemotherapy, with ibrutinib continuing in patients aged <65 years). The primary outcome was complete response on interim scan, achieved by 11 of 38 patients (29%; 95% confidence interval [CI], 15-46). This did not reach the prespecified threshold for clinically significant activity. Secondary outcomes included allocation to the low-risk arm (41% of patients), 2-year progression-free survival (58%; 95% CI, 44-76), and 2-year overall survival (76%; 95% CI, 63-91). Adverse events were mostly hematological, gastrointestinal, and infective. Although TIDaL does not support adding ibrutinib into first-line treatment of PTLD, increasing the proportion of patients who can be treated without cytotoxic chemotherapy remains an important aim of future research. This trial was registered at www.clinicaltrials.gov as #ISRCTN32667607.
Introduction
Posttransplant lymphoproliferative disorder (PTLD) is an uncommon complication of solid organ or allogeneic hematopoietic stem cell transplantation that carries a substantial risk of mortality. Long-term use of immunosuppressants, necessary to prevent transplant rejection, typically leads to unrestrained Epstein-Barr viremia, which drives an initial polyclonal and then later monoclonal B-cell lymphoproliferation. Although PTLD describes a spectrum of lymphoid or plasmacytoid proliferations, the most common manifestation is a monomorphic CD20+ B-cell lymphoma fulfilling the diagnostic criteria for diffuse large B-cell lymphoma.1 There is little prospective evidence to guide the optimal management of PTLD, due to the rarity and heterogeneity of the disease, as well as the complexity of the transplant-recipient patients.
A reduction of immunosuppressive therapy underpins the initial management of PTLD, although this alone is rarely adequate.2,3 Current recommendations mirror the pivotal PTLD-1 trial, which prospectively evaluated sequential treatment with rituximab monotherapy followed by cyclophosphamide, doxorubicin, vincristine, and prednisolone (CHOP) chemotherapy.4 This approach resulted in a high overall response rate (90%) and a median overall survival (OS) of 6.6 years. The subsequent introduction of a response assessment after initial monotherapy allowed for patients to be risk stratified, with low-risk patients receiving consolidation with rituximab alone, whereas high-risk patients were escalated to 4 cycles rituximab-CHOP (R-CHOP) immunochemotherapy.5 This risk-stratified approach allows for a proportion of patients to avoid CHOP chemotherapy, which is associated with a substantial risk of treatment-related morbidity and mortality in the PTLD setting.6 Strategies that improve responses to initial treatment and thereby spare more patients from cytotoxic chemotherapy would be an important advance.
Ibrutinib is a Bruton tyrosine kinase inhibitor and shows significant activity against a range of B-cell lymphoproliferative disorders.7-9 Ibrutinib is also active against non–germinal center diagnostic criteria for diffuse large B-cell lymphoma, which predominates in PTLD.10,11 The TIDaL trial investigated the addition of ibrutinib to rituximab (IR) for first-line treatment of adults with newly diagnosed CD20+ B-cell PTLD, followed by a risk-stratified allocation to IR consolidation (low risk) or IR-CHOP chemotherapy (high risk). By enhancing the efficacy of the first stage of treatment, it is hypothesized that an increased proportion of patients will achieve a good early response, making them eligible for low-risk consolidation, avoiding chemotherapy, and therefore reducing the risk of toxicity but without compromising progression-free survival (PFS).
Methods
Trial design
TIDaL is a prospective, multicenter, single arm, phase 2 trial evaluating the activity and tolerability of incorporating ibrutinib into risk-stratified sequential treatment of newly diagnosed PTLD, following a Simon 2-stage design (ISRCTN32667607 and EudraCT 2015-005454-35). The trial was approved by a UK Research Ethics Committee (16/NE/0279) and by institutional review at participating sites. All patients gave written informed consent for participation. The trial was conducted in accordance with the Declaration of Helsinki. Authors S.M. and A.J. analyzed the trial data, and all authors had access to the primary clinical trial data.
Participants
Adults with newly diagnosed, measurable (>2 cm diameter tumor or bone marrow involvement) B-cell PTLD without central nervous system involvement were eligible for this trial. Histological confirmation of CD20 positivity, with or without Epstein-Barr virus association, was required. Patients whose lymphoma showed Hodgkin-like or plasmacytic morphology were eligible if CD20-positivity was demonstrated, reflecting a lack of established alternative treatment regimens for these rare patient groups. When sufficient material was available, histology was centrally reviewed. Eligible patients had undergone a solid organ (heart, lung, liver, kidney, pancreas, small intestine, or combination thereof) transplant at any time. Upfront reduction of immunosuppression or use of antiviral therapy were permitted, but no other therapy for PTLD (including antibody, chemotherapy, or radiotherapy) was permitted ahead of trial entry. Participants required adequate baseline organ function, and ECOG performance status 0 to 2. Exclusion criteria included concomitant use of strong CYP3A4/5 enzyme inhibitors or inducers, treatment with a vitamin K antagonist within 1 week of trial entry, significant infection including active hepatitis B or HIV, pregnancy, or breastfeeding.
Treatment
See supplemental Figure 1, available on the Blood website, for a trial schema. Patients were initially treated with a 7-week cycle of continuous once-daily oral ibrutinib 560 mg, with 4 doses of rituximab 375 mg/m2 given IV on days 1, 8, 15, and 22 (IR). Response assessment was performed between days 42 and 47 by interim computed tomography (CT) scan, which determined whether patients were treated on the low- or high-risk arm. Patients with symptoms or signs clearly demonstrating a clinical progression during the initial IR treatment were immediately entered into the high-risk arm, and interim CT scan was not mandated in this group.
Low-risk patients comprised all those achieving complete response (CR) on interim assessment, as well as patients achieving partial response (PR) with a baseline International Prognostic Index (IPI) of 0 or 1. This group received 4 further 3-week cycles of continuous ibrutinib 560 mg once daily and rituximab 375 mg/m2 given on day 1 of each cycle.
High-risk patients comprised those achieving PR with a baseline IPI of 2 to 5 and all patients with stable disease or progressive disease (PD). This group went on to receive four 3-week cycles of IR-CHOP chemotherapy: continuous ibrutinib 560 mg once daily; rituximab 375 mg/m2 on day 1; cyclophosphamide 750 mg/m2 IV day 1; doxorubicin 50 mg/m2 IV day 1; vincristine 1.4 mg/m2 (capped at 2 mg) IV day 1; and prednisolone 100 mg orally on days 1 to 5. After evidence of increased toxicity in older patients receiving IR-CHOP,11 the protocol was amended: ibrutinib was not continued in patients aged ≥65 years allocated to the high-risk arm, who instead received R-CHOP.
Supportive treatment with granulocyte colony-stimulating factor was permitted for primary and secondary prophylaxis. Primary prophylaxis against Pneumocystis jirovecii pneumonia was recommended and mandated after a protocol amendment for all patients.
Outcomes
The primary outcome was CR after 6 to 7 weeks of therapy, evaluated by interim CT scan. Patients with clinical progression during the first 7 weeks, who required immediate treatment with IR-CHOP or R-CHOP but did not undergo interim CT scan, were deemed not to have met the primary outcome.
Secondary outcomes comprised the following: response on interim and end-of-treatment scans; OS from trial entry until death from any cause; PFS from trial entry to disease progression or death from any cause; post-IR PFS, which does not include disease progression during the initial 7-week IR treatment as an event; event-free survival from trial entry to treatment discontinuation due to toxicity, disease progression at any time, or death from any cause; entry into the low-risk arm after IR therapy; treatment tolerability quantified using the Common Toxicity Criteria of Adverse Events, version 4; and dose modifications or discontinuations.
Responses were assessed using the Lugano classification.12 Patients were followed up for 2 years.
Sample size and statistical analysis
A Simon 2-stage optimal design13 was used to determine the sample size and success criteria based on unacceptable and desirable response rates. After 7 weeks of IR treatment, a CR rate of ≤25% was considered unacceptable, reflecting the 25% CR rate achieved with rituximab monotherapy,5 whereas CR rate of 40% was considered clinically interesting. With a power of 85% and alpha of 20%, at least 7 of 24 patients achieving CR at interim analysis would indicate the trial should continue to its second stage, and at least 12 of 38 patients with CR at final analysis indicate that the treatment has sufficient activity to warrant further investigation in a phase 3 trial.
In the event of overrecruitment, the first 38 eligible patients starting treatment were included in the primary outcome analysis. All patients were included in secondary outcome analyses. The numbers and proportions of patients achieving overall response (CR + PR) are given; survival outcomes were calculated using the Kaplan-Meier method, and patients without an event were censored at date last seen. Confidence intervals (CIs) for binary outcomes are calculated using the Clopper-Pearson method. Outcomes are also presented by age, <65 years and ≥65 years. Survival outcomes stratified by risk allocation use landmark analysis such that survival times start from the date of risk group allocation. Secondary outcomes and subgroup comparisons are not powered, and statistical analysis is provided solely to help summarize the data. Contrary to the statistical analysis plan, the results presented are of the per-protocol rather than the intention-to-treat population. This population was considered more intuitive because this excludes 3 patients who were recruited to trial but did not commence treatment due to ineligibility and clinician choice.
The trial was approved by a UK Research Ethics Committee and by institutional review at participating sites; all patients gave written informed consent for participation; the trial was conducted in accordance with the Declaration of Helsinki.
Results
Patients
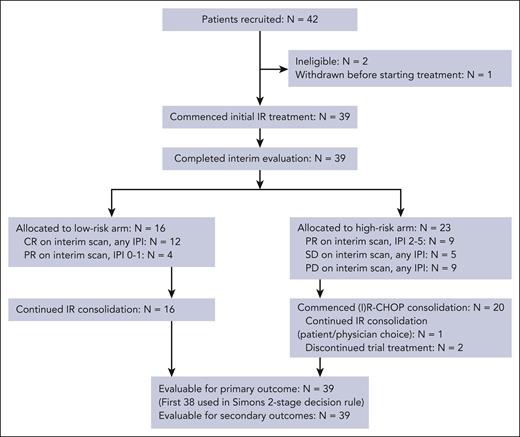
Between April 2017 and March 2020, a total of 42 patients were recruited from 17 sites, of whom 39 started treatment on the TIDaL regimen. Two patients were recruited but found to be ineligible, and 1 withdrew before receiving treatment. The patient flow diagram is shown in Figure 1. Baseline characteristics are shown in Table 1.
Patient flow diagram. The diagram outlines patient recruitment, treatment, and outcome evaluation. SD, stable disease.
Patient flow diagram. The diagram outlines patient recruitment, treatment, and outcome evaluation. SD, stable disease.
Baseline characteristics
| Characteristic . | Overall (n = 39) . |
|---|---|
| Median age, y (range) | 58 (22-75) |
| Sex, n (%) | |
| Male | 22 (56%) |
| Female | 17 (44%) |
| Transplanted organ, n (%) | |
| Kidney | 20 (51%) |
| Liver | 13 (33%) |
| Heart | 4 (10%) |
| Lung | 2 (5%) |
| Median time from transplant until PTLD diagnosis, y (range) | 8 (0.5-31) |
| ECOG performance status, n (%) | |
| 0 | 24 (62%) |
| 1 | 13 (33%) |
| 2 | 2 (5%) |
| Ann Arbor stage, n (%) | |
| I | 6 (15%) |
| II | 9 (23%) |
| III | 4 (10%) |
| IV | 20 (51%) |
| International prognostic index, n (%) | |
| 0 | 2 (5%) |
| 1 | 15 (38%) |
| 2 | 12 (31%) |
| 3 | 6 (15%) |
| 4 | 2 (5%) |
| 5 | 2 (5%) |
| Locally assessed histology, n (%) | |
| Diffuse large B-cell lymphoma | 32 (82%) |
| Burkitt lymphoma | 1 (3%) |
| Classical Hodgkin lymphoma-type PTLD | 1 (3%) |
| Polymorphic PTLD | 1 (3%) |
| Polymorphic PTLD, diffuse large B-cell lymphoma, and classical Hodgkin lymphoma-type PTLD | 1 (3%) |
| Plasmacytic hyperplasia, polymorphic PTLD, and other B-cell neoplasms | 1 (3%) |
| Unknown | 2 (5%) |
| Epstein-Barr virus-associated histology, n (%) | |
| Positive | 13 (33%) |
| Negative | 18 (46%) |
| Unknown | 8 (21%) |
| Prior reduction in immunosuppression, n (%) | |
| Yes | 21 (54%) |
| No | 18 (46%) |
| Characteristic . | Overall (n = 39) . |
|---|---|
| Median age, y (range) | 58 (22-75) |
| Sex, n (%) | |
| Male | 22 (56%) |
| Female | 17 (44%) |
| Transplanted organ, n (%) | |
| Kidney | 20 (51%) |
| Liver | 13 (33%) |
| Heart | 4 (10%) |
| Lung | 2 (5%) |
| Median time from transplant until PTLD diagnosis, y (range) | 8 (0.5-31) |
| ECOG performance status, n (%) | |
| 0 | 24 (62%) |
| 1 | 13 (33%) |
| 2 | 2 (5%) |
| Ann Arbor stage, n (%) | |
| I | 6 (15%) |
| II | 9 (23%) |
| III | 4 (10%) |
| IV | 20 (51%) |
| International prognostic index, n (%) | |
| 0 | 2 (5%) |
| 1 | 15 (38%) |
| 2 | 12 (31%) |
| 3 | 6 (15%) |
| 4 | 2 (5%) |
| 5 | 2 (5%) |
| Locally assessed histology, n (%) | |
| Diffuse large B-cell lymphoma | 32 (82%) |
| Burkitt lymphoma | 1 (3%) |
| Classical Hodgkin lymphoma-type PTLD | 1 (3%) |
| Polymorphic PTLD | 1 (3%) |
| Polymorphic PTLD, diffuse large B-cell lymphoma, and classical Hodgkin lymphoma-type PTLD | 1 (3%) |
| Plasmacytic hyperplasia, polymorphic PTLD, and other B-cell neoplasms | 1 (3%) |
| Unknown | 2 (5%) |
| Epstein-Barr virus-associated histology, n (%) | |
| Positive | 13 (33%) |
| Negative | 18 (46%) |
| Unknown | 8 (21%) |
| Prior reduction in immunosuppression, n (%) | |
| Yes | 21 (54%) |
| No | 18 (46%) |
Disease response and treatment allocation
At the protocol-defined stage 1 analysis of the first 24 treated patients, 7 (29%; 95% CI, 13-51) achieved CR on interim scan after 7 weeks of treatment, and the trial proceeded to stage 2. The primary outcome analysis was based on the first 38 treated patients and showed 11 (29%; 95% CI, 15-46) achieved CR after 7 weeks of initial IR treatment. This did not reach the prespecified threshold for clinically relevant activity, suggesting that further investigation of this treatment strategy is not supported. Despite this conclusion, of all 39 patients, 12 (31%; 95% CI, 17-48) achieved CR.
In addition to the evaluation of CR rate using Simon 2-stage success criteria, Bayesian analysis was performed using a beta-binomial conjugate model with a Beta(1,1) prior distribution. The posterior probability distribution of complete remission rate was plotted (supplemental Figure 2), and posterior probabilities were calculated. With 12 of 39 CRs, the probability of the true CR rate being >40% is only 0.13, whereas the probability that the true CR rate is <25% is 0.18. This reaffirms that the addition of ibrutinib to the risk-stratified sequential treatment of rituximab and R-CHOP has not shown enough activity to support further investigation in a larger trial.
Secondary outcome analysis of all 39 treated patients showed an overall response (CR or PR) on interim scan was achieved by 25 patients (64%; 95% CI, 47-79), comprising 12 CR and 13 PR. After the initial 7 weeks of IR therapy, 16 patients (41%) were allocated to the low-risk arm, comprising all 12 patients with CR and 4 patients with PR and a baseline IPI of 0 to 1. The remaining 23 patients (59%) were allocated to the high-risk arm, including 9 with PR and baseline IPI 2 or higher, 5 with stable disease, and 8 with PD on interim scan. An additional 1 patient was classified as having PD on clinical grounds without radiological confirmation, because rapidly enlarging cervical lymphadenopathy was causing dysphagia, and a delay before treatment escalation could not be justified. Seven patients allocated to the high-risk arm were aged ≥65 years: 3 patients received IR-CHOP before the protocol amendment; 1 patient received IR-CHOP for course 1 and then R-CHOP for the remaining 3 courses; 2 patients commenced R-CHOP without concurrent ibrutinib; and 1 discontinued after the interim assessment. One patient allocated to the high-risk group instead continued with IR therapy, due to patient and physician choice. Two additional patients in the high-risk group did not commence IR-CHOP but instead discontinued trial treatment and withdrew from the trial.
At the end of treatment, 26 of 39 patients (67%; 95% CI, 50-81) achieved an overall response, including 22 with CR (12 of 16 in the low-risk arm and 10 of 23 in the high-risk arm). Thirteen of 16 patients (81%) allocated to the low-risk group achieved an overall response at the end of treatment, compared with 13 of 23 (57%) allocated to the high-risk group.
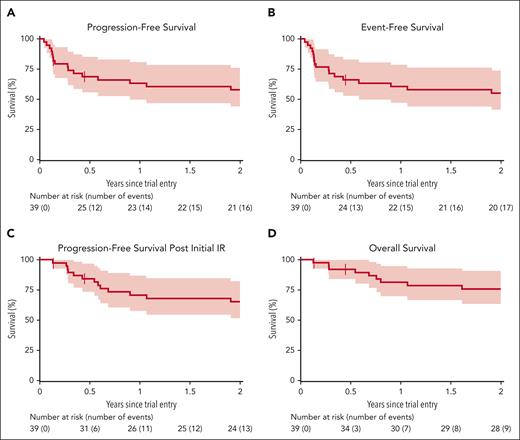
Median follow-up was 2.4 years. Kaplan-Meier plots of the secondary time-to-event outcomes for the whole trial cohort are shown in Figure 2. Estimates for 1-year and 2-year survival are shown in Table 2, which also includes estimates stratified according to whether patients were allocated to the low- or high-risk arm. In all cases, outcomes for patients in the low-risk arm were favorable compared with those allocated to the high-risk arm, although patient numbers are small. Risk-stratified Kaplan-Meier plots are provided in supplemental Figure 3.
Survival outcomes. Survival Kaplan-Meier curves of PFS (A), event-free survival (B), PFS after initial IR therapy (C), and OS (D).
Survival outcomes. Survival Kaplan-Meier curves of PFS (A), event-free survival (B), PFS after initial IR therapy (C), and OS (D).
Survival estimates
| . | Total trial cohort (n = 39) . | Low risk (n = 16) . | High risk (n = 23) . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of events . | 1 y . | 2 y . | No. of events . | 1 y . | 2 y . | No. of events . | 1 y . | 2 y . | |
| PFS | 18 | 63% (50-81) | 58% (44-76) | 6 | 75% (57-100) | 69% (49-96) | 9 | 63% (45-87) | 63% (45-87) |
| PFS post-IR | 15 | 71% (57-87) | 65% (52-82) | — | — | ||||
| EFS | 19 | 61% (47-78) | 55% (41-74) | 7 | 69% (49-96) | 63% (43-91) | 10 | 58% (40-83) | 58% (40-83) |
| OS | 10 | 81% (70-95) | 76% (63-91) | 2 | 88% (73-100) | 88% (73-100) | 8 | 72% (55-94) | 67% (50-90) |
| . | Total trial cohort (n = 39) . | Low risk (n = 16) . | High risk (n = 23) . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of events . | 1 y . | 2 y . | No. of events . | 1 y . | 2 y . | No. of events . | 1 y . | 2 y . | |
| PFS | 18 | 63% (50-81) | 58% (44-76) | 6 | 75% (57-100) | 69% (49-96) | 9 | 63% (45-87) | 63% (45-87) |
| PFS post-IR | 15 | 71% (57-87) | 65% (52-82) | — | — | ||||
| EFS | 19 | 61% (47-78) | 55% (41-74) | 7 | 69% (49-96) | 63% (43-91) | 10 | 58% (40-83) | 58% (40-83) |
| OS | 10 | 81% (70-95) | 76% (63-91) | 2 | 88% (73-100) | 88% (73-100) | 8 | 72% (55-94) | 67% (50-90) |
Data are survival estimate (95% CI).
Risk stratified survival times are calculated from the date of interim assessment.
Treatment tolerability
During initial IR treatment, dose interruptions affected 11 patients (28%), most commonly because of adverse events, after which treatment resumed. One patient (3%) required dose reduction due to interacting concomitant medications. One patient (3%) discontinued initial IR after withdrawing consent. For the 17 patients who proceeded to subsequent IR consolidation, 7 patients (41%) had temporary interruptions in treatment, mostly associated with adverse events or surgical procedures, and none had dose reductions. Two patients (12%) discontinued subsequent IR, because of disease progression (n = 1) and toxicity (n = 1). For the 20 patients who were treated with (I)R-CHOP, 7 patients (35%) had temporary treatment interruptions, and 8 (40%) had dose reductions, the most common reasons for both modifications were adverse event related. Eight patients (40%) discontinued (I)R-CHOP because of the following: death (n = 2); disease progression (n = 2); withdrawal of consent (n = 2); or toxicity (n = 2).
In total, 596 adverse events were reported in all 39 treated patients: 238 (40%) occurred during initial IR therapy, 89 (15%) during subsequent IR, and 268 (45%) during IR-CHOP chemotherapy; and 1 adverse event could not be classified due to missing data. Of the adverse events, 89 were grade ≥3, experienced by 23 patients (59%). Grade ≥3 adverse events are listed in Table 3. Supplemental Table 1 provides further detail on the common and severe adverse events.
Grade 3 or higher adverse events
| Adverse event . | Initial IR . | Subsequent IR . | IR-CHOP . |
|---|---|---|---|
| Blood and lymphatic system disorders | |||
| Anemia | 2 (n = 2 [5%]) | 0 (n = 0) | 6 (n = 4 [20%]) |
| Febrile neutropenia | 0 (n = 0) | 1 (n = 1 [6%]) | 8 (n = 6 [30%]) |
| Cardiac disorders | |||
| Pericardial effusion | 0 (n = 0) | 1 (n = 1 [6%]) | 0 (n = 0) |
| Gastrointestinal disorders | |||
| Abdominal distension | 1 (n = 1 [3%]) | 0 (n = 0) | 1 (n = 1 [5%]) |
| Abdominal pain | 4 (n = 4 [10%]) | 1 (n = 1 [6%]) | 2 (n = 2 [10%]) |
| Diarrhea | 1 (n = 1 [3%]) | 0 (n = 0) | 2 (n = 2 [10%]) |
| Dysphagia | 0 (n = 0) | 0 (n = 0) | 1 (n = 1 [5%]) |
| Nausea | 1 (n = 1 [3%]) | 0 (n = 0) | 0 (n = 0) |
| Other, proximal jejunitis | 0 (n = 0) | 0 (n = 0) | 1 (n = 1 [5%]) |
| Vomiting | 1 (n = 1 [3%]) | 0 (n = 0) | 2 (n = 2 [10%]) |
| General disorders and administration site conditions | |||
| Chills | 1 (n = 1 [3%]) | 0 (n = 0) | 0 (n = 0) |
| Fever | 1 (n = 1 [3%]) | 0 (n = 0) | 0 (n = 0) |
| Other, facial swelling | 1 (n = 1 [3%]) | 0 (n = 0) | 0 (n = 0) |
| Infections and infestations | |||
| Catheter related infection | 1 (n = 1 [3%]) | 0 (n = 0) | 0 (n = 0) |
| Device related infection | 0 (n = 0) | 0 (n = 0) | 1 (n = 1 [5%]) |
| Lung infection | 0 (n = 0) | 0 (n = 0) | 1 (n = 1 [5%]) |
| Nail infection | 0 (n = 0) | 0 (n = 0) | 1 (n = 1 [5%]) |
| Other, chest infection | 1 (n = 1 [3%]) | 0 (n = 0) | 0 (n = 0) |
| Other, neutropenic sepsis | 0 (n = 0) | 0 (n = 0) | 1 (n = 1 [5%]) |
| Other, Pneumocystis carinii pneumonia | 0 (n = 0) | 0 (n = 0) | 1 (n = 1 [5%]) |
| Other, shingles | 0 (n = 0) | 0 (n = 0) | 1 (n = 1 [5%]) |
| Sepsis | 0 (n = 0) | 0 (n = 0) | 4 (n = 2 [10%]) |
| Upper respiratory infection | 0 (n = 0) | 0 (n = 0) | 1 (n = 1 [5%]) |
| Urinary tract infection | 0 (n = 0) | 0 (n = 0) | 1 (n = 1 [5%]) |
| Injury, poisoning, and procedural complications | |||
| Hip fracture | 0 (n = 0) | 1 (n = 1 [6%]) | 0 (n = 0) |
| Investigations | |||
| Neutrophil count decreased | 0 (n = 0) | 3 (n = 2 [12%]) | 8 (n = 5 [25%]) |
| Other, neutropenic sepsis | 0 (n = 0) | 0 (n = 0) | 1 (n = 1 [5%]) |
| Other, raised amylase | 1 (n = 1 [3%]) | 0 (n = 0) | 0 (n = 0) |
| Metabolism and nutrition disorders | |||
| Acidosis | 0 (n = 0) | 0 (n = 0) | 1 (n = 1 [5%]) |
| Hyperglycemia | 1 (n = 1 [3%]) | 0 (n = 0) | 0 (n = 0) |
| Hypokalemia | 0 (n = 0) | 1 (n = 1 [6%]) | 0 (n = 0) |
| Hypomagnesemia | 0 (n = 0) | 0 (n = 0) | 1 (n = 1 [5%]) |
| Hypophosphatemia | 0 (n = 0) | 0 (n = 0) | 2 (n = 2 [10%]) |
| Tumor lysis syndrome | 0 (n = 0) | 0 (n = 0) | 1 (n = 1 [5%]) |
| Musculoskeletal and connective tissue disorders | |||
| Other, proximal myopathy secondary to steroids | 0 (n = 0) | 0 (n = 0) | 1 (n = 1 [5%]) |
| Pain in extremity | 0 (n = 0) | 1 (n = 1 [6%]) | 0 (n = 0) |
| Nervous system disorders | |||
| Ischemia, cerebrovascular | 0 (n = 0) | 0 (n = 0) | 1 (n = 1 [5%]) |
| Peripheral sensory neuropathy | 0 (n = 0) | 0 (n = 0) | 3 (n = 3 [15%]) |
| Somnolence | 0 (n = 0) | 0 (n = 0) | 1 (n = 1 [5%]) |
| Syncope | 1 (n = 1 [3%]) | 0 (n = 0) | 0 (n = 0) |
| Psychiatric disorders | |||
| Confusion | 0 (n = 0) | 0 (n = 0) | 1 (n = 1 [5%]) |
| Renal and urinary disorders | |||
| Acute kidney injury | 0 (n = 0) | 0 (n = 0) | 1 (n = 1 [5%]) |
| Respiratory, thoracic and mediastinal disorders | |||
| Dyspnea | 1 (n = 1 [3%]) | 0 (n = 0) | 1 (n = 1 [5%]) |
| Skin and subcutaneous tissue disorders | |||
| Other, erythematous rash | 1 (n = 1 [3%]) | 0 (n = 0) | 0 (n = 0) |
| Vascular disorders | |||
| Hypotension | 0 (n = 0) | 0 (n = 0) | 2 (n = 2 [10%]) |
| Adverse event . | Initial IR . | Subsequent IR . | IR-CHOP . |
|---|---|---|---|
| Blood and lymphatic system disorders | |||
| Anemia | 2 (n = 2 [5%]) | 0 (n = 0) | 6 (n = 4 [20%]) |
| Febrile neutropenia | 0 (n = 0) | 1 (n = 1 [6%]) | 8 (n = 6 [30%]) |
| Cardiac disorders | |||
| Pericardial effusion | 0 (n = 0) | 1 (n = 1 [6%]) | 0 (n = 0) |
| Gastrointestinal disorders | |||
| Abdominal distension | 1 (n = 1 [3%]) | 0 (n = 0) | 1 (n = 1 [5%]) |
| Abdominal pain | 4 (n = 4 [10%]) | 1 (n = 1 [6%]) | 2 (n = 2 [10%]) |
| Diarrhea | 1 (n = 1 [3%]) | 0 (n = 0) | 2 (n = 2 [10%]) |
| Dysphagia | 0 (n = 0) | 0 (n = 0) | 1 (n = 1 [5%]) |
| Nausea | 1 (n = 1 [3%]) | 0 (n = 0) | 0 (n = 0) |
| Other, proximal jejunitis | 0 (n = 0) | 0 (n = 0) | 1 (n = 1 [5%]) |
| Vomiting | 1 (n = 1 [3%]) | 0 (n = 0) | 2 (n = 2 [10%]) |
| General disorders and administration site conditions | |||
| Chills | 1 (n = 1 [3%]) | 0 (n = 0) | 0 (n = 0) |
| Fever | 1 (n = 1 [3%]) | 0 (n = 0) | 0 (n = 0) |
| Other, facial swelling | 1 (n = 1 [3%]) | 0 (n = 0) | 0 (n = 0) |
| Infections and infestations | |||
| Catheter related infection | 1 (n = 1 [3%]) | 0 (n = 0) | 0 (n = 0) |
| Device related infection | 0 (n = 0) | 0 (n = 0) | 1 (n = 1 [5%]) |
| Lung infection | 0 (n = 0) | 0 (n = 0) | 1 (n = 1 [5%]) |
| Nail infection | 0 (n = 0) | 0 (n = 0) | 1 (n = 1 [5%]) |
| Other, chest infection | 1 (n = 1 [3%]) | 0 (n = 0) | 0 (n = 0) |
| Other, neutropenic sepsis | 0 (n = 0) | 0 (n = 0) | 1 (n = 1 [5%]) |
| Other, Pneumocystis carinii pneumonia | 0 (n = 0) | 0 (n = 0) | 1 (n = 1 [5%]) |
| Other, shingles | 0 (n = 0) | 0 (n = 0) | 1 (n = 1 [5%]) |
| Sepsis | 0 (n = 0) | 0 (n = 0) | 4 (n = 2 [10%]) |
| Upper respiratory infection | 0 (n = 0) | 0 (n = 0) | 1 (n = 1 [5%]) |
| Urinary tract infection | 0 (n = 0) | 0 (n = 0) | 1 (n = 1 [5%]) |
| Injury, poisoning, and procedural complications | |||
| Hip fracture | 0 (n = 0) | 1 (n = 1 [6%]) | 0 (n = 0) |
| Investigations | |||
| Neutrophil count decreased | 0 (n = 0) | 3 (n = 2 [12%]) | 8 (n = 5 [25%]) |
| Other, neutropenic sepsis | 0 (n = 0) | 0 (n = 0) | 1 (n = 1 [5%]) |
| Other, raised amylase | 1 (n = 1 [3%]) | 0 (n = 0) | 0 (n = 0) |
| Metabolism and nutrition disorders | |||
| Acidosis | 0 (n = 0) | 0 (n = 0) | 1 (n = 1 [5%]) |
| Hyperglycemia | 1 (n = 1 [3%]) | 0 (n = 0) | 0 (n = 0) |
| Hypokalemia | 0 (n = 0) | 1 (n = 1 [6%]) | 0 (n = 0) |
| Hypomagnesemia | 0 (n = 0) | 0 (n = 0) | 1 (n = 1 [5%]) |
| Hypophosphatemia | 0 (n = 0) | 0 (n = 0) | 2 (n = 2 [10%]) |
| Tumor lysis syndrome | 0 (n = 0) | 0 (n = 0) | 1 (n = 1 [5%]) |
| Musculoskeletal and connective tissue disorders | |||
| Other, proximal myopathy secondary to steroids | 0 (n = 0) | 0 (n = 0) | 1 (n = 1 [5%]) |
| Pain in extremity | 0 (n = 0) | 1 (n = 1 [6%]) | 0 (n = 0) |
| Nervous system disorders | |||
| Ischemia, cerebrovascular | 0 (n = 0) | 0 (n = 0) | 1 (n = 1 [5%]) |
| Peripheral sensory neuropathy | 0 (n = 0) | 0 (n = 0) | 3 (n = 3 [15%]) |
| Somnolence | 0 (n = 0) | 0 (n = 0) | 1 (n = 1 [5%]) |
| Syncope | 1 (n = 1 [3%]) | 0 (n = 0) | 0 (n = 0) |
| Psychiatric disorders | |||
| Confusion | 0 (n = 0) | 0 (n = 0) | 1 (n = 1 [5%]) |
| Renal and urinary disorders | |||
| Acute kidney injury | 0 (n = 0) | 0 (n = 0) | 1 (n = 1 [5%]) |
| Respiratory, thoracic and mediastinal disorders | |||
| Dyspnea | 1 (n = 1 [3%]) | 0 (n = 0) | 1 (n = 1 [5%]) |
| Skin and subcutaneous tissue disorders | |||
| Other, erythematous rash | 1 (n = 1 [3%]) | 0 (n = 0) | 0 (n = 0) |
| Vascular disorders | |||
| Hypotension | 0 (n = 0) | 0 (n = 0) | 2 (n = 2 [10%]) |
Data are number of occurrences at grade ≥3. The number (n) and percent of patients affected are shown for each event. Safety populations include initial IR (n = 39), subsequent IR (n = 17), and IR-CHOP (n = 20).
Of the 55 serious adverse events experienced by 23 patients (59%), 16 (29%) occurred during initial IR therapy, 5 (9%) during subsequent IR, and 34 (62%) during IR-CHOP. Common serious adverse events were gastrointestinal issues (n = 17 [31%]), blood and lymphatic disorders (n = 10 [18%]), and infections (n = 9 [16%]).
There were 10 patient deaths among those who commenced treatment, of which 2 were treatment related, both during IR-CHOP: 1 due to neutropenia (resulting in sepsis) and 1 Pneumocystis pneumonia, the latter before anti-Pneumocystis prophylaxis was mandated. Five further deaths were due to lymphoma, and the remaining 3 were not cancer related: 1 due to stroke, 1 due to bowel obstruction, and 1 pulmonary thromboembolism. Two deaths due to PTLD occurred in the low-risk group; 1 late relapse after completing subsequent IR, and 1 relapse from CR during subsequent IR treatment. The other deaths were in the high-risk group.
Discussion
This study showed that adding ibrutinib to IV rituximab was feasible in the setting of first-line treatment of PTLD, with no signal for excess toxicity. However, addition of ibrutinib did not result in a sufficiently high CR rate on interim scan to warrant further investigation of this treatment strategy. Although the study was only 1 response off from reaching the prespecified threshold for acceptable activity according to Simon 2-stage design,13 the overall CR rate of 12 of 39 (31%) is short of the target rate of 40% and not discernibly different from the 25% CR rate seen with rituximab monotherapy in the PTLD-1 study.5 Although the TIDaL trial outcomes cannot be directly compared with those from the PTLD-1 trial, the baseline characteristics of the 2 groups were similar; TIDaL with fewer thoracic solid organ recipients and trends toward lower IPI and performance status validating the use of PTLD-1 as the historical comparator. Similarly, the overall response rate at the end of sequential treatment and OS of the whole TIDaL cohort are not overtly different from that of patients treated without ibrutinib. Data from the PTLD-2 trial (NCT02042391), which did not incorporate ibrutinib into risk-stratified treatment, showed comparable 2-year PFS (56%) and OS (68%), although further interpretation is limited between these trials that recruited separately from different populations.14
In the TIDaL study, 41% of patients were allocated to the low-risk arm, which included patients with PR and a baseline IPI of 0 to 1. The cutoff of IPI 1 for low risk is relatively conservative but reflects the original definition of “low-risk” IPI.15 At the time, the TIDaL protocol was under development, and evidence was emerging to suggest patients with IPI 2 could also be described as low risk and may be able to avoid escalation to immunochemotherapy, however, this was yet to be tested in an interventional setting.16,17 Our approach is similar to that of the PTLD-2 trial (NCT02042391), which did additionally consider patients with PR on interim scan and baseline IPI of 2 to be low risk. The baseline characteristics of this cohort align with those of the earlier PTLD-1 trial and are comparable with the TIDaL trial. In the recently completed trial, 21 of 58 patients (36%) in PTLD-2 were allocated to low risk after initial rituximab monotherapy.14 Using the PTLD-2 risk-stratification criteria, an additional 5 patients (total 21/39 [54%]) in the TIDaL cohort would have been considered low risk, however, the TIDaL design limits further interpretation of these data. Risk stratification is an attractive (and now recommended) strategy,18 because it spares patients with the lowest-risk disease from unnecessary treatment with cytotoxic chemotherapy. The favorable outcomes seen among low-risk patients treated solely with IR underscores the importance of both remission induction during initial treatment, together with the accurate identification of low-risk patients who would otherwise be overtreated with chemotherapy.
The rationale underpinning the stratified sequential treatment approach is that a proportion of patients can be treated without cytotoxic chemotherapy. This depends on both the successful induction of remission with initial treatment and the accurate ascription of disease risk at interim assessment. Alternative agents to consider incorporating into initial treatment could include a later-generation Bruton tyrosine kinase inhibitor (with a more favorable side effect profile), newer B-cell–targeting monoclonal antibodies (eg, polatuzumab and tafasitamab), or a novel mechanism for engaging immune effectors (eg, with bispecific antibodies). Increasing the reliability of interim assessment could be explored through imaging, such as advanced positron emission tomography (PET)/CT techniques.19 Circulating tumor DNA technology is emerging as a powerful tool for monitoring patients with more common B-cell lymphomas.20 However, its application during the treatment of PTLD has not been well described.
As expected in a population of patients with significant comorbidities, immunochemotherapy was associated with considerable risk of toxicity, with infective complications common. Both treatment-related deaths were due to infection, 1 necessitating a protocol amendment to mandate the use of Pneumocystis prophylaxis. The treatment-related deaths in this trial were at a rate similar to that seen with risk-stratified R-CHOP consolidation5,14; there is no conspicuous increase in toxicity seen with the addition of ibrutinib to conventional treatment.
One potential criticism of TIDaL is its rule-based statistical design, which categorically determines whether the primary end point has been met according to a prespecified and inflexible response rate. Overrecruitment meant more patients were evaluable for secondary outcomes than the primary response outcome, and had the 12 interim CRs been observed in the first-recruited 38 patients, the trial would have concluded that the TIDaL regimen was deserving of further evaluation in a phase 3 trial. Bayesian approaches are being increasingly adopted in place of frequentist trial designs, in which conclusions are informed by prior understanding and the newly acquired data. This can allow for more clinically relevant interpretation of data, without necessarily changing the overall conclusion of a trial.21 In TIDaL, post hoc analysis using Bayesian methods showed no clinically meaningful response rate whichever population was considered, and the likelihood of a true response >40% remained low.
Compared with current practice, 1 weakness of the TIDaL trial is the reliance on CT imaging for interim assessment, whereas PET/CT has been largely adopted for this purpose. However, at the time of trial design, the role of interim PET had not been defined in the PTLD setting. Moreover, CT was favored to more closely reflect the PTLD-1 trial, which was also used to inform the predefined thresholds for clinically interesting response rate.
In this prospective trial of risk-stratified sequential treatment, initial IR did not show sufficient activity to suggest an improvement on rituximab monotherapy, and further investigation of this combination is not supported. Survival outcomes were similar to those reported previously,5 using sequential rituximab and R-CHOP in a risk-stratified manner. The significant risk of toxicity with CHOP chemotherapy, including treatment-related mortality,6 means alternative treatment options are urgently needed. Therefore, the incorporation of novel agents in the management of PTLD merits further investigation to reduce treatment-related toxicity and improve survival.
Acknowledgments
The authors thank Bindu Vydianath and Chris Bacon, who performed the central histopathology review. The authors also thank Richard Szydlo, Nick Morley, Christopher Fox, and Simon Rule for their support as the Trial Steering Committee and all members of the Trial Management Group.
This work is supported by Bloodwise (now Blood Cancer UK), award number 15044, and Janssen Pharmaceuticals. Janssen also provided Ibrutinib to participating sites free of charge. Staff at the CRCTU are supported by a core funding grant from Cancer Research UK (C22436/A25354 and C22436/A28028).
Authorship
Contribution: T.M., S.C., and A.L. were responsible for study conception, design, and protocol development; S.M., A.J., and K.W. were responsible for study design, statistical plan development, and data analysis; G.M. was involved in data analysis and manuscript preparation; R.B. and S.J. were responsible for trial management, protocol development and regulatory approvals; and T.M., S.C., E.K., S.K., K.C., D.W., A.A., C.P.F., R.J., P.M., S.P., C.R., C.B., G.P.C., A.D., J.W., and S.B. were involved in patient recruitment and data collection.
Conflict-of-interest disclosure: S.C. reports research grants from Janssen and Kite-Gilead; consulting fees from Takeda, Kite-Gilead, Roche, Atara Bio, Orion Pharma, Adicet Bio, Incyte, AbbVie, Novartis, Pierre Fabre, Miltenyi Bio, and Bristol Myers Squibb (BMS)-Celgene; honoraria from Takeda, Kite-Gilead, Incyte, AbbVie, and Pierre Fabre; and travel support from Takeda, Kite-Gilead, AbbVie, and Pierre Fabre. K.C. reports consulting fees from Roche, Takeda, BMS, Atara, KITE, Incyte, and AbbVie; honoraria from Roche, Takeda, KITE, and Incyte; and travel support from Roche, Takeda, BMS, and KITE. A.A. reports travel support from Kyowa Kirin, and AbbVie; and advisory board fees from Kyowa Kirin. P.M. reports consulting fees from Roche and Janssen; honoraria from Janssen; travel support from Janssen; and advisory board participation for Roche and Janssen. S.P. reports honoraria from Gilead, AstraZeneca, AbbVie, Beigene, Takeda, and Janssen. G.P.C. reports research grants from BeiGene, BMS, and AstraZeneca; consulting fees from Roche, Takeda, AstraZeneca, Secura Bio, Incyte, Sobi, Beigene, and AbbVie; honoraria from Takeda, Roche, Incyte, Kite, and AbbVie; travel support from Roche and Takeda; and advisory board fee from BMS. A.D. reports research grants from Roche, MSD, and AstraZeneca; consulting fees from Roche, AbbVie, Genmab, Kite, and Sobi; honoraria from Roche and AstraZeneca; travel support from Roche; and being the Chair of the UK National Cancer Research Institute Lymphoma Group. T.M. reports research grants from Janssen, AstraZeneca, and Novartis; honoraria for advisory board meetings from Kite/Gilead, Amgen, Novartis, Pfizer, Celgene/BMS, Daiichi Sankyo, Atara, Roche, and Janssen; honoraria for lectures from Kite/Gilead, Takeda, Janssen, Roche, Servier, Novartis, Celgene/BMS, Pfizer, and Incyte; and travel grants from Amgen, Jazz, Pfizer, Bayer, Kyowa Kirin, Celgene/BMS, Kite/Gilead, Janssen, and Takeda. The remaining authors declare no competing financial interests.
Correspondence: Tobias Menne, Northern Centre for Cancer Care, Freeman Hospital, Newcastle upon Tyne Hospitals NHS Foundation Trust, Freeman Rd, Newcastle upon Tyne, NE7 7DN, United Kingdom; email: tobiasmenne@nhs.net.
References
Author notes
Presented in abstract form at the 63rd annual meeting of the American Society of Hematology, 11-14 December 2021, Atlanta, GA.
Data requests should be made to the Trial Management Group via the corresponding author, Tobias Menne (tobiasmenne@nhs.net).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal