In this issue of Blood, Locatelli and colleagues reports the results of a phase 1/2, open-label, single-arm, international multicenter study (REACH4) evaluating the pharmacokinetics (PK), efficacy, and safety of ruxolitinib in pediatric patients with treatment-naïve and steroid-refractory (SR) grade II to IV acute graft-versus-host disease (aGVHD).1 The data demonstrate that ruxolitinib is effective for pediatric patients with either treatment-naïve or SR-GVHD.
Allogeneic hematopoietic cell transplantation (allo-HCT) is a curative therapy for high-risk hematopoietic malignancies, nonmalignant hematologic disorders, and congenital immunodeficiencies. However, aGVHD remains one of the major obstacles to successful allo-HCT. The standard first-line treatment for aGVHD is corticosteroids, but up to 50% of cases are refractory. SR aGVHD has poor outcomes including 6-month survival of approximately 50% and decreased quality of life.2 Therefore, second-line treatments are unmet need.
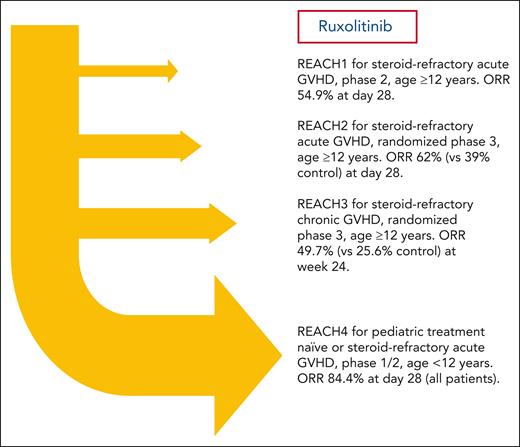
Janus kinase (JAK) is an intracellular nonreceptor tyrosine kinase that mediates signaling downstream of cytokine receptors critical for the development and function of immune cells, such as T cells, B cells, and antigen-presenting cells.3 Allogeneic donor T cells are critical mediators of both acute and chronic GVHD, and preclinical studies implicated JAK 1 and JAK 2 signaling in immune cells in the pathogenesis of both types of GVHD. Ruxolitinib is an oral JAK1/2 inhibitor that ameliorated GVHD while maintaining graft-versus-tumor effect in preclinical models.4 A large retrospective clinical study demonstrated potential efficacy of ruxolitinib for adult patients with SR GVHD.5 Therefore, the prospective multicenter REACH studies were conducted to evaluate ruxolitinib in patients with SR aGVHD (REACH1 and REACH2) or SR chronic GVHD (REACH3).6-8 In the phase 3 REACH2 study, the overall response rate (ORR) at day 28 and day 56 was significantly higher for the ruxolitinib group compared with the group treated with best available therapy in patients 12 years or older with SR aGVHD (grade II-IV).7 In the REACH3 study, the overall response at week 24 was greater in the ruxolitinib group than in those treated with investigator’s choice of therapy.8 Notable side effects of ruxolitinib included an increased incidence of thrombocytopenia and anemia.7,8 However, the REACH2 and 3 studies were limited to adults and adolescents. To date, there is no established standard therapy for SR aGVHD in pediatric patients and all current options rely on data extrapolated from adults.
In this phase 1/2 study, Locatelli et al assessed ruxolitinib PK parameters in phase 1 to establish an age-appropriate recommended phase 2 dose. In phase 2, the median duration of ruxolitinib exposure was 3.8 months (range 0.3-11.2). Comparable to REACH2, clinical response was assessed on days 28 and 56. Impressively, the ORR at day 28 was 84.4% (90% confidence interval [CI], 72.8-92.5) with a durable ORR at day 56 of 66.7% (90% CI, 53.4-78.2). High response rates were observed in all age groups and in both treatment-naïve and SR aGVHD subgroups. Overall, 22 patients completed the 24-week treatment period: however, 23% discontinued ruxolitinib early due to lack of efficacy (n = 12), adverse event (AE) (n = 10), and relapse (n = 1). AEs were very similar to those observed in the previous REACH1 to 3 studies. Importantly, no new critical AEs were reported in this pediatric population. Therefore, these REACH4 data support the use of ruxolitinib for pediatric patients with treatment-naïve and SR aGVHD. An additional important contribution of this study was the rigorous establishment of ruxolitinib doses for children younger than 12 years of age.
Overall, these data now extend the “REACH” of ruxolitinib to pediatric patients <12 years old with treatment-naïve aGVHD or SR aGVHD (see figure). Despite the important contributions reported here, this study also has some limitations. First, because REACH4 was not randomized and included only 13 patients with SR aGVHD, the optimal second-line therapy for SR aGVHD in pediatric patients is still unknown. Second, the current standard first-line therapy in aGVHD is corticosteroids, either alone or with calcineurin inhibitors. REACH4 showed a high ORR in treatment-naïve patients, but the definitive role of ruxolitinib for treatment-naïve aGVHD will need further study in appropriately powered, randomized trials. Third, the ORR at day 28 was higher in the SR aGVHD group compared with the treatment-naïve group. However, this difference was not present at day 56. Whether this is a reproducible phenomenon, and if so, why, needs further study. Fourth, this study was not powered to detect differences in outcomes among important subgroups such as those with different aGVHD organ stages and overall grades. Fifth, proportionately high early discontinuation rates may have influenced outcomes observed in REACH4. Sixth, unlike the REACH2 study, there was no association between biomarkers and outcomes at day 28 or day 56 adjusted on key clinical covariables in REACH4. Granted, this would be a challenging analysis using the REACH4 cohort due to its small sample size and high response rates. Seventh, important outcomes such as GVHD-free, relapse-free survival, in pediatric patients are still unknown. Thus, important clinical questions regarding the application of ruxolitinib in pediatric patients with GVHD will need to be addressed in future studies.
REACH studies conducted to evaluate ruxolitinib for SR acute or chronic GVHD up to the present time.
REACH studies conducted to evaluate ruxolitinib for SR acute or chronic GVHD up to the present time.
Nevertheless, REACH4 demonstrates promising results for ruxolitinib in the treatment of patients <12 years old with treatment-naïve and SR aGVHD. Ruxolitinib will be a valuable treatment option for pediatric patients with aGVHD.
Conflict-of-interest disclosure: T.T. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal