In this issue of Blood, John et al apply spatial transcriptomics to gain insights into the spatial complexity of extramedullary disease (EMD) in multiple myeloma (MM).1 Location, location, location, are the top 3 considerations in real estate. In their work, the authors illustrate how features of EMD may impact MM biology.
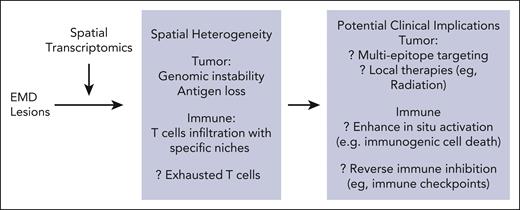
MM is characterized by multifocal growth of malignant plasma cells in the bone marrow. However, in a subset of patients, tumor cells exhibit a clinically visible extramedullary growth pattern with tumor cells located outside the bone marrow; patients with such EMD lesions experience worse outcomes after therapy, even including the newer T-cell–redirecting immune therapies.2 The mechanisms underlying the development of EMD lesions and resistance to therapy in these patients remain to be clarified. Application of spatial transcriptomic tools to EMD tissues has revealed spatial heterogeneity for both tumor and immune cells within these lesions (see figure). Spatial heterogeneity was also manifest as differences in the expression of targets of current T-cell–redirecting immune therapies in some patients. Analysis of immune infiltration demonstrated spatial heterogeneity within EMD lesions, similar to findings in spatial analyses of trephine marrow biopsies in MM.3 Interestingly, T cells were found to infiltrate EMD lesions but with considerable phenotypic heterogeneity. T cells consistent with functionally active phenotypes were found in localized niches in proximity to myeloid M1 cells, again consistent with earlier data on the role of in situ antigen presentation impacting biology of tumor-infiltrating T cells.3 Detection of T cells within EMD lesions suggests that these lesions are not immune excluded, but rather immune suppressed.4 Mechanisms suppressing T-cell function may therefore be operative in vivo and could be targeted to improve outcomes.
Spatial heterogeneity in extramedullary myeloma and potential clinical opportunities. Application of spatial transcriptomics to EMD lesions in myeloma revealed heterogeneity in both tumor and immune compartments. In turn, this has implications for consideration of strategies targeting features of EMD to improve outcomes. EMD, extramedullary disease.
Spatial heterogeneity in extramedullary myeloma and potential clinical opportunities. Application of spatial transcriptomics to EMD lesions in myeloma revealed heterogeneity in both tumor and immune compartments. In turn, this has implications for consideration of strategies targeting features of EMD to improve outcomes. EMD, extramedullary disease.
Although limited by small sample size, these data provide an initial glimpse into the spatial complexity of EMD lesions in MM, resembling that seen in metastatic solid tumors. Spatial evaluation is an important step, but methods used in these studies still have limited resolution; newer tools with improved resolution can be applied to address this limitation. Intralesional heterogeneity within EMD also implores the need to examine different regions from the entire EMD tissue. Based on earlier studies of tissue-based immunity, differentiating tumor/tissue-resident T cells from transiting T cells may require model systems or in vivo labeling.5 Immune phenotypes of T-cell exhaustion derived largely from animal models of chronic viral infection may not apply to MM.6 Functional analyses and antigen specificity of T cells in these lesions are therefore essential to better understand this biology.
Can location of tumors or patterns of immune infiltration impact outcomes and cure rates in MM? This concept has been supported by several studies showing worse outcomes in patients with EMD lesions, including those outcomes seen with newer T-cell–redirecting immune therapies.2 At present, it is not known whether EMD serves simply as a biomarker for more aggressive disease or as a specific site for residual/resistant disease. However, if the latter is true, then including pathologic and advanced radiologic evaluation of EMD lesions during initial diagnostic staging and after disease recurrence may be needed for improving long-term outcomes and cure rates. Features of EMD lesions may then also guide choices of specific therapies. For example, detection of antigenic heterogeneity or antigen-loss phenotypes in EMD may support early consideration of multiepitope targeting for T-cell redirection, as currently being tested in clinical trials.7 Residual EMD lesions may in principle also be amenable to localized therapies such as radiation. Spatial immune considerations may also impact optimal application of immune therapies or combinations in MM. For example, appreciation of potential importance of in situ antigen presentation3,8 supports combinations of T-cell redirection with strategies that induce immunogenic cell death, such as antibody-drug conjugates. Targeting inhibitory immune checkpoints such as TIGIT or Lag-39 with potential clinical activity in MM may also be considered to reinvigorate T cells already in EMD lesions. As with real estate, paying attention to location may well be the key to improving outcomes and achieving cures in MM.
Conflict-of-interest disclosure: M.V.D. has served on the advisory boards of Bristol Meyers Squibb, Janssen, Lava Therapeutics, and Sanofi.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal