Key Points
Ruxolitinib demonstrated clinically meaningful efficacy in pediatric patients with either treatment-naïve or steroid-refractory aGVHD.
We identified the ruxolitinib dosage across the pediatric age range treated, and no unexpected safety signals were observed in children.
Visual Abstract
In REACH4, a phase 1/2, open-label, single-arm, multicenter study, the pharmacokinetics (PK), efficacy, and safety of ruxolitinib were evaluated in treatment-naïve and steroid-refractory pediatric patients with grade 2 to 4 acute graft-versus-host disease (aGVHD; n = 45). Ruxolitinib dosing was based on age and targeted the exposure in adults receiving 10 mg twice daily; group 1 (aged ≥12 to <18 years) received 10 mg twice daily and preliminary starting doses for groups 2 (aged ≥6 to <12 years) and 3 (aged ≥2 to <6 years) were 5 mg twice daily and 4 mg/m2 twice daily, respectively. The phase 1 primary objective was to assess ruxolitinib PK parameters and define an age-appropriate recommended phase 2 dose (RP2D) for patients aged <12 years. The phase 2 primary objective was to measure the activity of ruxolitinib as assessed by overall response rate (ORR) at day 28; the key secondary objective was to assess the durable ORR at day 56. Ruxolitinib exposure was comparable across age groups; starting doses were confirmed as the RP2D. The median duration of ruxolitinib exposure was 3.8 months (range, 0.3-11.2). ORR in all patients was 84.4% (90% confidence interval [CI], 72.8-92.5) at day 28, with a durable ORR at day 56 of 66.7% (90% CI, 53.4-78.2); high response rates were observed across age groups and in both treatment-naïve and steroid-refractory subgroups. Adverse events were consistent with those expected in patients with aGVHD (anemia, decreased neutrophil and leukocyte count) treated with ruxolitinib. In pediatric patients with aGVHD, ruxolitinib showed clinically meaningful efficacy with no new safety signals. This trial was registered at www.clinicaltrials.gov as #NCT03491215.
Introduction
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a potentially curative treatment for patients with hematologic malignancies, as well as for patients with many nonmalignant hematologic disorders.1 The success of allo-HSCT is hindered by acute graft-versus-host disease (aGVHD), in which donor-derived immune cells cause inflammation, tissue and organ damage, or death.2 aGVHD mainly affects the skin, liver, and upper and lower gastrointestinal (GI) tracts. Symptoms include erythroderma, a maculopapular skin rash, nausea, vomiting, abdominal pain, secretory diarrhea, and jaundice due to cholestasis.3 The incidence of grade 2 to 4 aGVHD varies, affecting 40% to 85% of children with unrelated donors.4
The initial treatment for aGVHD includes systemic corticosteroids and calcineurin inhibitors.5 Unfortunately, one-third of pediatric patients show no response, necessitating second-line therapies.6 Most published studies focus on the management of aGVHD in adults, with assumptions of efficacy extrapolated to pediatric patients.7 Additionally, long-term use of corticosteroid therapy in children with aGVHD can lead to various consequences (eg, Cushing syndrome, infections, hyperglycemia, hypertension, muscle wasting, cutaneous atrophy, alterations in bone mineral density, cataracts, and metabolic syndrome) impairing the normal development of the organs and tissues of a growing body.7
Ruxolitinib, a Janus kinase 1/2 inhibitor, is approved for the treatment of patients aged ≥12 years with aGVHD or chronic GVHD (cGVHD) who have an inadequate response to corticosteroids or other systemic therapies.8,9 In the phase 3 REACH2 study (ClinicalTrials.gov identifier: NCT02913261), ruxolitinib demonstrated superiority vs best available therapy in steroid-refractory patients aged ≥12 years with grade 2 to 4 aGVHD, with a superior overall response rate (ORR) at day 28 and a significantly higher durable ORR at day 56.10 However, no definitive conclusions could be made about potential survival benefits.10 Pediatric data on aGVHD therapies from prospective studies are very limited. Currently, ruxolitinib clinical trials have been conducted in adult cohorts including adolescents. Here, we report the results of a phase 1/2 multicenter study (REACH4) of ruxolitinib added to the immunosuppressive regimen of pediatric patients with grade 2 to 4 aGVHD.
Methods
Study design
REACH4 (ClinicalTrials.gov identifier: NCT03491215) was an open-label, single-arm, phase 1/2 multicenter study aimed at assessing the pharmacokinetics (PK), safety, and efficacy of ruxolitinib in pediatric patients with treatment-naïve or steroid-refractory aGVHD. Eligible patients were aged ≥28 days and <18 years, had undergone allo-HSCT from any donor source, been diagnosed with either treatment-naïve or steroid-refractory (based on institutional criteria or per physician judgement) grade 2 to 4 aGVHD, and had evidence of myeloid engraftment with an absolute neutrophil count of >1000/μL and a platelet count of >20 000/μL. Key exclusion criteria are detailed in the supplemental Methods, available on the Blood website.
Phase 1 (dose finding and safety) and 2 (safety and efficacy) ran concurrently (supplemental Figure 1). The phase 1 primary objective was to determine the recommended phase 2 dose (RP2D) for children aged <12 years. Patients were grouped by age to allow appropriate ruxolitinib dosing (group 1: ≥12 to <18 years; group 2: ≥6 to <12 years; group 3: ≥2 to <6 years; and group 4: ≥28 days to <2 years). Ruxolitinib was given orally as a 5-mg tablet or as a liquid pediatric formulation twice a day at the assigned starting dose for up to 24 weeks or until early discontinuation. Group 1 patients (aged ≥12 to <18 years) received ruxolitinib 10 mg twice daily, the adult dose from the phase 3 REACH 2 study.10 Preliminary ruxolitinib starting doses for patients aged <12 years were derived using physiologically based PK modeling and predicted to yield an exposure equivalent to that of a 10 mg twice daily dose in adults. The provisional ruxolitinib starting doses for groups 2 and 3 were 5 mg twice daily and 4 mg/m2 twice daily, respectively. Phase 2 aimed to measure the efficacy (as assessed by ORR at day 28 [week 4]) and safety of ruxolitinib over a 24-week treatment period.
In addition to ruxolitinib, patients also received methylprednisolone (or equivalent prednisolone) with/without cyclosporine or tacrolimus. Standard supporting therapy (including anti-infective medications and transfusion support) was used according to institutional guidelines. Medications that would be expected to reduce platelet function and adversely affect blood coagulation, and fluconazole at doses of >6 mg/kg (maximum 200 mg) daily, were prohibited. Patients demonstrating a response (partial response [PR] or complete response [CR]) to ruxolitinib treatment at day 28 (week 4), continued ruxolitinib treatment until day 56 (week 8) after which ruxolitinib tapering could commence (supplemental Methods). Ruxolitinib treatment was discontinued in patients without a response at day 28 and these patients entered the long-term follow-up period (from end of treatment up to month 24).
Patient visits occurred weekly until day 56, then 4-weekly until week 24. Those needing prolonged tapering or who experienced an aGVHD flare also had 4-weekly visits up to week 48. A safety follow-up visit occurred 30 days after last ruxolitinib dose, with follow-ups at months 12, 18, and 24 to collect survival, progression, and safety data. Guidance on dose modification was provided in case of adverse events (AEs; supplemental Methods).
The study protocol and all amendments were approved by the independent ethics committee and/or institutional review board for each study center as listed below: (1) Comisse voor medische ethiek, Ghent 9000, Belgium; (2) Comité d'éthique hospitalier, Brussels 1020, Belgium; (3) Nagoya University Hospital institutional review board, Nagoya Aichi 466 8560, Japan; (4) Saitama Children's Medical Center, institutional review board, Saitama 330-8777, Japan; (5) Comité de protection des personnes Sud Ouest et Outre Mer IV, Limoges 87025, France; (6) Comité de Ética de la Investigación con Medicamenteos del Hospital Universitario de La Paz, Madrid 28046, Spain; (7) Comité d'éthique de la recherche du CHU Sainte-Justine, Montreal Quebec H3T 1C5, Canada; (8) Comitato Etico Regionale Della Liguria C/O Irccs Aou San Martino, Genova 16132, Italy; (9) Comitato Etico per la Sperimentazione Clinica dell'Irccs Ospedale Pediatrico Bambin Gesu', Roma 00146, Italy; (10) Seoul National University, Hospital Institutional Review Boards, Seoul Jongno-gu 03080, Republic of Korea; and (11) De Videnskabsetiske Komieer, Hilleroed Region Hovedstaden 2100, Denmark.
Study end points, assessments, and definitions
The phase 1 primary end point was determining the RP2D of ruxolitinib by measuring PK parameters (area under the concentration-time curve [AUC], trough plasma concentration, maximum plasma concentration, and half-life). Predose blood samples were collected on days 1, 7, 14, and 28. Postdose samples were collected at 0.5, 1, 1.5, 2, 4, 6, and 9 hours on day 1, and at 2 hours after dose on days 7, 14, and 28. Plasma samples were assayed for ruxolitinib concentrations using a validated liquid chromatography–tandem mass spectrometry method. PK parameters, safety, and efficacy data were reviewed by an independent committee before confirming the RP2D.
Phase 2 primary end point was the ORR at day 28 (week 4), the proportion of patients with CR or PR compared with baseline organ staging without the use of additional systemic therapy (supplemental Methods). A key secondary end point was the durable ORR at day 56 (week 8; defined as the proportion of patients who had a response at day 28 that was maintained at day 56). Other end points included the ORR at day 14, best overall response (BOR; the proportion of patients with CR or PR at any time point up to and including day 28 and before the start of additional systemic therapy for aGVHD), duration of response, overall survival (OS), failure-free survival (FFS), event-free survival (EFS), incidence of cGVHD, cumulative corticosteroid dosing up to day 56, graft failure, nonrelapse mortality (NRM), and malignancy relapse/progression. Organ-specific response rates were calculated at day 28. Subgroup analysis by baseline aGVHD severity and landmark analysis of time-to-event end points by day 28 responder status were performed.
Safety was assessed by the frequency, duration, and severity of AEs, including monitoring of infections and secondary primary malignancies by means of routine physical examination and laboratory assessments. AEs were assessed and graded according to common terminology criteria for adverse events, version 4.03.
Growth and development
Assessment of growth and sexual maturation were performed throughout the study (supplemental Methods).
Acceptability and palatability
The acceptability and palatability of ruxolitinib, administered as an oral pediatric liquid formulation, were evaluated using a questionnaire completed by parents or caregivers on day 1 and at weeks 4 and 24 (after either morning or evening dose).
Exploratory biomarker analysis
Biomarker analysis was performed as previously described by Socie et al.11
Statistical analysis
Phase 1 required a minimum of 5 patients in each age group (except group 4) with an evaluable PK profile. If the RP2D could not be confirmed in at least 5 patients per group, additional patients could be enrolled until the dose/exposure was confirmed.
The sample size for phase 2 was based on the primary efficacy end point (ORR at day 28). A target of 45 patients (regardless of age) was expected to provide 90% power to yield a 90% confidence interval (CI) for ORR with a lower limit of ≥60%. To represent the study population, at least 20% of patients had to be treatment naïve and 40% had to be steroid refractory.
The full analysis set included all patients assigned to study treatment and who received at least 1 dose of ruxolitinib. The safety analysis set included all patients who received at least 1 dose of ruxolitinib. The PK analysis set included all patients with at least 1 evaluable PK concentration. A concentration was considered evaluable if the patient had received the planned dose of ruxolitinib before collecting a PK sample and had not vomited within 2 hours of ruxolitinib dosing.
Baseline characteristics were presented as frequencies and percentages for categorical data and median, minimum, and maximum for continuous data. PK parameters were calculated by noncompartmental methods using Phoenix WinNonlin (Pharsight, Mountain View, CA) software for patients with extensive sampling. To determine the RP2D, PK data across the groups were compared with those observed in adults and adolescents with aGVHD receiving ruxolitinib 10 mg twice daily in the REACH2 study.10
For the primary and key secondary end points, outcomes were summarized by treatment group with the use of descriptive statistics with a 2-sided exact binomial 90% CI. Patients with missing assessments for the evaluation of the ORR were considered nonresponders. The cumulative incidence for the following events was calculated: duration of response, FFS, NRM, malignancy relapse or progression, and cGVHD. Kaplan-Meier curves for OS and EFS were plotted, and the Kaplan-Meier medians, and 1-, 2-, 6-, 12-, 18- and 24-month survival estimates calculated along with 95% CI.
Biomarker changes were evaluated over time for all patients. Proportional odds logistic regression models assessed the correlation between biomarker levels and efficacy end point response categories (CR, PR, no response/progression) as dependent variables.
Ethics
This study was conducted in accordance with the guidelines for good clinical practice of the International Council for Harmonization, the principles of the Declaration of Helsinki, and local laws and regulations. Before inclusion, patients, parents, or guardians provided written informed consent, and patient assent was obtained if capable. The study protocol and all amendments were approved by the independent ethics committee and/or institutional review board for each study center. A data monitoring committee reviewed phase 1 PK parameters, safety, and efficacy data, and reviewed safety data biannually in phase 2.
Results
Patients
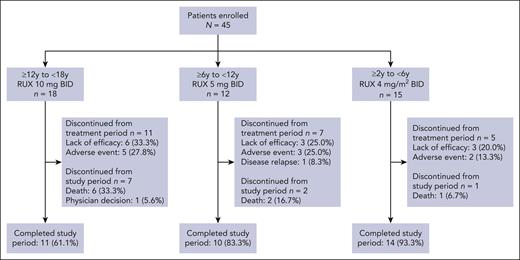
Between 21 February 2019 and 2 February 2023, 45 patients were treated with ruxolitinib at 19 centers across Belgium, Canada, Denmark, France, Italy, Japan, Republic of Korea, and Spain. Patients were treated and analyzed in 3 age groups (Figure 1); no patients were enrolled into group 4.
Patient disposition. Full analysis set. BID, twice daily; RUX, ruxolitinib; y, years.
Patient disposition. Full analysis set. BID, twice daily; RUX, ruxolitinib; y, years.
Overall, 22 (48.9%) patients completed the 24-week treatment period. Of the 23 patients who discontinued ruxolitinib treatment early, 12 (26.7%) patients discontinued because of a lack of efficacy, 10 (22.2%) patients because of an AE, and 1 (2.2%) patient because of disease relapse.
Patient demographics and baseline characteristics by age group are presented in Table 1 and supplemental Table 1. The median time from transplant to the start of study treatment was 29.0 days (range, 13.0-103.0 in the treatment-naïve group and 50.5 days (range, 21.0-509.0) in the steroid-refractory group. At the time of transplant, 15 (33.3%) patients were cytomegalovirus (CMV)-positive and 30 (66.7%) were CMV-negative. Additionally, 21 (46.7%) donors were CMV-positive, and 24 (53.3%) donors were CMV-negative.
Patient demographics and baseline characteristics
| . | Group 1 ≥12 y to <18 y n = 18 . | Group 2 ≥6 y to <12 y n = 12 . | Group 3 ≥2 y to <6 y n = 15 . | All patients N = 45 . |
|---|---|---|---|---|
| Median age (range), y | 14.3 (12.2-17.4) | 7.3 (6.1-8.8) | 3.6 (2.3-5.8) | 7.6 (2.3-17.4) |
| Male, n (%) | 13 (72.2) | 5 (41.7) | 10 (66.7) | 28 (62.2) |
| Race, n (%) | ||||
| White | 11 (61.1) | 5 (41.7) | 4 (26.7) | 20 (44.4) |
| Asian | 3 (16.7) | 3 (25.0) | 5 (33.3) | 11 (24.4) |
| Missing | 4 (22.2) | 4 (33.3) | 6 (40.0) | 14 (31.1) |
| Median weight (range), kg | 44.5 (36.9-85.5) | 22.4 (17.8-27.6) | 14.5 (9.0-22.5) | 23.8 (9.0-85.5) |
| Median BMI (range), kg/m2 | 18.6 (15.4-28.2) | 16.1 (11.9-19.1) | 16.7 (12.8-18.9) | 17.4 (11.9-28.2) |
| Steroid refractory, n (%) | 15 (83.3) | 6 (50.0) | 11 (73.3) | 32 (71.1) |
| Treatment-naïve patients, n (%) | 3 (16.7) | 6 (50.0) | 4 (26.7) | 13 (28.9) |
| aGVHD grade at baseline, n (%) | ||||
| Grade 2 | 12 (66.7) | 8 (66.7) | 9 (60.0) | 29 (64.4) |
| Grade 3 | 5 (27.8) | 4 (33.3) | 3 (20.0) | 12 (26.7) |
| Grade 4 | 1 (5.6) | 0 | 3 (20.0) | 4 (8.9) |
| aGVHD organ involvement at baseline, n (%) | ||||
| Skin | 13 (72.2) | 9 (75.0) | 12 (80.0) | 34 (75.6) |
| Liver | 2 (11.1) | 0 | 1 (6.7) | 3 (6.7) |
| Upper GI | 3 (16.7) | 5 (41.7) | 2 (13.3) | 10 (22.2) |
| Lower GI | 6 (33.3) | 5 (41.7) | 7 (46.7) | 18 (40.0) |
| . | Group 1 ≥12 y to <18 y n = 18 . | Group 2 ≥6 y to <12 y n = 12 . | Group 3 ≥2 y to <6 y n = 15 . | All patients N = 45 . |
|---|---|---|---|---|
| Median age (range), y | 14.3 (12.2-17.4) | 7.3 (6.1-8.8) | 3.6 (2.3-5.8) | 7.6 (2.3-17.4) |
| Male, n (%) | 13 (72.2) | 5 (41.7) | 10 (66.7) | 28 (62.2) |
| Race, n (%) | ||||
| White | 11 (61.1) | 5 (41.7) | 4 (26.7) | 20 (44.4) |
| Asian | 3 (16.7) | 3 (25.0) | 5 (33.3) | 11 (24.4) |
| Missing | 4 (22.2) | 4 (33.3) | 6 (40.0) | 14 (31.1) |
| Median weight (range), kg | 44.5 (36.9-85.5) | 22.4 (17.8-27.6) | 14.5 (9.0-22.5) | 23.8 (9.0-85.5) |
| Median BMI (range), kg/m2 | 18.6 (15.4-28.2) | 16.1 (11.9-19.1) | 16.7 (12.8-18.9) | 17.4 (11.9-28.2) |
| Steroid refractory, n (%) | 15 (83.3) | 6 (50.0) | 11 (73.3) | 32 (71.1) |
| Treatment-naïve patients, n (%) | 3 (16.7) | 6 (50.0) | 4 (26.7) | 13 (28.9) |
| aGVHD grade at baseline, n (%) | ||||
| Grade 2 | 12 (66.7) | 8 (66.7) | 9 (60.0) | 29 (64.4) |
| Grade 3 | 5 (27.8) | 4 (33.3) | 3 (20.0) | 12 (26.7) |
| Grade 4 | 1 (5.6) | 0 | 3 (20.0) | 4 (8.9) |
| aGVHD organ involvement at baseline, n (%) | ||||
| Skin | 13 (72.2) | 9 (75.0) | 12 (80.0) | 34 (75.6) |
| Liver | 2 (11.1) | 0 | 1 (6.7) | 3 (6.7) |
| Upper GI | 3 (16.7) | 5 (41.7) | 2 (13.3) | 10 (22.2) |
| Lower GI | 6 (33.3) | 5 (41.7) | 7 (46.7) | 18 (40.0) |
Full analysis set. The last available assessment on, or before, the date of start of study treatment is defined as baseline assessment.
BMI, body mass index.
PK and RP2D
Overall, the exposure in terms of AUC, trough plasma concentration, maximum plasma concentration, and half-life was similar across age groups (Table 2) and within the exposure range observed in adult patients with aGVHD receiving ruxolitinib at 10 mg twice daily. Geometric mean AUC was 269 ng∗hr/mL with high variability (78%) and the geometric mean peak plasma ruxolitinib concentration was 72.2 ng/mL, with a between patient variability of 84%. Median elimination half-life was similar in all age groups (range, 1.36-1.85 hours). Ruxolitinib starting doses were confirmed as the RP2D for groups 2 and 3 based on the PK, safety, and efficacy.
PK parameters
| . | Group 1 ≥12 y to <18 y n = 14 . | Group 2 ≥6 y to <12 y n = 11 . | Group 3 ≥2 y to <6 y n = 15 . | All patients N = 40 . |
|---|---|---|---|---|
| AUClast(ng∗h/mL) | ||||
| n | 5 | 10 | 15 | 30 |
| Mean (SD) | 412 (403) | 365 (195) | 286 (166) | 333 (224) |
| CV% | 97.9 | 53.6 | 58.2 | 67.2 |
| Geo-mean | 252 | 311 | 249 | 269 |
| Geo-CV% | 186.6 | 70.2 | 56.9 | 78.2 |
| Median (range) | 358 (45.1-1070) | 337 (105-676) | 231 (94.8-702) | 267 (45.1-1070) |
| Cmax(ng/mL) | ||||
| n | 5 | 10 | 15 | 30 |
| Mean (SD) | 96.3 (68.5) | 110 (72.7) | 78.1 (58.8) | 91.7 (64.6) |
| CV% | 71.2 | 66.2 | 75.3 | 70.5 |
| Geo-mean | 66.1 | 90.3 | 64.0 | 72.2 |
| Geo-CV% | 169.8 | 74.6 | 67.5 | 83.8 |
| Median (range) | 102 (9.9-184) | 72.5 (36.3-257) | 58.4 (31.1-229) | 69.4 (9.9-257) |
| Ctrough(ng/mL) | ||||
| n | 7 | 8 | 14 | 29 |
| Mean (SD) | 22.1 (38.7) | 14.3 (17.6) | 8.7 (12.7) | 13.5 (22.4) |
| CV% | 175.3 | 122.4 | 146.0 | 166.4 |
| Geo-mean | 9.27 | 6.31 | 4.79 | 6.07 |
| Geo-CV% | 379.4 | 266.0 | 225.1 | 252.1 |
| Median (range) | 6.1 (0.0-108) | 5.6 (1.3-48.8) | 3.4 (0.0-44.3) | 4.33 (0.0-108) |
| T1/2(h) | ||||
| n | 2 | 7 | 10 | 19 |
| Mean (SD) | 1.36 (0.414) | 1.63 (0.259) | 1.99 (0.873) | 1.79 (0.682) |
| CV% | 30.5 | 15.8 | 43.9 | 38.1 |
| Geo-mean | 1.33 | 1.62 | 1.82 | 1.68 |
| Geo-CV% | 31.7 | 15.4 | 47.1 | 36.3 |
| Median (range) | 1.36 (1.07-1.65) | 1.58 (1.31-2.12) | 1.85 (0.998-3.40) | 1.58 (0.998-3.40) |
| . | Group 1 ≥12 y to <18 y n = 14 . | Group 2 ≥6 y to <12 y n = 11 . | Group 3 ≥2 y to <6 y n = 15 . | All patients N = 40 . |
|---|---|---|---|---|
| AUClast(ng∗h/mL) | ||||
| n | 5 | 10 | 15 | 30 |
| Mean (SD) | 412 (403) | 365 (195) | 286 (166) | 333 (224) |
| CV% | 97.9 | 53.6 | 58.2 | 67.2 |
| Geo-mean | 252 | 311 | 249 | 269 |
| Geo-CV% | 186.6 | 70.2 | 56.9 | 78.2 |
| Median (range) | 358 (45.1-1070) | 337 (105-676) | 231 (94.8-702) | 267 (45.1-1070) |
| Cmax(ng/mL) | ||||
| n | 5 | 10 | 15 | 30 |
| Mean (SD) | 96.3 (68.5) | 110 (72.7) | 78.1 (58.8) | 91.7 (64.6) |
| CV% | 71.2 | 66.2 | 75.3 | 70.5 |
| Geo-mean | 66.1 | 90.3 | 64.0 | 72.2 |
| Geo-CV% | 169.8 | 74.6 | 67.5 | 83.8 |
| Median (range) | 102 (9.9-184) | 72.5 (36.3-257) | 58.4 (31.1-229) | 69.4 (9.9-257) |
| Ctrough(ng/mL) | ||||
| n | 7 | 8 | 14 | 29 |
| Mean (SD) | 22.1 (38.7) | 14.3 (17.6) | 8.7 (12.7) | 13.5 (22.4) |
| CV% | 175.3 | 122.4 | 146.0 | 166.4 |
| Geo-mean | 9.27 | 6.31 | 4.79 | 6.07 |
| Geo-CV% | 379.4 | 266.0 | 225.1 | 252.1 |
| Median (range) | 6.1 (0.0-108) | 5.6 (1.3-48.8) | 3.4 (0.0-44.3) | 4.33 (0.0-108) |
| T1/2(h) | ||||
| n | 2 | 7 | 10 | 19 |
| Mean (SD) | 1.36 (0.414) | 1.63 (0.259) | 1.99 (0.873) | 1.79 (0.682) |
| CV% | 30.5 | 15.8 | 43.9 | 38.1 |
| Geo-mean | 1.33 | 1.62 | 1.82 | 1.68 |
| Geo-CV% | 31.7 | 15.4 | 47.1 | 36.3 |
| Median (range) | 1.36 (1.07-1.65) | 1.58 (1.31-2.12) | 1.85 (0.998-3.40) | 1.58 (0.998-3.40) |
PK analysis set. N is the number of patients with corresponding evaluable PK parameters.
AUClast, AUC to last quantifiable concentration; Cmax, maximum plasma concentration; Ctrough, trough plasma concentration; CV%, coefficient of variation; Geo-mean, geometric mean; Geo-CV%, geometric coefficient of variation; SD, standard deviation; T1/2 half-life.
Efficacy
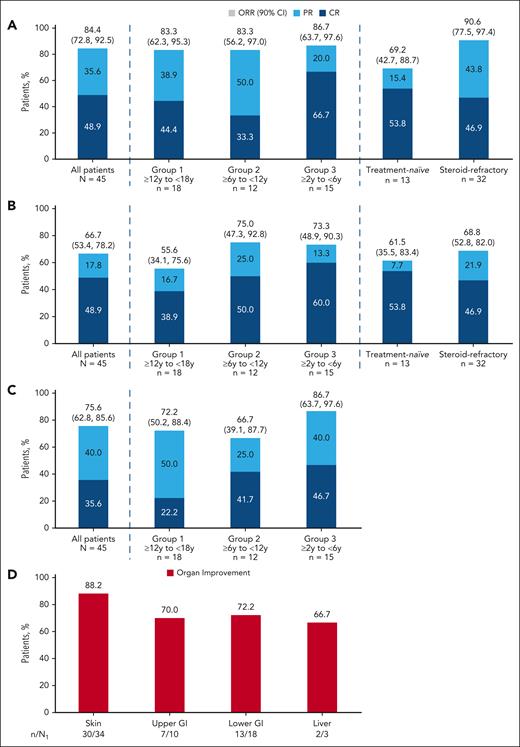
The ORR at day 28 was 84.4% (90% CI, 72.8-92.5) and the durable ORR at day 56 was 66.7% (90% CI, 53.4-78.2; Figure 2). Responses in treatment-naïve and steroid-refractory patients are shown in Figure 2. Overall, up to day 28, 93.3% of patients had either CR (60.0%) or PR (33.3%) at any time (supplemental Figure 2). Up to data cutoff, 93.3% of patients had either CR (75.6%) or PR (17.8%). By the data cutoff, 7 patients had improved their response from PR to CR compared with the BOR up to day 28. At day 28 (week 4) and up to data cutoff, BOR was similar across age groups. The ORR at day 14 was 75.6% (90% CI, 62.8-85.6; Figure 2C). At baseline, the most involved organ was the skin (34 [75.6%]), followed by the lower GI (18 [40.0%]), upper GI (10 [22.2%]), and the liver (3 [6.7%]). Responses were observed regardless of baseline organ involvement, and organ improvement was reported for all organs (Figure 2D; supplemental Table 2). Patients with grade 2 aGVHD (n = 29) achieved an ORR of 93.1% (90% CI, 79.8-98.8) at day 28 and a durable ORR of 72.4% (90% CI, 55.7-85.5) at day 56. In contrast, patients with grade 3/4 aGVHD (n = 16) had an ORR of 68.8% (90% CI, 45.2-86.8) at day 28 and a durable ORR of 56.3% (90% CI, 33.3-77.3) at day 56.
ORR. (A) ORR at day 28. (B) Durable overall response at day 56. (C) Overall response rate at day 14. (D) Organ response at day 28. Full analysis set. (A and C) ORR is defined as the proportion of patients achieving CR or PR. (B) Durable ORR at day 56 is defined as the proportion of patients who achieved a CR or PR at day 28 and maintained a CR or PR at day 56. The 2-sided 90% CI for the response rate was calculated using the Clopper-Pearson exact method. (D) Baseline involvement if respective stage at baseline is >0. n, number of patients with the respective organ response; N, number of patients in the treatment group; N1, number of patients with the respective organ involvement at baseline excluding patients in n with change/addition of new systemic GVHD treatment by day 28 who have a reduction in organ stage of ≥1; y, years.
ORR. (A) ORR at day 28. (B) Durable overall response at day 56. (C) Overall response rate at day 14. (D) Organ response at day 28. Full analysis set. (A and C) ORR is defined as the proportion of patients achieving CR or PR. (B) Durable ORR at day 56 is defined as the proportion of patients who achieved a CR or PR at day 28 and maintained a CR or PR at day 56. The 2-sided 90% CI for the response rate was calculated using the Clopper-Pearson exact method. (D) Baseline involvement if respective stage at baseline is >0. n, number of patients with the respective organ response; N, number of patients in the treatment group; N1, number of patients with the respective organ involvement at baseline excluding patients in n with change/addition of new systemic GVHD treatment by day 28 who have a reduction in organ stage of ≥1; y, years.
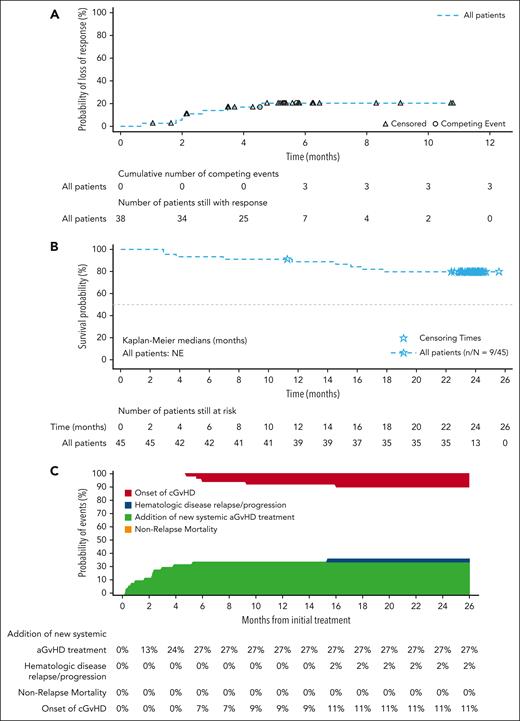
Among the 38 day-28 responders, 7 patients had an aGVHD progression or additional new systemic therapy. Up to the data cutoff, most responders maintained their response. The estimated cumulative incidence of loss of response was 20.4% (95% CI, 8.7-35.4) at 6 months (Figure 3A; supplemental Table 3).
Duration of response, OS, and FFS. (A) Cumulative incidence curve of duration of response. (B) Kaplan-Meier plot of OS. (C) FFS. Full analysis set, responders only. (A) Duration of response only includes patients whose overall response is CR or PR; the start date is the date of the first documented response of CR or PR, which could be before or at day 28. For duration of response analysis an event was defined as the progression of aGVHD or the addition of systemic therapies for aGVHD after day 28; the competing risks include death without prior observation of aGVHD progression and onset of cGVHD was censored at the last response assessment before or at the analysis cutoff date if no events or competing risks occurred on or before 4 weeks after the last response assessment. (B) OS was defined as the time from the date of the start of study treatment to the date of death from any cause; data were censored at the latest date that the patient was known to be alive. (C) FFS events include hematologic disease relapse/progression, NRM, or the addition of new systemic aGVHD treatment; the competing risk was the onset of cGVHD. Median and quartiles are provided using the Kaplan-Meier method. NE, not estimable.
Duration of response, OS, and FFS. (A) Cumulative incidence curve of duration of response. (B) Kaplan-Meier plot of OS. (C) FFS. Full analysis set, responders only. (A) Duration of response only includes patients whose overall response is CR or PR; the start date is the date of the first documented response of CR or PR, which could be before or at day 28. For duration of response analysis an event was defined as the progression of aGVHD or the addition of systemic therapies for aGVHD after day 28; the competing risks include death without prior observation of aGVHD progression and onset of cGVHD was censored at the last response assessment before or at the analysis cutoff date if no events or competing risks occurred on or before 4 weeks after the last response assessment. (B) OS was defined as the time from the date of the start of study treatment to the date of death from any cause; data were censored at the latest date that the patient was known to be alive. (C) FFS events include hematologic disease relapse/progression, NRM, or the addition of new systemic aGVHD treatment; the competing risk was the onset of cGVHD. Median and quartiles are provided using the Kaplan-Meier method. NE, not estimable.
OS analysis was performed using follow-up data up to the end of the study, including 9 (20%) deaths that occurred after the treatment period. Of the 9 deaths, 6 occurred in steroid-refractory patients and 3 in treatment-naïve patients. Among all patients treated with ruxolitinib, the median OS was not reached and the 12-month survival probability was 88.8% (95% CI, 75.2-95.2; Figure 3B; supplemental Table 4). The estimated survival probability at 12 months was 92.3% (95% CI, 56.6-98.9) among treatment-naïve patients and 87.4% (95% CI, 69.8-95.1) in steroid-refractory patients (supplemental Figure 3; supplemental Table 4). Four responders and 5 nonresponders died, resulting in estimated survival probabilities at 12 months after day 28 of 94.7% (95% CI, 80.6-98.7) and 57.1% (95% CI, 17.2-83.7), respectively (supplemental Table 4). Furthermore, by baseline aGVHD severity, 4 patients with grade 2 aGVHD patients and 5 with grade 3/4 aGVHD died. Neither severity subgroup reached median OS, and the probability of 12-month OS was 93.1% (95% CI, 75.1-98.2) for patients with grade 2 aGVHD and 81.3% (95% CI, 52.5-93.5) for those with grade 3/4 aGVHD (supplemental Table 4). The estimated 6-month cumulative incidence rate of any FFS event was 26.7% (95% CI, 14.7-40.2; Figure 3C; supplemental Table 5). Thirteen (28.9%) patients had ≥1 event, most commonly addition of a new systemic therapy, with a 24% estimated likelihood up to month 4. At 6 months after day 28, the estimated rate of any FFS event among responders was 15.8% (95% CI, 6.3-29.2; supplemental Figure 4; supplemental Table 5). However, because of the limited number of nonresponders (n = 3), it is not possible to interpret the FFS rate after day 28. Notably, all 3 nonresponders experienced an FFS event: 2 of them received additional new systemic therapy within 1 month after day 28, whereas 1 patient experienced hematologic disease relapse/progression at 14.5 months after day 28. Cumulative incidence rates of any FFS event at 6 months were 17.2% (95% CI, 6.1-33.1) for patients with grade aGVHD and 43.8% (95% CI, 18.9-66.4) for those with grade 3/4 aGVHD (supplemental Figure 5; supplemental Table 5).
By data cutoff, 11 (24.4%) patients had developed cGVHD; 5 (11.1%) had severe disease, 4 (8.9%) moderate disease, and 2 (4.4%) mild disease. The estimated cumulative incidence of cGVHD at 6 and 12 months was 11.1% (95% CI, 4.0-22.3) and 20.1% (95% CI, 9.8-32.9), respectively (supplemental Figure 6; supplemental Table 6). Furthermore, the incidence rates of cGVHD at 6 and 12 months after day 28 were 10.5% (95% CI, 3.3-22.7) and 18.5% (95% CI, 8.0-32.4) in responders, respectively, and 28.6% (95% CI, 2.8-64.6) and 42.9% (95% CI, 6.5-76.9), in nonresponders, respectively.
Among the 27 patients with an underlying hematologic malignancy, 3 (11.1%) had a relapse. The cumulative incidence of malignancy relapse or progression was 7.4% (95% CI, 1.2-21.4) at 12 months (supplemental Figure 7; supplemental Table 7).
The cumulative incidence of NRM at 12 months was 9.0% (95% CI, 2.8-19.6), 5.3% (95% CI, 0.9-15.7), and 28.6% (95% CI, 2.9-64.1) in all patients, responders, and nonresponders, respectively (supplemental Figure 8; supplemental Table 8).
Duration of treatment with corticosteroids and ruxolitinib
By day 56, 75.6% (95% CI, 60.5-87.1) of patients had a ≥50% reduction in corticosteroid dose, and 37.8% (95% CI, 23.8-53.5) of patients were able to taper off corticosteroids completely (Table 3). By the end of treatment, 86.7% (95% CI, 73.2-94.9) of patients had a ≥50% reduction in corticosteroid dose, and 62.2% (95% CI, 46.5-76.2) of patients were able to discontinue corticosteroids (Table 3). Change in corticosteroid dose over the duration of the study is shown in supplemental Figure 9.
Corticosteroid dosing
| . | Group 1 ≥12 y to <18 y n = 18 . | Group 2 ≥6 y to <12 y n = 12 . | Group 3 ≥2 y to <6 y n = 15 . | All patients N = 45 . |
|---|---|---|---|---|
| Duration of exposure to corticosteroids (days) | ||||
| Mean (SD) | 70.7 (73.9) | 76.6 (56.7) | 86.3 (55.6) | 77.5 (62.8) |
| Median (range) | 46.5 (8.0-342.0) | 68.5 (18.0-196.0) | 77.0 (19.0-195.0) | 65.0 (8.0-342.0) |
| Completely tapered off by, n (%) [95% CI] | ||||
| Day 28 | 2 (11.1) [1.4-34.7] | 1 (8.3) [0.2-38.5] | 1 (6.7) [0.2-31.9] | 4 (8.9) [2.5-21.2] |
| Day 56 | 9 (50.0) [26.0-74.0] | 4 (33.3) [9.9-65.1] | 4 (26.7) [7.8-55.1] | 17 (37.8) [23.8-53.5] |
| EOT | 11 (61.1) [35.7-82.7] | 6 (50.0) [21.1-78.9] | 11 (73.3) [44.9-92.2] | 28 (62.2) [46.5-76.2] |
| ≥50% dose reduction by, n (%) [95% CI] | ||||
| Day 28 | 10 (55.6) [30.8-78.5] | 6 (50.0) [21.1-78.9] | 8 (53.3) [26.6-78.7] | 24 (53.3) [37.9-68.3] |
| Day 56 | 15 (83.3) [58.6-96.4] | 7 (58.3) [27.7-84.8] | 12 (80.0) [51.9-95.7] | 34 (75.6) [60.5-87.1] |
| EOT | 15 (83.3) [58.6-96.4] | 10 (83.3) [51.6-97.9] | 14 (93.3) [68.1-99.8] | 39 (86.7) [73.2-94.9] |
| Mean (SD) percentage dose change at | ||||
| Day 28 | −39.2 (52.1) | −41.7 (36.3) | −25.3 (71.6) | −35.2 (55.3) |
| Day 56 | −56.8 (49.6) | −52.7 (46.7) | −47.4 (64.2) | −52.6 (53.2) |
| EOT | −56.6 (52.0) | −63.2 (33.2) | −61.8 (63.4) | −60.1 (51.1) |
| . | Group 1 ≥12 y to <18 y n = 18 . | Group 2 ≥6 y to <12 y n = 12 . | Group 3 ≥2 y to <6 y n = 15 . | All patients N = 45 . |
|---|---|---|---|---|
| Duration of exposure to corticosteroids (days) | ||||
| Mean (SD) | 70.7 (73.9) | 76.6 (56.7) | 86.3 (55.6) | 77.5 (62.8) |
| Median (range) | 46.5 (8.0-342.0) | 68.5 (18.0-196.0) | 77.0 (19.0-195.0) | 65.0 (8.0-342.0) |
| Completely tapered off by, n (%) [95% CI] | ||||
| Day 28 | 2 (11.1) [1.4-34.7] | 1 (8.3) [0.2-38.5] | 1 (6.7) [0.2-31.9] | 4 (8.9) [2.5-21.2] |
| Day 56 | 9 (50.0) [26.0-74.0] | 4 (33.3) [9.9-65.1] | 4 (26.7) [7.8-55.1] | 17 (37.8) [23.8-53.5] |
| EOT | 11 (61.1) [35.7-82.7] | 6 (50.0) [21.1-78.9] | 11 (73.3) [44.9-92.2] | 28 (62.2) [46.5-76.2] |
| ≥50% dose reduction by, n (%) [95% CI] | ||||
| Day 28 | 10 (55.6) [30.8-78.5] | 6 (50.0) [21.1-78.9] | 8 (53.3) [26.6-78.7] | 24 (53.3) [37.9-68.3] |
| Day 56 | 15 (83.3) [58.6-96.4] | 7 (58.3) [27.7-84.8] | 12 (80.0) [51.9-95.7] | 34 (75.6) [60.5-87.1] |
| EOT | 15 (83.3) [58.6-96.4] | 10 (83.3) [51.6-97.9] | 14 (93.3) [68.1-99.8] | 39 (86.7) [73.2-94.9] |
| Mean (SD) percentage dose change at | ||||
| Day 28 | −39.2 (52.1) | −41.7 (36.3) | −25.3 (71.6) | −35.2 (55.3) |
| Day 56 | −56.8 (49.6) | −52.7 (46.7) | −47.4 (64.2) | −52.6 (53.2) |
| EOT | −56.6 (52.0) | −63.2 (33.2) | −61.8 (63.4) | −60.1 (51.1) |
Safety set; 95% confidence limits were calculated from the Fisher exact test.
EOT, end of treatment; SD, standard deviation.
Overall, the median duration of ruxolitinib exposure was 3.8 months (range, 0.3-11.2), with 15.2 patient-treatment-years (defined as the sum of each patient’s treatment exposure in years) of exposure.
Safety
All patients experienced at least 1 AE and 23 (51.1%) patients experienced a treatment-related AE of any grade, with the most common (occurring in ≥15% of patients) being anemia (20.0%), decreased neutrophil count (17.8%), and decreased white blood cell count (15.6%) (Table 4). Thirty-nine (86.7%) patients had an AE of grade ≥3, of which 18 (40.0%) patients had grade ≥3 AEs that were considered treatment related, with the most common event being neutrophil count decreased.
Summary of AEs
| . | Group 1 ≥12 y to <18 y n = 18 . | Group 2 ≥6 y to <12 y n = 12 . | Group 3 ≥2 y to <6 y n = 15 . | All patients N = 45 . | ||||
|---|---|---|---|---|---|---|---|---|
| All grades, n (%) . | Grade ≥3, n (%) . | All grades, n (%) . | Grade ≥3, n (%) . | All grades, n (%) . | Grade ≥3, n (%) . | All grades, n (%) . | Grade ≥3, n (%) . | |
| AEs | 18 (100) | 16 (88.9) | 12 (100) | 11 (91.7) | 15 (100) | 12 (80.0) | 45 (100) | 39 (86.7) |
| Treatment related | 10 (55.6) | 8 (44.4) | 7 (58.3) | 4 (33.3) | 6 (40.0) | 6 (40.0) | 23 (51.1) | 18 (40.0) |
| Most frequently (>4%) reported treatment-related AEs by PT | ||||||||
| Anemia | 1 (5.6) | 1 (5.6) | 4 (33.3) | 2 (16.7) | 4 (26.7) | 3 (20.0) | 9 (20.0) | 6 (13.3) |
| Neutrophil count decreased | 3 (16.7) | 3 (16.7) | 1 (8.3) | 1 (8.3) | 4 (26.7) | 4 (26.7) | 8 (17.8) | 8 (17.8) |
| White blood cell count decreased | 0 | 0 | 2 (16.7) | 2 (16.7) | 5 (33.3) | 4 (26.7) | 7 (15.6) | 6 (13.3) |
| Platelet count decreased | 0 | 0 | 2 (16.7) | 1 (8.3) | 4 (26.7) | 4 (26.7) | 6 (13.3) | 5 (11.1) |
| Neutropenia | 2 (11.1) | 2 (11.1) | 0 | 0 | 1 (6.7) | 1 (6.7) | 3 (6.7) | 3 (6.7) |
| Blood creatinine increased | 0 | 0 | 1 (8.3) | 0 | 1 (6.7) | 0 | 2 (4.4) | 0 |
| Constipation | 0 | 0 | 1 (8.3) | 0 | 1 (6.7) | 0 | 2 (4.4) | 0 |
| SAEs | 11 (61.1) | 10 (55.6) | 7 (58.3) | 6 (50.0) | 6 (40.0) | 4 (26.7) | 24 (53.3) | 20 (44.4) |
| Treatment related | 4 (22.2) | 4 (22.2) | 1 (8.3) | 1 (8.3) | 2 (13.3) | 1 (6.7) | 7 (15.6) | 6 (13.3) |
| Fatal SAEs | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Treatment related | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| AEs leading to discontinuation | 5 (27.8) | 4 (22.2) | 3 (25.0) | 3 (25.0) | 2 (13.3) | 2 (13.3) | 10 (22.2) | 9 (20.0) |
| Treatment related | 4 (22.2) | 3 (16.7) | 2 (16.7) | 2 (16.7) | 2 (13.3) | 2 (13.3) | 8 (17.8) | 7 (15.6) |
| AEs leading to dose adjustment/interruption | 11 (61.1) | 10 (55.6) | 5 (41.7) | 3 (25.0) | 7 (46.7) | 7 (46.7) | 23 (51.1) | 20 (44.4) |
| AEs requiring additional therapy | 16 (88.9) | 14 (77.8) | 12 (100) | 9 (75.0) | 15 (100) | 12 (80.0) | 43 (95.6) | 35 (77.8) |
| . | Group 1 ≥12 y to <18 y n = 18 . | Group 2 ≥6 y to <12 y n = 12 . | Group 3 ≥2 y to <6 y n = 15 . | All patients N = 45 . | ||||
|---|---|---|---|---|---|---|---|---|
| All grades, n (%) . | Grade ≥3, n (%) . | All grades, n (%) . | Grade ≥3, n (%) . | All grades, n (%) . | Grade ≥3, n (%) . | All grades, n (%) . | Grade ≥3, n (%) . | |
| AEs | 18 (100) | 16 (88.9) | 12 (100) | 11 (91.7) | 15 (100) | 12 (80.0) | 45 (100) | 39 (86.7) |
| Treatment related | 10 (55.6) | 8 (44.4) | 7 (58.3) | 4 (33.3) | 6 (40.0) | 6 (40.0) | 23 (51.1) | 18 (40.0) |
| Most frequently (>4%) reported treatment-related AEs by PT | ||||||||
| Anemia | 1 (5.6) | 1 (5.6) | 4 (33.3) | 2 (16.7) | 4 (26.7) | 3 (20.0) | 9 (20.0) | 6 (13.3) |
| Neutrophil count decreased | 3 (16.7) | 3 (16.7) | 1 (8.3) | 1 (8.3) | 4 (26.7) | 4 (26.7) | 8 (17.8) | 8 (17.8) |
| White blood cell count decreased | 0 | 0 | 2 (16.7) | 2 (16.7) | 5 (33.3) | 4 (26.7) | 7 (15.6) | 6 (13.3) |
| Platelet count decreased | 0 | 0 | 2 (16.7) | 1 (8.3) | 4 (26.7) | 4 (26.7) | 6 (13.3) | 5 (11.1) |
| Neutropenia | 2 (11.1) | 2 (11.1) | 0 | 0 | 1 (6.7) | 1 (6.7) | 3 (6.7) | 3 (6.7) |
| Blood creatinine increased | 0 | 0 | 1 (8.3) | 0 | 1 (6.7) | 0 | 2 (4.4) | 0 |
| Constipation | 0 | 0 | 1 (8.3) | 0 | 1 (6.7) | 0 | 2 (4.4) | 0 |
| SAEs | 11 (61.1) | 10 (55.6) | 7 (58.3) | 6 (50.0) | 6 (40.0) | 4 (26.7) | 24 (53.3) | 20 (44.4) |
| Treatment related | 4 (22.2) | 4 (22.2) | 1 (8.3) | 1 (8.3) | 2 (13.3) | 1 (6.7) | 7 (15.6) | 6 (13.3) |
| Fatal SAEs | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Treatment related | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| AEs leading to discontinuation | 5 (27.8) | 4 (22.2) | 3 (25.0) | 3 (25.0) | 2 (13.3) | 2 (13.3) | 10 (22.2) | 9 (20.0) |
| Treatment related | 4 (22.2) | 3 (16.7) | 2 (16.7) | 2 (16.7) | 2 (13.3) | 2 (13.3) | 8 (17.8) | 7 (15.6) |
| AEs leading to dose adjustment/interruption | 11 (61.1) | 10 (55.6) | 5 (41.7) | 3 (25.0) | 7 (46.7) | 7 (46.7) | 23 (51.1) | 20 (44.4) |
| AEs requiring additional therapy | 16 (88.9) | 14 (77.8) | 12 (100) | 9 (75.0) | 15 (100) | 12 (80.0) | 43 (95.6) | 35 (77.8) |
Safety Set. A patient with multiple severity grades for an AE is only counted under the maximum grade. AEs occurring during treatment or within 30 days of the last study medication are summarized. AEs were coded according to MedDRA version 25.1 and common toxicity criteria for adverse events version 4.03.
PT, preferred term.
Serious AEs (SAEs) occurred in 24 (53.3%) patients, of which 20 (44.4%) had a SAE of grade ≥3. Most SAEs were reported in 1 patient each. The most common SAEs were pyrexia (4 patients; 8.9%), and acute kidney injury, febrile neutropenia, sepsis, septic shock, and viral hemorrhagic cystitis (2 patients each; 4.4%; supplemental Table 9). Treatment-related SAEs occurred in 7 patients (15.6%) and included neutropenia, acute pancreatitis, CMV viremia (the patient and the donor were both CMV-positive at the time of transplant), skin infection, transplant dysfunction, CMV test positive (the patient was CMV-negative and the donor was CMV-positive at the time of transplant; the event prolonged hospitalization), neutrophil count decreased, and white blood cell count decreased; except for CMV test positive, all were SAEs of grade ≥3.
In total, 8 (17.8%) patients had treatment-related AEs leading to treatment discontinuation, of which 7 (15.6%) had grade ≥3 events. Infections were reported in 32 (71.1%) patients; 14 patients had grade ≥3 infections (supplemental Table 10). CMV infection was reported in 4 (8.9%) patients and 3 (6.7%) patients had CMV reactivation. Additionally, 6 (13.3%) patients had a positive CMV test (between study days 5 and 14), and 1 (2.2%) patient had CMV viremia (study day 7). AEs leading to dose adjustment or interruption occurred in 23 (51.1%) patients, of which 20 (44.4%) had an AE of grade ≥3. No cases of graft failure were observed.
No on-treatment deaths (within 30 days from end of treatment) occurred, and of the 9 posttreatment deaths, none were related to study treatment. Underlying leukemia was the primary cause of death (3 patients) and aGVHD was the cause of death in 2 patients. The causes of death in the remaining patients were cGVHD, thrombotic microangiopathy, sepsis, and septic shock (1 patient each).
Growth and development
Most patients remained in the same percentile for height and weight as they were at baseline. One female and 2 male patients experienced precocious puberty. Two male patients had delayed puberty; no female patients had delayed puberty. All the other patients showed advancing Tanner stages with increasing age.
Biomarkers
Longitudinal analysis of inflammatory cytokines showed no change from baseline for interleukin-6 (IL-6), IL-8, and tumor necrosis factor α with ruxolitinib treatment; however, an overall trend of decrease was observed for IL-10 (supplemental Figure 10). Steady increases in all immune cell populations assessed (CD8+, CD4+, regulatory T cells, natural killer cells, and type 17 T helper cells), indicative of immune reconstitution during ruxolitinib treatment, were observed (supplemental Figure 11). Models relating to ORR at day 28 or durable response at day 56 to baseline biomarker levels indicated no evidence that these variables at baseline have predictive power in this study population (data not shown).
Acceptability and palatability
Most patients answered the acceptability and palatability questionnaire with neutral or positive responses (supplemental Table 11).
Discussion
This study evaluated the PK, safety, and efficacy of ruxolitinib therapy in treatment-naïve or steroid-refractory pediatric patients with aGVHD. Ruxolitinib exposure was similar across age groups and the PK parameters were within the range of those observed in adult patients with aGVHD treated with ruxolitinib 10 mg twice daily in REACH2 (data on file). Based on the observed PK, safety, and efficacy of ruxolitinib, the preliminary starting doses were confirmed as the RP2D.
The standard initial systemic treatment for aGVHD is corticosteroids, either alone or with calcineurin inhibitors. However, there is no standardized second-line strategy for pediatric patients with aGVHD and treatment options rely on data from adult populations. Previous retrospective trials have demonstrated that ruxolitinib is effective in treating steroid-refractory pediatric patients with aGVHD, with an ORR ranging from 45% to 87%.12-17 The results of this trial confirm previous findings, showing that ruxolitinib treatment leads to high response rates across all age groups and in both treatment-naïve and steroid-refractory patients. Overall, the ORR rate at day 28 was 84.4% and durable ORR at day 56 showed that 66.7% of patients maintained an ORR from day 28 to day 56. The ORR at day 28 was higher in steroid-refractory patients compared with treatment-naïve patients. However, this difference is not noted at day 56. This study was not designed to compare the 2 groups and factors like baseline organ involvement and early discontinuation rates complicate interpretation. Thus, more in-depth research involving a larger population is needed. These REACH4 findings echo previous REACH2 data for which the ORR at day 28 was 62.3%.10
The therapy goals for aGVHD are to control acute manifestations, prevent life-threatening organ damage, and gradually reduce corticosteroid dose. In this study, skin was the main target organ of aGVHD; however, improvement was observed in all affected organs. Treatment of intestinal aGVHD has shown conflicting outcomes with ruxolitinib, with some studies noting a poor response in patients with GI involvement16,18 and others reporting successful treatment.13,19 We observed organ improvement in 70.0% and 72.2% of patients with upper or lower GI involvement, respectively. The number of patients with liver involvement is too low to draw firm conclusions on ruxolitinib efficacy in children with liver aGVHD. A high proportion of patients in REACH4 were able to reduce their corticosteroid dose and completely taper off corticosteroids by the end of treatment. Furthermore, additional benefits of ruxolitinib treatment were seen in terms of BOR, EFS, and FFS at 12 and 18 months. No incidences of graft failure were reported and the estimated cumulative incidence of NRM was 13.5% at 18 and 24 months.
The safety profile of ruxolitinib in this patient population was consistent with what is already known. The most common treatment-related AEs were cytopenias, which aligns with the mechanism of action of ruxolitinib and previous studies.10,12,15,16,20 Ruxolitinib use has been associated with an increase in opportunistic infections; in this study >70% of patients experienced at least 1 infection. CMV infection has been associated with ruxolitinib treatment.19 However, a recent study observed a similar incidence of CMV reactivation both before and following ruxolitinib initiation.21 Although 7 patients presented with CMV infection/reactivation, it was not the cause of any death. None of the 9 deaths occurred on treatment and none were suspected to be related to study treatment; 2 deaths were reported to be due to aGVHD.
In this pediatric population, ruxolitinib treatment was associated with a reduction in IL-10 and an increase in various immune cell populations, suggestive of disease resolution and restoration of immune cells; however, further investigation is required to validate these findings. Unlike the findings of REACH2,11 there was no association between biomarkers and outcomes at day 28 or 56 adjusted on key clinical covariates. There are notable differences between the two studies. Firstly, REACH4 had a smaller sample size (n = 45) compared with REACH2 (n = 295). Secondly, in REACH4, most patients demonstrated a CR or PR at day 28, whereas in REACH2, a higher proportion of patients were nonresponders. Using biomarkers to differentiate between a CR and PR may be more challenging than distinguishing between a response and a nonresponse.
REACH4 was a single-arm phase 1/2 study with no control arm, the absence of which is an inherent limitation of the study. There were a limited number of patients across the individual age groups and in the treatment-naïve group, hence data from the individual subgroups should be interpreted with caution. Assessment of treatment outcomes in treatment-naïve and steroid-refractory groups based on disease severity was limited because of low patient numbers in these groups. Future studies should consider this aspect. Furthermore, comparison with previously published response rates is limited because of the variation in reported response rates, which are difficult to interpret because of differences in study design, varied criteria for steroid refractoriness, lack of standardized end points and efficacy assessments, and small sample sizes.
Apart from steroids, few oral treatments are available for aGVHD in pediatric patients. This is an advantage of ruxolitinib for the pediatric population, because the oral liquid formulation used in this study demonstrated acceptability and palatability. However, some patients with mucositis and aGVHD may have limited oral tolerability and/or erratic intestinal absorption; the conditions could affect their ability to take both the tablet and oral liquid formulations.
Ruxolitinib treatment in juvenile rats was associated with growth and bone effects in a dose-dependent manner.8,9 In REACH4, most patients remained in the same height and weight percentile as at baseline. Growth and bone development should in any case be monitored when using ruxolitinib in children.
In conclusion, the study confirmed the recommended starting doses of ruxolitinib for different age groups (aged ≥12 to <18 years: 10 mg twice daily; ≥6 to <12 years: 5 mg twice daily; and ≥2 to <6 years: 4 mg/m2 twice daily). The ruxolitinib dose for patients aged <2 years will be determined through modeling. Ruxolitinib therapy had a high ORR at day 28, which was durable at day 56 and beyond, in treatment-naïve and steroid-refractory pediatric patients with grade 2 to 4 aGVHD. There were no deaths or instances of graft failure during treatment. The safety profile of ruxolitinib in pediatric patients was consistent with what is already known in adults. No new safety concerns were identified. Ruxolitinib has shown promising results in the treatment of steroid-refractory aGVHD, and the role of ruxolitinib in the first-line treatment will continue to be evaluated in future studies. The data presented here demonstrate that, overall, ruxolitinib provides a valuable treatment option for pediatric patients with aGVHD.
Acknowledgments
The authors thank the patients and their families for participating in REACH4 and all medical staff involved in the trial. They also thank Pantelia Roussou of Novartis Pharma AG for her contribution to the statistical analysis. Medical writing support was provided by Helen Findlow, of Novartis Pharmaceuticals UK Ltd., London, United Kingdom, in accordance with the good publication practice 2022 guidelines (https://www.ismpp.org/gpp-2022).
Authorship
Contribution: All authors provided substantial contribution to the study design, and/or collection, analysis, and/or interpretation of data; were involved in the drafting and/or critical reviewing of the manuscript; provided final approval of the version to be published; and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflict-of-interest disclosure: F.L. has participated in speaker bureaus for Amgen, Bluebird Bio, Bristol Myers Squibb, medac Pharma, Miltenyi Biotec, Neovii, Novartis, and Sobi. H.J.K. has received research funding, honoraria, and fees for being part of a speakers bureau from Amgen, Jazz Pharmaceutical, and Novartis, and has been a member of their board of directors or advisory committee. H.B. has provided consultancy to, and received fees from, Amgen, Jazz Pharmaceutical, and Novartis for being part of a speaker's bureau. G.C., C.R., X.L., A.S.P., and A.P. are employees of Novartis. C.D.-d.-H. has been a consultant for, and/or received travel expenses from, Biotest, Jazz Pharmaceuticals, Novartis, and Vertex. The remaining authors declare no competing financial interests.
Correspondence: Franco Locatelli, Department of Pediatric Hematology and Oncology, IRCCS Ospedale Pediatrico Bambino Gesù, Piazza Sant'Onofrio, 4, 00165 Rome, Italy; email: franco.locatelli@opbg.net.
References
Author notes
Presented in part at the 64th annual meeting of the American Society of Hematology, New Orleans, LA, 10 to 13 December 2022.
Novartis will not provide access to patient-level data if there is a reasonable likelihood that individual patients could be reidentified. Phase 1 studies, by their nature, present a high risk of patient reidentification; therefore, patient individual results for phase 1 studies cannot be shared. In addition, clinical data, in some cases, have been collected subject to contractual or consent provisions that prohibit transfer to third parties. Such restrictions may preclude granting access under these provisions. In instanced in which codevelopment agreements or other legal restrictions prevent companies from sharing particular data, companies will work with qualified requestors to provide summary information where possible.
The online version of this article contains a data supplement
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal