In this issue of Blood, Young et al reveal that a reduction in growth signals from mesenchymal stromal cells (MSCs) to hematopoietic stem cells (HSCs) underlies impaired lymphoid differentiation and increased transcriptional heterogeneity of HSCs in aging mice.1
Aging-related changes in HSCs include impaired lymphoid differentiation relative to myeloid lineages (known as myeloid skewing2), and an increase in cell-to-cell heterogeneity in gene expression.3 It remains a long-standing, open question to what extend these phenotypes are primarily caused by intrinsic changes in aging HSCs or by other changes in the aging organism that induce changes in HSCs.4 The latter could be mediated either by niche cells (neighboring cells that control HSC function) or by systemically acting factors in the blood (see figure).
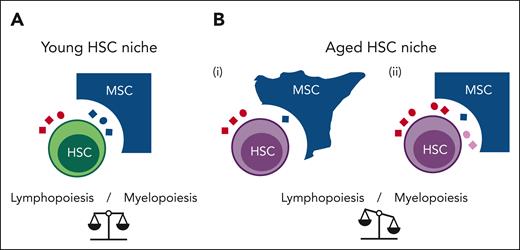
Age-related impairment in cell-cell communication in the HSC niche correlates with a decrease in lymphoid differentiation of HSCs. (A) In the young HSC niche, HSCs receive signals from MSCs (blue ligands) and from the blood (red ligands). (B) In the aged HSC niche, HSC-MSC ligand-receptor interactions are impaired. These impairments may be induced by: (i) cell intrinsic changes in MSCs (aged cell morphology), leading to reduced secretion of MSC-derived ligands (blue ligands); or (ii) changes in signaling factors that enter the niche from the blood (red ligands) and/or by retrograde signaling from the aged HSCs (pink ligands), thereby perturbing signaling from MSC-derived ligands to HSCs.
Age-related impairment in cell-cell communication in the HSC niche correlates with a decrease in lymphoid differentiation of HSCs. (A) In the young HSC niche, HSCs receive signals from MSCs (blue ligands) and from the blood (red ligands). (B) In the aged HSC niche, HSC-MSC ligand-receptor interactions are impaired. These impairments may be induced by: (i) cell intrinsic changes in MSCs (aged cell morphology), leading to reduced secretion of MSC-derived ligands (blue ligands); or (ii) changes in signaling factors that enter the niche from the blood (red ligands) and/or by retrograde signaling from the aged HSCs (pink ligands), thereby perturbing signaling from MSC-derived ligands to HSCs.
Young and colleagues take strides toward answering this question by conducting a single-cell analysis of age-related changes in the bone marrow. Crucially, they analyze both bone marrow–derived hematopoietic stem and progenitor cells and various nonhematopoietic cell types comprising the HSC niche (MSCs, differentiated blood cells, endothelial cells, and osteoclasts) from the same individual young or middle-aged mice. The authors interrogate these data to identify age-related changes in interactions between HSCs and niche cells. HSCs show the most age-related changes in gene expression among all investigated hematopoietic cell types. The gene expression changes at this early phase of HSC aging already indicate a significant decline in lymphoid differentiation—a marker of advanced HSC aging. Cell-cell communication analysis reveals that HSCs are most strongly connected to MSCs via receptor-ligand interactions. Of note, these interactions markedly decline with age, especially those mediated by the insulin-like growth factor-1 (IGF-1) and KIT-ligand (also known as stem cell factor or steel factor) signaling pathways. Using genetic approaches, the authors show that deletion of Igf1 specifically in MSCs of young mice results in a decrease in lymphoid output and expansion of HSCs in the bone marrow. This provides genetic evidence that the Igf1 knockout in MSCs phenocopies a reduction of lymphoid differentiation capacity that is observed in HSCs of aging mice.
The study by Young et al is of general interest for HSC biology and organismal aging. First, it provides direct evidence that receptor-ligand interactions can induce phenotypes of HSC aging—an important piece of information in the debate on environment vs cell-intrinsic signaling. Based on single-cell association studies, alterations in HSC-niche interactions could represent a therapeutic target to ameliorate HSC aging and its consequences, that is, the declines in immune function and increased disease risk that come with age. Vice versa, an aged niche may limit therapeutic approaches that aim to rejuvenate the HSC pool by transplantation of young HSCs or by selective depletion of subpopulations of aged HSCs, such as myeloid-biased HSCs. The latter have recently been shown to lead to improved immune functions in aged mice.5 It remains to be seen whether age-related cell-cell communication changes in the HSC niche—as observed by Young and colleagues—would compromise the duration of such therapeutic effects. It is also quite possible that age-related changes in cell-cell communication between HSCs and niche cells are bidirectional, that is, also involving signals sent from HSCs to MSCs. In that case, the transplantation or selection of young HSCs could even rejuvenate the aged niche. An intriguing next step will be to use similar computational approaches as in the current work to determine the consequences of ablating aged, myeloid-biased HSCs on the most prominent cell-cell communication interactions in aged mice.
Second, the authors provide the impetus for future investigations into the relative roles of niche-derived vs systemically acting ligands in controlling HSC function (see figure). There are 2 isoforms of KIT-receptor ligands. Here, the systemic form of the ligand (sKIT) is shown to be more important for the control of HSC maintenance and function than the membrane-bound form (mKIT).6 It will be important to delineate how each of these ligands change with age and study the effects of these changes locally in the niche. Similar studies for IGF-1 may also be enlightening: IGF-1 sources are both local and systemic. The main source of IGF-1 in blood serum is the liver. Which has the greater influence on HSC function via growth signaling: the bone marrow niche or the liver? To add to this complexity, reductions in IGF-1 signaling can delay organismal7 and HSC aging.8,9 Reductions in IGF signaling caused by dietary restriction or by genetic ablation of Igf2bp2 lead to an amelioration of the aging-associated selection of myeloid-biased HSCs.8,9 Thus, it is of paramount importance to resolve the ambivalence of IGF signaling in this context: Is it accelerating or decelerating trajectories of HSC aging? It may also be acting differently at different stages of the life cycle.
Third, the authors underscore the need to analyze mechanisms of niche cell aging. Do niche cells age due to cell intrinsic processes or due to changes in signals that come from the aged environment or from aged HSCs?
Last, it is possible that HSC-intrinsic and -extrinsic environmental factors or the niche cooperatively impact HSC aging. Along these lines, IGF-mediated signaling is predicted to activate an HSC-intrinsic circuit of Cdk6/Mecom signaling, which controls the balance of self-renewal and differentiation at the HSC-to-multipotent transition and, crucially, this IGF-induced signaling circuit is attenuated in HSCs of aging mice.10
Together, Young et al provide evidence that the age-related decline in cell-cell communication in the murine HSC niche contributes to the impairment of lymphoid differentiation and to the increased transcriptional heterogeneity of HSCs in middle age. Given the essential roles that HSCs occupy, namely controlling hematopoiesis, immune function, inflammation, and age-associated diseases, these findings spark interest in exploring further mechanisms of HSC aging induced by altered intercellular communication in the HSC niche. It is conceivable that interventions designed to target the age-associated cell-cell communication impairments in the HSC niche could also have positive effects on the organism, delaying organismal aging and potentially mitigating the increased disease risks that come with advanced age.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal