In this issue of Blood,1Chaganti et al present the results of a phase 2 study (TIDaL) combining ibrutinib with risk-stratified therapy as first-line treatment of CD20+ posttransplant lymphoproliferations (PTLDs). The goal of the study was to increase the complete remission (CR) rate after initial therapy to at least 40% using a 7-week cycle of continuous once-daily oral ibrutinib, 560 mg, with 4 doses of weekly rituximab (IR).
Low-risk patients included all those achieving CR on interim assessment, as well as patients achieving partial response (PR) with a baseline international prognostic index (IPI) score of 0 or 1. This group received 4 further 3-week cycles of continuous ibrutinib and rituximab. High-risk patients comprised those achieving PR with a baseline IPI score of 2 to 5, and all patients with stable disease or progressive disease. This group received four 3-week cycles of IR-CHOP (cyclophosphamide, doxorubicin hydrochloride, vincristine [Oncovin], and prednisone) chemotherapy. The primary outcome was complete response on interim scan after IR, achieved by 11 of 38 patients (29%). This level did not reach the prespecified threshold of 40% for clinically significant activity. Secondary outcomes in the study included allocation to the low-risk arm (41% of patients), 2-year progression-free survival (PFS) (58%), and 2-year overall survival (76%). Adverse events were mostly hematological, gastrointestinal, and infection. The trial results do not support adding ibrutinib into first-line treatment of PTLD.
In its latest classification,2 the World Health Organization places lymphoproliferative disorders of immunocompromised patients, no matter the cause of the immune suppression, in the same category. Although this grouping seems logical to the pathologist, this is not the case for clinicians. In patients living with HIV, treatment focuses on HIV therapy to maximally improve immunity, with standard therapy given to immunocompetent patients. For PTLD, the level of immune competency must be balanced to avoid the risk of rejection and therapy adapted to a posttransplant population. After solid organ transplantation, the standard first-line treatment for systemic CD20+ PTLD is that proposed by the PTLD-1 study.3 After a reduction of the immunosuppression, the first phase includes 4 weekly injections of rituximab with subsequent treatment based on the initial response: 4 more doses of rituximab every 3 weeks in patients in CR, otherwise 4 cycles of RCHOP21. In the PTLD-1 study, 25% of patients achieved CR after the initial phase, and median overall survival was 6.6 years. In the PTLD-2 study,4 3 modifications were made to the schedule: (1) rituximab was given subcutaneously, (2) patients with IPI <3 and with PR after 4 rituximab doses received the same treatment as the patients in CR, and (3) patients with progression and those who underwent transplant with a thoracic organ who did not obtain CR are treated with alternating R-CHOP and rituximab–dexamethasone–high-dose cytarabine–oxaliplatin (R-DHAOx). The results are satisfactory, but the CR rate was only 9%. The benefit of the subcutaneous form of rituximab is questionable. The expansion of the low-risk group by including patients with PR and IPI <3 increases the number of chemotherapy-free patients compared with the PTLD-1 study, keeping the same response rate. In the current study, ibrutinib did not add any benefit, similar to the Phoenix study in immunocompetent patients, when given in combination with R-CHOP.5 It is possible that, as in immunocompetent patients, the addition of ibrutinib to R-CHOP is of benefit in those patients with coexpression of BCL2 and MYC.6 The TIDaL study is, however, not completely negative; it partly confirms the finding from the PTLD-2 study that it is possible to avoid chemotherapy for patients with an IPI score of 0 or 1 with PR after the 4 initial rituximab doses but cannot address the inclusion of IPI 2 in PR like in the PTLD-2 study. The results of the PTLD-1, PTLD-2, and TIDaL studies are based on an evaluation by computed tomography (CT) scan and not positron emission tomography (PET)–CT. In a retrospective real-life study, Morland et al7 showed a CR rate of 30% and overall response (OR) of 61%, by PET-CT after 4 cycles of rituximab, compared with 45% OR for PTLD-2 and 64% for TIDaL. These similar results increase the interest in further investigating the IPI stratification to adapt the treatment.
In second-line therapy, new therapeutic options are appearing. Tabelecleucel is an Epstein-Barr virus (EBV)–specific T-cell immunotherapy, composed of nongenetically modified anti-EBV T lymphocytes harvested from human donors, that showed an objective response rate of 51%,8 and a 70% OR for central nervous system (CNS) disease. Tabelecleucel obtained approval in Europe at the end of 2022 as monotherapy for treatment of adult and pediatric patients with EBV+ relapse/refractory PTLD. When tabelecleucel is not available or not indicated (EBV-negative PTLD), brentuximab vedotin appears particularly attractive in the CD30+ PTLD setting.9
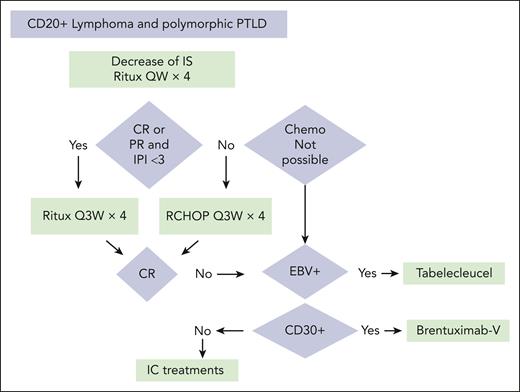
For other situations, if no study is available, treatments classically recommended in immunocompetent patients can be offered. In view of these studies, it is possible to propose a treatment algorithm for CD20+ PTLD, as presented (see figure).
Treatment algorithm for CD20+ PTLD. Chemo, chemotherapy; IC treatments, recommended treatments for immunocompetent patients; IS, immunosuppression; QW, once a week; Q3W, every 3 weeks; RCHOP, rituximab–cyclophosphamide–doxorubicin hydrochloride–vincristine–prednisone; Ritux, rituximab; V, vedotin.
Treatment algorithm for CD20+ PTLD. Chemo, chemotherapy; IC treatments, recommended treatments for immunocompetent patients; IS, immunosuppression; QW, once a week; Q3W, every 3 weeks; RCHOP, rituximab–cyclophosphamide–doxorubicin hydrochloride–vincristine–prednisone; Ritux, rituximab; V, vedotin.
For PTLD after allogeneic stem cell transplants, first-line treatment includes the 4 initial cycles of rituximab. Chemotherapy is often impossible because of its toxicity in this setting. Tabelecleucel or brentuximab vedotin may be valuable treatment options in second-line treatment.
Clinical studies like the one presented in this issue are essential to make tangible progress in the management of PTLD. Future front-line studies will likely test combining tabelecleucel with initial rituximab if the PTLD is EBV+ or brentuximab vedotin if it is CD30+, to further reduce the use of chemotherapy and potentially increase the response rate and PFS. In relapse, promising studies are underway to put EBV+ PTLDs into the lytic phase to make them sensitive to antivirals. Finally, specific forms of PTLD (CNS, CD20 negative) need to be considered separately in light of their guarded prognosis and their usual exclusion from studies in the field. The high rate of tolerance of ibrutinib in the TIDaL study suggests that testing ibrutinib for immunocompromised patients with CNS disease is feasible.
Conflict-of-interest disclosure: S.C. served on the scientific advisory board for Roche, Sandoz, Takeda, Atara, and Pierre Fabre.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal