In this issue of Blood, Hu and colleagues show that reduced Yes-associated protein 1 (YAP1) expression in megakaryocytes (MK), via an impaired interaction with myosin heavy chain 9 (MYH9), induces MK-cytoskeletal defects and suppresses thrombopoiesis in immune thrombocytopenia (ITP).1
ITP is the most common hematological autoimmune disorder where immune-mediated destruction of platelets and MKs can lead to bleeding, fatigue, and sometimes thrombosis.2 Both autoantibodies and cytotoxic T cells enhance platelet destruction in the spleen and liver and inhibit MK maturation in the bone marrow (BM), thus reducing platelet production.3-5 The mechanisms underlying this reduced platelet production in ITP, however, remains poorly understood.
YAP1 is an evolutionally conserved transcription coactivator and member of the hippo signaling pathway and is primarily involved in the regulation of stem cell self-renewal and organ volume and tissue regeneration.6 Although most of the literature pertaining to YAP1 is related to solid neoplasms, dysfunction of YAP1 has now been implicated in an increasing number of human diseases such as hematological cancers and autoimmunity.7,8 Thus, pharmacologic interventions of the YAP1 system may have beneficial effects in these disorders. The role of YAP1 in megakaryopoiesis has previously been studied but has remained inconclusive.9,10 Here, Hu et al shed further light on the role of YAP1 in MK development by studying patients with ITP and by using an animal model of the disease.
Flow cytometric analysis of MKs from the BM of patients with ITP displayed a lower percentage of YAP1+ MKs. Furthermore, RNA sequencing analysis of total BM RNA and gene ontology enrichment revealed that compared with healthy controls, at least 42% of patients with ITP showed a reduction in YAP1 expression. Using a murine model of passive ITP, based on injection of a monoclonal anti-CD41, they also found that the expression of YAP1, along with CD41 and CD42a, was reduced in MKs after ITP induction.
As YAP1 knockouts are embryonically lethal, they used CRISPR/Cas9 technology to generate a heterozygous YAP1 mouse model. After ITP induction, lower platelet recovery was observed in YAP1+/− mice compared with wild-type (WT) mice with ITP. Furthermore, compared with WT mice, the YAP1+/− mice exhibited decreased numbers of total MKs and reduced localization around the sinusoidal endothelium. Furthermore, there were disruptions in proplatelet formation in YAP1+/− MKs. Conversely, using a CD34+ hematopoietic stem cell–based in vitro induction system, they found that inhibiting YAP1 with peptide 17 significantly decreased MK maturation indicators such as size, CD41/CD42 expression, and the proportion of CD42a+ MKs and CD41a+CD42a+ mature MKs. Associated with these findings, they observed lower GATA1 messenger RNA levels in the BM of patients with ITP compared with healthy controls. By creating GATA1 overexpressing and knockdown mutants in HEK293T cells, the levels of YAP1 positively correlated with the expression levels of GATA1.
They then examined the potential role of YAP1 on MK cytoskeletal activation. Pretreatment of MKs with the YAP1 activator XMU-MP-1 stimulated actin polymerization and abundant stress fiber formation, while in contrast, after inhibition of YAP1, they found that F-actin presented as an abnormal entwined peripheral pattern in the MKs with disrupted assembly of F-actin. Intriguingly, they found similar findings of defective cytoskeletal activation in YAP1-deficient MKs derived from YAP1+/− mice. These findings suggested a critical role of YAP1 in MK maturation, where it may function as a key regulator of actin dynamics during platelet biogenesis.
Furthermore, by conducting coimmunoprecipitation experiments, the authors established that only phosphorylated YAP1 interacted with MYH9, which facilitated activation of the cytoskeleton in MKs. Using mutant constructs, they investigated the potential binding sites of this interaction and confirmed that the WW2 domain of YAP1 was the functional binding site.
As decreased YAP1 and GATA1 expression correlates closely with differentiation defects of MKs in ITP, it was shown that the thrombopoietic defects triggered by GATA1 deficiency could be rescued with the YAP1 activator XMU-MP-1 in established GATA1-knockdown mice. To further investigate this in an experimental in vivo ITP setting, XMU-MP-1 was administered to the YAP1+/− mice for 3 consecutive days following ITP induction, and this restored platelet counts to levels comparable with WT mice. It was concluded that administration of YAP1 can effectively correct the MK cytoskeletal dysregulation resulting from reduced YAP1 expression during ITP development, highlighting the therapeutic potential of activating YAP1.
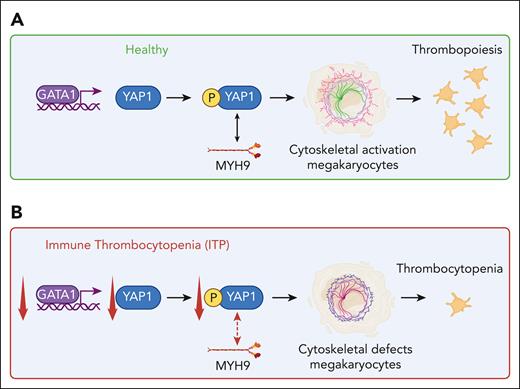
This work by Hu and colleagues suggests that in ITP, due to decreased GATA1 and YAP1 expression, the interaction between phosphorylated YAP1 and MYH9 is impaired, which results in cytoskeletal misalignment in MKs translating to defective MK-platelet production (see figure). What is not known is whether the deficiency is intrinsic to the MKs or if it is due to the autoimmune attack on the MK; the latter scenario appears plausible as passive induction of ITP reduced the YAP1 levels in BM MKs. It may be possible that besides platelet autoantibodies, cytotoxic T cells may be able to trigger this MK defect, but further investigations are warranted. The importance of this article is that a new therapeutic potential has been uncovered, aimed at targeting transcriptional factors such as YAP1 in MKs, which may restore the cytoskeletal actin defects and restore platelet counts in ITP. More research is needed to further assess this potential.
The role of YAP in ITP. In a healthy setting (A), YAP1 expression is upregulated through binding of GATA1 to its promoter in MKs. Phosphorylated YAP1, through its interaction with MYH9, promotes MK-cytoskeletal activation, which enables thrombopoiesis. In ITP (B), GATA1 and YAP1 expression is decreased, which impairs the interaction between phosphorylated YAP1 and MYH9, resulting in MK-cytoskeletal defects with subsequent development of thrombocytopenia. Figure created with BioRender.com.
The role of YAP in ITP. In a healthy setting (A), YAP1 expression is upregulated through binding of GATA1 to its promoter in MKs. Phosphorylated YAP1, through its interaction with MYH9, promotes MK-cytoskeletal activation, which enables thrombopoiesis. In ITP (B), GATA1 and YAP1 expression is decreased, which impairs the interaction between phosphorylated YAP1 and MYH9, resulting in MK-cytoskeletal defects with subsequent development of thrombocytopenia. Figure created with BioRender.com.
Conflict-of-interest disclosure: The authors declare no competing financial interest.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal