Key Points
Thrombosis in atypical sites is often the first sign of PNH, and anticoagulation/anticomplement combination is the mainstay of treatment.
DOACs are potential options for PNH-related thrombosis; discontinuation is safe in cases with complete control of intravascular hemolysis.
Visual Abstract
Thrombophilia is one of the principal features of paroxysmal nocturnal hemoglobinuria (PNH) and constitutes the main cause of disease morbidity/mortality. Anticomplement treatment has revolutionized the natural history of PNH, with control of the hemolytic process and abolition of thrombotic events (TEs). However, no guidelines exist for the management of thromboembolic complications in this setting, with type and duration of anticoagulation depending on individual practices. Besides, a scarcity of data is present on the efficacy of direct oral anticoagulants (DOACs). Herein, we accrued a large real-world cohort of patients with PNH from 4 US centers to explore features, predictors of TE, and anticoagulation strategies. Among 267 patients followed up for a total of 2043 patient-years, 56 (21%) developed TEs. These occurred at disease onset in 43% of cases, involving more frequently the venous system, typically as Budd-Chiari syndrome. Rate of TEs was halved in patients receiving complement inhibitors (21 vs 40 TEs per 1000 patient-years in untreated cases, with a 2-year cumulative incidence of thrombosis of 3.9% vs 18.3%, respectively), and varied according to PNH granulocytes and erythrocytes clone size, type, disease activity parameters, as well as number (≥2 mutations, or less) and variant allelic frequency of PIGA mutations. Anticoagulation with warfarin (39%), DOACs (37%), and low-molecular weight heparin (16%) was administered for a median of 29 months (interquartile range [IQR], 9-61.8). No thrombotic recurrence was observed in 19 patients treated with DOACs at a median observation of 17.1 months (IQR, 8.9-45) whereas 14 cases discontinued anticoagulation without TE recurrence at a median time of 51.4 months (IQR, 29.9-86.8).
Introduction
Increased propensity for thrombotic events (TEs) is well established in patients with paroxysmal nocturnal hemoglobinuria (PNH) and represents the main cause of disease morbidity and mortality.1-3 In the pre-complement blocker era, the lifetime risk of TEs was in the order of 50%,4 with patients presenting such complication at disease onset having 4-year survival of only 40%.5-8 In fact, TEs are the cause of mortality in up to 67% of deaths of which the cause is known.6,9 Although the precise pathogenesis of TEs remains ill-defined and likely multifactorial,10 cases with classical hemolytic PNH show the highest risk for thrombosis, often occurring in atypical localizations including the hepatic circulation (eg, Budd-Chiari syndrome).6,11
The introduction of anticomplement therapy greatly abated hemolysis and thrombosis, demonstrating the crucial role of the complement cascade in triggering TEs.12,13 Because of the disease rarity, its latency, and natural history, placebo-controlled studies looking at TEs in PNH are not feasible, but the antithrombotic effects of inhibitors such as eculizumab have been demonstrated using the patient-years concept. This post-hoc analysis of trials data revealed a decrease of TEs from 7.37 to 1.07 per 100 patient-years, resulting in a relative reduction of 82%.14,15 During the past 5 years, new anticomplement treatments have been approved for PNH and many are under investigation.16 These new complement inhibitors are characterized by at least as good control of the hemolytic process as compared with eculizumab, thereby having the potential to completely abate the clinical signs and symptoms of the disease, including thrombosis. Because anticoagulation can usually be stopped after 3 to 6 months in case of TEs provoked by a reversible risk factor, one could theoretically also discontinue anticoagulation therapy in the setting of PNH.17 Indeed, once the patient achieves a good control of the hemolytic process with adequate treatment, the provoking thrombophilic factor represented by the untreated PNH would ideally be removed.18,19 Notwithstanding this, despite the evolving therapeutic armamentarium of PNH-directed treatments, a scarcity of data exists on selection and duration of anticoagulation therapy or duration and general need of anticoagulant prevention in patients on complement inhibitors. This is particularly true for the newer class of direct oral anticoagulants (DOACs) in this setting.20 Specifically, guidelines on management of PNH-related TEs are lacking, and patients are treated in a heterogeneous fashion, borrowing strategies that most commonly align with general recommendations for venous thromboembolism. Therefore, clinical management can be highly diverse, emphasizing the need for an official consensus based on retrospective analyses in lieu of the paucity of controlled trial data.
Herein, we took advantage of a large, real-life cohort of patients with PNH to describe PNH-related TEs, unravel determinants of thrombosis, and decipher anticoagulation strategies.
Methods
Patient cohort and definitions
Retrospective chart review was performed to retrieve clinical and molecular information of all consecutive patients diagnosed with PNH clones and/or treated for hemolytic disease at the participating institutions. A total of 267 patients with a diagnosis of classical hemolytic PNH21-23 (PNH granulocyte clonal size of ≥20% and/or lactate dehydrogenase [LDH] levels of >2.5 upper limit of normal) or aplastic anemia (AA) with clinically significant PNH clones (>1%) were accrued via a collaboration of 4 centers.
A concomitant diagnosis of AA was established according to standard guidelines.22 Post-AA PNH was defined as the new onset of intravascular hemolysis in the presence of a PNH granulocyte clone size of ≥20% in a patient with prior AA diagnosis.
Patients were followed-up from diagnosis of AA/PNH until death or last follow-up, and were treated with a variety of complement inhibitors, either anti-C5 (eg, eculizumab and ravulizumab) or the anti-C3, pegcetacoplan, according to on-label indications.12,24-26 Thrombotic complications were managed according to the nature of the TE (arterial/venous) and anticoagulation included either warfarin, DOACs, or low-molecular weight heparin (LMWH), as per physician’s choice and institutional practices. LMWH was preferred if platelet counts were consistently <100 × 109/L in cases with concomitant AA. Discontinuation of anticoagulation was based on physician’s choice, taking into account bleeding risk, parameters of disease control (eg, LDH), and patient preference.
Radiologic studies were reviewed by an experienced radiologist (C.C.) to identify imaging features of thrombotic manifestations.
PNH flow cytometry and molecular studies
PNH clone size of both granulocytes and red blood cells (RBCs) was defined as the percentage of glycosyl phosphatidylinositol (GPI)–deficient cells and was assessed via flow cytometric assays, as previously described.23,27 Briefly, a 5-color cocktail (CD15-V450, CD45-PC7, CD64-APC, CD157-PE, and FLAER-Alexa 488) based on Sutherland et al28 (alternative protocol 2: “Simultaneous High-Sensitivity Detection of PNH Neutrophils and Monocytes Using FLAER and CD157-Based 5-Color Assay”) was used to identify GPI-deficient granulocytes and RBCs and to determine PNH granulocyte clone size. Thiazole orange along with assessment of CD59-PE and CD235a–FITC isothiocyanate was the combination used to identify PNH RBCs. Specifically, RBCs were then classified based on the degree of GPI deficiency (and thereby vulnerability to complement-mediated destruction) into PNH type II with a partial reduction, and PNH type III with a total absence of GPI-linked proteins, as previously described.23,29
Peripheral blood specimens were subjected to DNA extraction, and PIGA gene molecular status was investigated by using a multi-amplicon next-generation sequencing (NGS) panel on a Nextera Rapid Capture Custom Enrichment kit (Illumina), as previously reported.23 Clonal burden (eg, variant allelic frequency [VAF] adjusted according to X-chromosome ploidy) and number of PIGA mutations were investigated, given previous reports on PIGA mosaicism (>1 alteration) in patients with PNH supporting the concept of positive selection mechanisms underpinning the survival advantage of GPI anchor–deficient hemopoietic cells in the context of AA immune attack.30-32
DNA extracted from peripheral blood or bone marrow specimens was collected and subjected to myeloid genes NGS platforms, as recently described. 22
Statistical analysis
Median and interquartile ranges (IQRs) were used to tabulate continuous variables, whereas numbers and percentages were used for categorical variables. Accordingly, 2-sided Wilcoxon test was used for comparative analyses between 2 groups in case of quantitative variables, and Fisher exact or χ2 tests were applied for testing independence between groups, for qualitative variables.
Logistic regression univariable and multivariable models were applied to investigate the likelihood of association between relevant clinical variables and the occurrence of TEs. Poisson regression, using a time-dependent approach (based on patient-year analysis), was applied to estimate the thrombosis risk adjusted for relevant clinical and molecular covariates. Cumulative incidences of thrombosis were calculated in a competing risk setting, with death and anticomplement inhibitor treatment initiation as competing events. The latter was also used as landmark time for treated patients.
All analyses and data visualization were generated using the statistical computing environment R (4.0.0 R Core Team, R Foundation for Statistical Computing, Vienna, Austria), and Excel Microsoft Office 365. All statistical tests were 2-sided, and a P value <.05 was considered as a threshold for statistical significance.
All patients consented to participate in clinical and translational studies, according to each local institutional review board, and this research was conducted following the principles and the ethics of the Declaration of Helsinki. Review and collection of clinical and molecular data were performed in accordance with the protocols and written consent approved by the institutional review boards of each participating institutions and guidelines set forth by the Declaration of Helsinki.
Results
Patient characteristics
Among 267 patients (male-to-female ratio, 0.77), the median age at PNH diagnosis was 46 years (IQR, 27-61). Combined, our study encompassed 2043 patient-years since first PNH clone detection (of which 667.9 patient-years were under anti-PNH treatment), corresponding to a median follow-up of 5.9 years (2.1-11.7) for the entire cohort. Overall, 55% were de novo PNH whereas 45% had an antecedent diagnosis of AA with a median time to PNH evolution of 2.8 years (0.3-8). Within the cases with antecedent AA, 67.9% showed a severe AA phenotype at initial bone marrow failure diagnosis. At last follow-up, 4 patients experienced spontaneous PNH remission, as previously described.33
Median granulocyte clone size at initial PNH detection was 43.9% (IQR, 1.3%-81.9%), whereas median total RBC clone size was 5% (IQR, 0.6%-25.1%), with the majority of cases classified as type III–dominant RBC clones (78%) at flow cytometric analysis (Table 1).23PIGA mutations using whole-blood NGS were detected in 49% of cases with available multi-amplicon deep NGS at a median ploidy-adjusted VAF of 18.8% (IQR, 6.8%-40.7%), of whom 46.5% harbored multiple PIGA clones (eg, clonal mosaicism).23 Most of the PIGA mutations were frameshift (37%), followed by missense (32%), splicing sites (15%), nonsense (10%), and insertions/deletions (6%).
Demographic and clinical characteristics at diagnosis
| Variable . | All PNH cases (N = 267) . |
|---|---|
| Follow-up, median (IQR), y | 5.9 (2.1-11.7) |
| Age at diagnosis, median (IQR), y | 46 (27-61) |
| Sex, n (%) | |
| Male | 116 (43.4) |
| Female | 151 (56.6) |
| Total PNH RBC clone, median (IQR) | 5 (0.6-25.1) |
| Type II–dominant RBC clone, n (%) | 26 (22)∗ |
| Type III–dominant RBC clone, n (%) | 92 (78)∗ |
| Total PNH WBC clone, median (IQR) | 43.9 (1.3-81.9) |
| PIGA, n (%) | |
| Wild-type | 89 (33.6) |
| Mutated | 86 (32.2) |
| Unavailable | 92 (34.2) |
| Post-AA PNH, n (%) | 85 (45.2) |
| Spontaneous remission, n (%) | 4 (1.5) |
| Thrombosis, n (%) | 56 (21) |
| Time to thrombosis, median (IQR), mo | 8 (0-58) |
| Variable . | All PNH cases (N = 267) . |
|---|---|
| Follow-up, median (IQR), y | 5.9 (2.1-11.7) |
| Age at diagnosis, median (IQR), y | 46 (27-61) |
| Sex, n (%) | |
| Male | 116 (43.4) |
| Female | 151 (56.6) |
| Total PNH RBC clone, median (IQR) | 5 (0.6-25.1) |
| Type II–dominant RBC clone, n (%) | 26 (22)∗ |
| Type III–dominant RBC clone, n (%) | 92 (78)∗ |
| Total PNH WBC clone, median (IQR) | 43.9 (1.3-81.9) |
| PIGA, n (%) | |
| Wild-type | 89 (33.6) |
| Mutated | 86 (32.2) |
| Unavailable | 92 (34.2) |
| Post-AA PNH, n (%) | 85 (45.2) |
| Spontaneous remission, n (%) | 4 (1.5) |
| Thrombosis, n (%) | 56 (21) |
| Time to thrombosis, median (IQR), mo | 8 (0-58) |
WBC, white blood cell.
Missing data (PIGA molecular information available in 91 patients, and PNH RBC clone type available in 100 patients)
Consistent with a non-negligible AA overlap and the presence of multiple post-AA PNH cases, the majority of the patients had at least bilineage cytopenia (81.8%) at diagnosis, with a median hemoglobin of 9.2 g/dL (IQR, 8.1-10.8 g/dL), platelet count of 43.5 × 109/L (IQR, 20-109.5× 109/L), and absolute neutrophil count (ANC) of 1.59 × 109/L (IQR, 0.9-2.9× 109/L).
Overall, 53% of patients did not receive complement inhibitors because of absence of a severe hemolytic picture (mild anemia, lack of symptoms including TE, normal D-dimers, only moderate increase in LDH, and personal choice). Given the long follow-up of this multicenter cohort, the majority of patients requiring anticomplement therapy received eculizumab (76.2%; 587.6 patient-years), followed by ravulizumab (23%; 73.25 patient-years) after its approval in 2019, and, only recently, pegcetacoplan (0.8%; 7 patient-years). Despite these treatments, 28 patients still had symptomatic anemia (defined as transfusion dependence and presence of the aforementioned PNH symptoms), and were thereby deemed poor responders and switched to ravulizumab (64%) or pegcetacoplan (36%). In 2 cases, pegcetacoplan was given after eculizumab and ravulizumab treatment.
A summary of PNH-related TEs
Overall, 211 patients (1357.5 patient-years) did not experience any thrombotic complications (of whom 474 patient-years on complement inhibitors). A total of 56 patients (21%) experienced TEs at a median of 8 months from diagnosis (0-56): 43% occurred as the initial disease manifestation requiring urgent medical evaluation (or workup for PNH evolution in former AA cases), 7% during a temporary discontinuation of anticomplement treatment (eg, preparation to allogeneic bone marrow transplant, or other circumstances), and only 5% during treatment (eculizumab in all 3 cases; n = 1 had heterozygosity for factor V Leiden; and n = 2 received the drug at longer time intervals). Notably, 8 patients (14%) had >1 TE, with 1 patient experiencing 3 TEs, and another patient experiencing 5 TEs. Patients experiencing TEs under anticomplement treatment were more likely poor responders (20% vs 8%, P = .3285).
Most TEs originated in the venous system (89.3%) whereas 8.9% of the cases involved the cerebrovascular arteries, and 1.8% affected both arterial and venous compartments. Remarkably, TEs occurred in unusual sites in the majority of cases, with Budd-Chiari syndrome as the most frequent (37.5%), followed by deep vein thrombosis (DVT, 26.7%), DVT complicated by pulmonary embolism (17.9%), cerebrovascular (9.1%), splanchnic (7.1%), and superficial veins (1.7%, Figure 1).
Features of Budd-Chiari syndrome in PNH. (A) A pie chart illustrates the type of TEs. (B) A cartoon showcases the anatomic site of origin of thromboses. (C) Left panel shows magnetic resonance imaging (MRI) of a 59-year-old man, whereby on the axial postcontrast VIBE (volumetric interpolated breath-hold examination), the hepatic veins are decreased in caliber (thin arrows) and demonstrate peripheral pruning, however there is no residual hepatic venous filling defect to suggest thrombus. The intrahepatic inferior vena cava (IVC; thick arrow) is significantly narrowed without IVC thrombosis. On the right, a Doppler ultrasound image of the IVC of an 89-year-old male shows the hypoechoic and nonocclusive thrombus in the lumen (arrow). (D) A swimmer plot illustrates the longitudinal follow-up, and anticomplement and anticoagulation strategies in patients experiencing TEs in our cohort. ∗Patients for whom precise dates on subsequent thrombotic events (5 episodes for UPN 9 and 3 episodes for UPN 13) are lacking.
Features of Budd-Chiari syndrome in PNH. (A) A pie chart illustrates the type of TEs. (B) A cartoon showcases the anatomic site of origin of thromboses. (C) Left panel shows magnetic resonance imaging (MRI) of a 59-year-old man, whereby on the axial postcontrast VIBE (volumetric interpolated breath-hold examination), the hepatic veins are decreased in caliber (thin arrows) and demonstrate peripheral pruning, however there is no residual hepatic venous filling defect to suggest thrombus. The intrahepatic inferior vena cava (IVC; thick arrow) is significantly narrowed without IVC thrombosis. On the right, a Doppler ultrasound image of the IVC of an 89-year-old male shows the hypoechoic and nonocclusive thrombus in the lumen (arrow). (D) A swimmer plot illustrates the longitudinal follow-up, and anticomplement and anticoagulation strategies in patients experiencing TEs in our cohort. ∗Patients for whom precise dates on subsequent thrombotic events (5 episodes for UPN 9 and 3 episodes for UPN 13) are lacking.
Rate of TEs correlated with the size of both PNH granulocytes and RBC clones (Figure 2 A-B). Indeed, patients with classical hemolytic disease had higher thrombotic rates, with up to 54.4 TEs per 1000 patient-years in the groups with PNH granulocyte clone size of >70% (odds ratio [OR], 3.28; 95% confidence interval [CI], 1.75-6.12; P = .0002). Controlling for the different follow-up time, Poisson regression revealed that rate of TEs increased from 8.5% to 34% in patients with PNH RBC clone size below or ≥5% (β = 1.386, 95% CI, 0.7780-2.072; P < .001), and from 6.4% to 34% for those with granulocyte clone size below or ≥20% (β = 1.677; 95% CI, 0.9934-2.498; P < .001). Overall, rate of TEs was halved in patients receiving complement inhibitors (21 vs 40 TEs per 1000 patient-years in untreated cases) and varied according to type of treatment received despite not being significant (22.1 and 13.7 per 1000 patient-years in eculizumab- and ravulizumab-treated cases, respectively; Figure 2C). No event was registered in the group treated with pegcetacoplan (any line) although only 7 patients were under this drug (7 patient-years) at the time of this analysis, thereby limiting any general conclusions. When focusing on patients with classical hemolytic diseases, 2-year cumulative incidence of thrombosis changed from 18.3% (12.8%-24.7%) in untreated patients to 3.9% (1.3%-9.1%) upon treatment initiation (Figure 2D).
Rate of TEs in our PNH cohort. (A-B) Bar charts show thrombosis rates per 1000 patient-years in our cohort according to PNH granulocytes and RBC clone sizes. (C) Bar chart on the left indicates rate of thrombosis based on receiving or not receiving anticomplement treatment, and on the right a subanalysis on treated cases according to the type of complement inhibitors received is shown. (D) Cumulative incidence of thrombosis in hemolytic disease is shown. At 2 years, the incidence of thrombosis was 18.3% (12.8%-24.7%) in untreated (death and anticomplement treatment initiation as competing events) vs 3.9% (1.3%-9.1%) upon therapy commencement (landmark time = treatment start, death as competing event). Numbers at risk are indicated below the curve.
Rate of TEs in our PNH cohort. (A-B) Bar charts show thrombosis rates per 1000 patient-years in our cohort according to PNH granulocytes and RBC clone sizes. (C) Bar chart on the left indicates rate of thrombosis based on receiving or not receiving anticomplement treatment, and on the right a subanalysis on treated cases according to the type of complement inhibitors received is shown. (D) Cumulative incidence of thrombosis in hemolytic disease is shown. At 2 years, the incidence of thrombosis was 18.3% (12.8%-24.7%) in untreated (death and anticomplement treatment initiation as competing events) vs 3.9% (1.3%-9.1%) upon therapy commencement (landmark time = treatment start, death as competing event). Numbers at risk are indicated below the curve.
Clinical-molecular predictors of thrombosis
We then sought to find possible clinical and molecular markers associated with a higher risk of thrombosis. The baseline characteristics of patients who developed TEs as compared to those not experiencing thrombosis are presented in Table 2. No differences in baseline variables such as age, sex, presence of PIGA mutation, hemoglobin counts, aspartate aminotransferase (AST), and alanine aminotransferase were observed upon comparison of the 2 groups. Interestingly, patients experiencing TEs were more commonly presenting with type II RBC phenotype (39.4% vs 15.1%; P = .006), had larger PNH granulocyte clone (median, 71% vs 13%; P < .0001) and RBC clone size (median, 17% vs 2%; P < .0001), higher ANC (median, 2.4 × 109/L vs 1.4 × 109/L; P = .021) and platelet counts (median, 85 × 109/L vs 37 × 109/L; P = .006), reticulocytes (median, 106 × 103/μL vs 77 × 103/μL; P = .0392), increased LDH levels (median, 603 U/L vs 318 U/L; P = .0070) and PIGA VAF (median, 35.4% vs 11.6%; P = .0172) and mosaicism (≥2 mutations; 40% vs 18% in those with <2; P = .011) when compared with those not developing TEs. There was no statistical difference in frequency of missense mutations (43% vs 26% in TE vs no-TE groups, respectively). Furthermore, patients with thrombosis were noted to have lower albumin levels (median, 4 g/dL vs 4.3 g/dL; P = .003). Notably, no difference in rate of complement inhibitor switch was observed between patients developing or not developing TEs (20% vs 32%; P = .335).
Association of TEs and clinical characteristics at diagnosis
| Variable . | TE (n = 56) . | No TE (n = 211) . | P value . |
|---|---|---|---|
| Demographics | |||
| Age at diagnosis, median (IQR), y | 42 (26.3-58) | 46 (27-62) | .5353 |
| Sex, n (%) | .0964 | ||
| Male | 30 (53.6) | 86 (40.8) | |
| Female | 26 (46.4) | 125 (59.2) | |
| Laboratory markers, median (IQR) | |||
| Hb (g/dL) | 9.5 (8.1-11.2) | 9.2 (8.1-10.7) | .8396 |
| ANC (×109/L) | 2.4 (1.2-3.9) | 1.4 (0.8-2.5) | .0212 |
| Platelets (×109/L) | 85 (28-137.5) | 37 (18-93) | .0064 |
| Absolute reticulocyte count (×103/μL) | 106 (61-184) | 77.5 (33.8-142) | .0392 |
| D-dimer (mg/L) | 1335 (337.5-3023) | 360 (237-820) | .0004 |
| LDH (U/L) | 603 (362-939) | 318 (311-902) | .0070 |
| AST (U/L) | 37 (18.5-61.5) | 25 (18-42.3) | .1701 |
| ALT (U/L) | 24 (17.5-56) | 20.5 (14-31.8) | .0606 |
| Creatinine (mg/dL) | 0.8 (0.66-1.07) | 0.8 (0.69-1.0) | .7700 |
| Albumin (g/dL) | 4 (3.8-4.5) | 4.3 (4.1-4.7) | .0030 |
| C-reactive protein (mg/dL) | 7.65 (2.15-19.45) | 0.7 (0.2-3) | .0459 |
| Presence of CHIP, n (%)∗ | 8 of 35 (23%) | 27 of 151 (19%) | .4798 |
| Disease characteristics | |||
| Prior AA, n (%) | 31 (55.4) | 161 (76.3) | .5505 |
| AA to PNH, time in mo, median (IQR) | 23.4 (1.5-126.4) | 36.9 (3.7-85.2) | .1385 |
| PIGA mutant, n (%) | 23 (67.7) | 124 (61.4) | .5684 |
| PIGA VAF, % (IQR) | 35.4 (14.6-47.4) | 11.6 (5.8-36.2) | .0172∗ |
| Granulocyte clone size, median (IQR) | 71.3 (42.7-92.2) | 12.8 (0.6-70.2) | <.0001 |
| RBC clone size, median (IQR) | 17.3 (5.9-49.3) | 1.8 (0.2-19.6) | <.0001 |
| Type II–dominant RBC clone, n (%) | 13 (39.4) | 13 (15.1) | .0064∗ |
| Type III–dominant RBC clone, n (%) | 20 (60.6) | 73 (84.9) |
| Variable . | TE (n = 56) . | No TE (n = 211) . | P value . |
|---|---|---|---|
| Demographics | |||
| Age at diagnosis, median (IQR), y | 42 (26.3-58) | 46 (27-62) | .5353 |
| Sex, n (%) | .0964 | ||
| Male | 30 (53.6) | 86 (40.8) | |
| Female | 26 (46.4) | 125 (59.2) | |
| Laboratory markers, median (IQR) | |||
| Hb (g/dL) | 9.5 (8.1-11.2) | 9.2 (8.1-10.7) | .8396 |
| ANC (×109/L) | 2.4 (1.2-3.9) | 1.4 (0.8-2.5) | .0212 |
| Platelets (×109/L) | 85 (28-137.5) | 37 (18-93) | .0064 |
| Absolute reticulocyte count (×103/μL) | 106 (61-184) | 77.5 (33.8-142) | .0392 |
| D-dimer (mg/L) | 1335 (337.5-3023) | 360 (237-820) | .0004 |
| LDH (U/L) | 603 (362-939) | 318 (311-902) | .0070 |
| AST (U/L) | 37 (18.5-61.5) | 25 (18-42.3) | .1701 |
| ALT (U/L) | 24 (17.5-56) | 20.5 (14-31.8) | .0606 |
| Creatinine (mg/dL) | 0.8 (0.66-1.07) | 0.8 (0.69-1.0) | .7700 |
| Albumin (g/dL) | 4 (3.8-4.5) | 4.3 (4.1-4.7) | .0030 |
| C-reactive protein (mg/dL) | 7.65 (2.15-19.45) | 0.7 (0.2-3) | .0459 |
| Presence of CHIP, n (%)∗ | 8 of 35 (23%) | 27 of 151 (19%) | .4798 |
| Disease characteristics | |||
| Prior AA, n (%) | 31 (55.4) | 161 (76.3) | .5505 |
| AA to PNH, time in mo, median (IQR) | 23.4 (1.5-126.4) | 36.9 (3.7-85.2) | .1385 |
| PIGA mutant, n (%) | 23 (67.7) | 124 (61.4) | .5684 |
| PIGA VAF, % (IQR) | 35.4 (14.6-47.4) | 11.6 (5.8-36.2) | .0172∗ |
| Granulocyte clone size, median (IQR) | 71.3 (42.7-92.2) | 12.8 (0.6-70.2) | <.0001 |
| RBC clone size, median (IQR) | 17.3 (5.9-49.3) | 1.8 (0.2-19.6) | <.0001 |
| Type II–dominant RBC clone, n (%) | 13 (39.4) | 13 (15.1) | .0064∗ |
| Type III–dominant RBC clone, n (%) | 20 (60.6) | 73 (84.9) |
ALT, alanine aminotransferase; CHIP, clonal hematopoiesis of indeterminate potential; Hb, hemoglobin.
Bold values indicate statistical significance.
Missing data (PIGA molecular information available in 91 patients, and PNH RBC clone type available in 100 patients; myeloid NGS available for 186 patients).
Univariate logistic regression analysis further confirmed higher odds of TEs for patients presenting with type II–dominant RBC phenotype (OR, 3.7; 95% CI, 1.46-9.23; P = .0056), RBC clone size of >20% (OR, 2.5; 95% CI, 1.31-4.86; P = .0053), granulocyte clone size of >70% (OR, 3.3; 95% CI, 1.76-6.16; P = .0057), PIGA VAF of >15% (OR, 3.36; 95% CI, 1.17-9.9; P = .0274), ANC of <1.5 × 109/L (OR, 0.42; 95% CI, 0.21-0.82; P = .0110), reticulocyte count of >80 × 103/μL (OR, 2.18; 95% CI, 1.10-4.61; P = .0369), platelets of <100 × 109/L (OR, 0.43; 95% CI, 0.22-0.85; P = .0152), LDH of >400 U/L (OR, 3.5; 95% CI, 1.73-7.4; P = .0004), albumin of <4.2 g/dL (OR, 3.1; 95% CI, 1.58-6.33; P = .0011), as well as AST of >40 U/L (OR, 2.3; 95% CI, 1.14-4.55; P = .0203; Table 3). Subsequent multivariate logistic analysis confirmed type II–dominant RBC phenotype (OR, 4.09; 95% CI, 1.32-13.19; P = .0160) as an independent factor associated with TEs (Table 4).
Univariate logistic regression analysis of predictors of thromboembolism in patients with PNH
| Variable . | OR . | Low 95% CI . | High 95% CI . | P value . |
|---|---|---|---|---|
| AST >40 U/L | 2.28 | 1.14 | 4.55 | .0203 |
| ALT >40 U/L | 2.15 | 0.99 | 4.59 | .0543 |
| Age >40 y | 0.81 | 0.45 | 1.47 | .4853 |
| Albumin <4.2 g/dL | 3.14 | 1.58 | 6.33 | .0011 |
| Creatinine >1 mg/dL | 1.2 | 0.54 | 2.53 | .6457 |
| D-dimers >1000 mg/L | 3.92 | 1.47 | 10.71 | .0064 |
| Hb <10 g/dL | 0.83 | 0.44 | 1.59 | .5726 |
| Reticulocytes >80 × 103/μL | 2.19 | 1.07 | 4.48 | .0320 |
| ANC <1.5 × 109/L | 0.42 | 0.21 | 0.82 | .0110 |
| Platelets <100 × 109/L | 0.43 | 0.22 | 0.85 | .0152 |
| LDH >400 U/L | 3.49 | 1.73 | 7.40 | .0004 |
| Type II–dominant RBC PNH | 3.65 | 1.46 | 9.23 | .0057 |
| RBC clonal size >20% | 2.54 | 1.31 | 4.86 | .0053 |
| Granulocytic clonal size >70% | 3.28 | 1.76 | 6.16 | .0002 |
| PIGA VAF >15% | 3.36 | 1.16 | 9.72 | .0254 |
| Variable . | OR . | Low 95% CI . | High 95% CI . | P value . |
|---|---|---|---|---|
| AST >40 U/L | 2.28 | 1.14 | 4.55 | .0203 |
| ALT >40 U/L | 2.15 | 0.99 | 4.59 | .0543 |
| Age >40 y | 0.81 | 0.45 | 1.47 | .4853 |
| Albumin <4.2 g/dL | 3.14 | 1.58 | 6.33 | .0011 |
| Creatinine >1 mg/dL | 1.2 | 0.54 | 2.53 | .6457 |
| D-dimers >1000 mg/L | 3.92 | 1.47 | 10.71 | .0064 |
| Hb <10 g/dL | 0.83 | 0.44 | 1.59 | .5726 |
| Reticulocytes >80 × 103/μL | 2.19 | 1.07 | 4.48 | .0320 |
| ANC <1.5 × 109/L | 0.42 | 0.21 | 0.82 | .0110 |
| Platelets <100 × 109/L | 0.43 | 0.22 | 0.85 | .0152 |
| LDH >400 U/L | 3.49 | 1.73 | 7.40 | .0004 |
| Type II–dominant RBC PNH | 3.65 | 1.46 | 9.23 | .0057 |
| RBC clonal size >20% | 2.54 | 1.31 | 4.86 | .0053 |
| Granulocytic clonal size >70% | 3.28 | 1.76 | 6.16 | .0002 |
| PIGA VAF >15% | 3.36 | 1.16 | 9.72 | .0254 |
ALT, alanine aminotransferase; Hb, hemoglobin.
Bold values indicate statistical significance.
Multivariate analysis of predictors of thromboembolism in patients with PNH
| Variable . | OR . | Low 95% CI . | High 95% CI . | P value . |
|---|---|---|---|---|
| AST >40 U/L | 0.73 | 0.25 | 2.02 | .5434 |
| ALT >40 U/L | 1.79 | 0.66 | 4.81 | .2472 |
| Albumin <4.2 g/dL | 0.49 | 0.21 | 1.11 | .0911 |
| D-dimers >1000 mg/L | 1.96 | 0.54 | 7.19 | .3026 |
| Reticulocytes >80 × 103/μL | 1.05 | 0.39 | 2.76 | .9271 |
| ANC <1.5 × 109/L | 0.82 | 0.32 | 2.13 | .6881 |
| Platelets <100 × 109/L | 0.69 | 0.25 | 1.93 | .4859 |
| LDH >400 U/L | 2.84 | 1.01 | 8.30 | .0514 |
| Type II–dominant RBC PNH | 4.09 | 1.32 | 13.19 | .0160 |
| RBC clonal size >20% | 0.70 | 0.22 | 2.16 | .5438 |
| Granulocyte clonal size >70% | 2.05 | 0.77 | 5.56 | .1536 |
| PIGA VAF >15% | 2.59 | 0.72 | 10.44 | .1600 |
| Variable . | OR . | Low 95% CI . | High 95% CI . | P value . |
|---|---|---|---|---|
| AST >40 U/L | 0.73 | 0.25 | 2.02 | .5434 |
| ALT >40 U/L | 1.79 | 0.66 | 4.81 | .2472 |
| Albumin <4.2 g/dL | 0.49 | 0.21 | 1.11 | .0911 |
| D-dimers >1000 mg/L | 1.96 | 0.54 | 7.19 | .3026 |
| Reticulocytes >80 × 103/μL | 1.05 | 0.39 | 2.76 | .9271 |
| ANC <1.5 × 109/L | 0.82 | 0.32 | 2.13 | .6881 |
| Platelets <100 × 109/L | 0.69 | 0.25 | 1.93 | .4859 |
| LDH >400 U/L | 2.84 | 1.01 | 8.30 | .0514 |
| Type II–dominant RBC PNH | 4.09 | 1.32 | 13.19 | .0160 |
| RBC clonal size >20% | 0.70 | 0.22 | 2.16 | .5438 |
| Granulocyte clonal size >70% | 2.05 | 0.77 | 5.56 | .1536 |
| PIGA VAF >15% | 2.59 | 0.72 | 10.44 | .1600 |
ALT, alanine aminotransferase.
Bold indicates statistical significance.
Management of TEs
Apart from patients with TEs of superficial veins (1.7%), who were promptly started on anticomplement treatment, all cases improved with addition of anticoagulation. One patient with portal vein thrombosis had severe thrombocytopenia precluding effective anticoagulant treatment and eventually died due to progressive Budd-Chiari syndrome. A coronary stent was placed in a patient experiencing acute coronary syndrome; and low-dose aspirin was started in all cases experiencing arterial cerebrovascular events. Profound anemia/thrombocytopenia inherent to the concomitant AA did not allow anticoagulation in 4 patients because of the high risk of bleeding, whereas in 1 patient an inferior vena cava filter was positioned. In the remaining cases, anticoagulation strategy consisted of warfarin (45%), DOACs (41%), and LMWH (14%), with a median treatment duration of 46 months (15.3-113.6). In 1 patient, warfarin was switched to DOACs for prolonged treatment, 2 Budd-Chiari syndrome cases underwent transjugular intrahepatic portosystemic shunt, and in 1 case of DVT plus a concomitant diagnosis of myeloproliferative neoplasm with MPLW515L an inferior vena cava filter was placed.
Recurrence of TEs under anticoagulants was noted in only 1 case receiving enoxaparin and 3 cases receiving warfarin, of whom 1 was found to have subtherapeutic (eg, <2) international normalized ratio levels at the time of TE recurrence. While on anticoagulation, 7 patients (5 on warfarin, and 2 on DOACs) experienced bleeding. Among them, the 2 patients on DOACs experienced an acute rectal hemorrhage but had additional bleeding risk factors (1 was on concomitant clopidogrel treatment, and the other had an additional diagnosis of ulcerative colitis). No thrombotic recurrence was observed in all 19 patients treated with DOACs at a median observation of 17.1 months (8.9-45). Because of achievement of adequate control of the manifestations of the underlying disease and considering patient choice, a discontinuation of anticoagulation was attempted in 14 patients, without any TE recurrence at a median time of 51.4 months (29.9-86.8). Of note is that radiologic studies were performed to follow-up TEs according to standard guidelines for thrombosis management outside of PNH diagnosis,34 and were deemed helpful in guiding treatment discontinuation in the absence of active hemolysis and stable disease parameters (Figure 3).
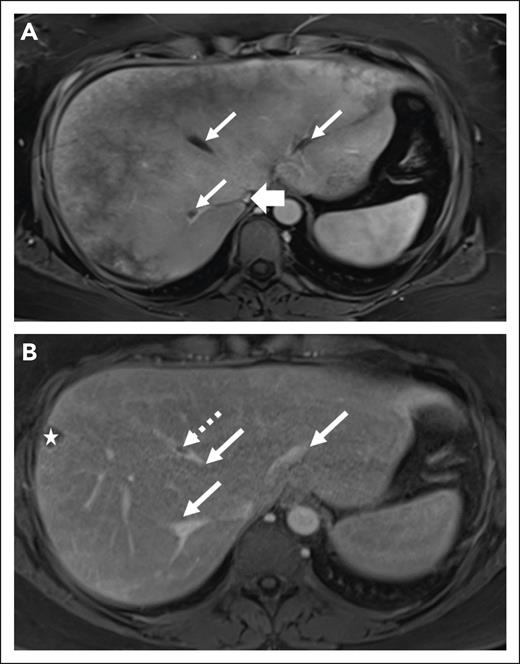
Radiologic features of thrombosis at diagnosis and follow-up of a 45-year-old woman with PNH. (A) Axial postcontrast VIBE MRI of a 45-year-old woman demonstrates heterogeneous enhancement of the liver. There is bland thrombus in 3 hepatic veins (thin arrows) and decreased IVC caliber without discrete thrombus (thick arrow). (B) After treatment, axial postcontrast VIBE image demonstrates significantly decreased thrombus burden from the prior (thin arrows) with persistent thrombus within middle hepatic vein (dashed arrow). The star indicates an incidental cyst in the liver periphery.
Radiologic features of thrombosis at diagnosis and follow-up of a 45-year-old woman with PNH. (A) Axial postcontrast VIBE MRI of a 45-year-old woman demonstrates heterogeneous enhancement of the liver. There is bland thrombus in 3 hepatic veins (thin arrows) and decreased IVC caliber without discrete thrombus (thick arrow). (B) After treatment, axial postcontrast VIBE image demonstrates significantly decreased thrombus burden from the prior (thin arrows) with persistent thrombus within middle hepatic vein (dashed arrow). The star indicates an incidental cyst in the liver periphery.
Discussion
Nowadays, the management of PNH-related thrombosis remains a clinical conundrum. Given the lack of randomized clinical trials aimed at assessing this specific instance and the orphan nature of the disease, retrospective analysis of large cohorts with prolonged follow-up are critical to guide clinical practice. Here, we report the largest PNH series to date (267 patients with a 5.9 years of median follow-up), detailing clinical-molecular risk factors, features of TEs, and their management in the era of novel anticomplement therapy and DOACs.
In line with previous reports,21,35-39 TEs occurred most commonly at disease onset or in cases secondarily evolving to classical hemolytic disease, as shown by the analyses accounting for increasing PNH granulocytes and RBC clone sizes and disease activity parameters (LDH, reticulocyte counts, and D-dimers). Conversely, TEs occurred less in patients with lower platelet counts and ANCs, chiefly those with concomitant AA, as previously demonstrated.21,37,40 Overall, TEs mainly involved the venous system, and specifically the intra-abdominal veins as the most common site. Notably, also patients with <20% PNH granulocyte clone size experienced TEs, in line with a very recent analysis from the International PNH Registry.41 Anticoagulation strategies were heterogeneous, with increasing use of DOACs in the most recent years.
Analysis of factors associated with TEs revealed that patients with a type II–dominant RBC clones were at higher risk of thrombotic complications. This observation is in line with a previous study from the United Kingdom reporting that cases with a higher proportion of type II vs type III RBCs (eg, what we called “type II dominant”) had increased risk of TEs, regardless of the degree of the hemolytic process.42 Patients with type II–dominant RBC PNH generally display lower LDH levels, typically resulting from an incomplete GPI deficiency due to missense PIGA mutation as compared with deleterious mutations in type III cases, whereby the deficiency is absolute and RBCs are completely vulnerable to hemolysis.23 In our cohort, not the type but rather the clonal burden of PIGA mutations as well as the presence of mosaicism (≥2 hits) associated with TE occurrence. Paralleling the molecular findings, a higher disease burden/activity as per clinical parameters such as LDH, reticulocytes, and D-dimer levels, correlated with higher TE odds. Furthermore, the higher levels of AST and the lower albumin concentrations in patients experiencing TEs may be the epiphenomenon of an underlying liver disease, especially in cases developing Budd-Chiari syndrome.
Multiple studies have shown the noninferiority, safety, and ease of administration of DOACs as compared with vitamin K antagonists in the treatment of acute TEs, with decrease of bleeding complications and need for a patient’s routine laboratory controls.43 Their application in acquired thrombophilias is theoretically possible, but recent data on antiphospholipid syndrome have shown that disease-specific characteristics may preclude their use in all prothrombotic states.44 However, in other conditions such as cancer-associated TEs, efficacy and safety of DOACs have been proven by several randomized trials when compared with traditional antivitamin K or LMWH, leading to their increased use and opening an opportunity for applications in other scenarios.20
A matter of controversy is the duration of anticoagulation treatment in PNH. Indeed, only anecdotal reports have described the discontinuation of anticoagulation in such a setting.19 Although greatly diminished, rates of TEs were not completely abated upon initiation of anticomplement therapy, as also shown by a recent US-based analysis.45 However, once the disease is under control (LDH of <1.5 upper limit of normal) on a complement inhibitor, one could envision discontinuing anticoagulation after 3 to 6 months, if radiological and clinical signs of thrombosis are absent. It is important to emphasize that this decision making should be shared between the treating physician and the patient, acknowledging the scarcity of available data in the literature.18 Anticoagulation discontinuation has been deemed safe in a recently described small cohort of patients with PNH from Johns Hopkins Hospital, whose rate of thrombosis appeared similar regardless of anticoagulation, if complete PNH control was achieved.46 Accordingly, our data show that discontinuation was feasible and safe in patients who had reached a complete control of PNH manifestations and a radiologic resolution of the TE. Indeed, no recurrence was registered at a median follow-up of 4 years. Obvious are the implications of such an approach on potential complications of prolonged anticoagulation (eg, bleeding) and quality of life of patients, already subjected to life-long infusions and potential hospital visits and admissions. Even more attractive is the possibility of discontinuing anticoagulation in patients with PNH and concomitant AA. As shown in our cohort, these cases were mostly treated with LMWH, because thrombocytopenia would constitute additional risk factor for bleeding complications, especially if other predisposing conditions (eg, inflammatory bowel disease or antiplatelet agents, as in our data) are present.
In conclusion, thrombosis in PNH is directly linked to the disease phenotype and activity, and its management includes both anticoagulation and disease-specific therapy. In a real-life cohort with an extensive follow-up (2043 patient-years vs the 1683.4 of the seminal meta-analytic study14 conducted before DOACs and novel anti-PNH treatments, and based on trial data), we show that rate of TEs varies according to not only granulocytes but also RBC clone size, type of RBC dominant clone, concomitant AA presence, hemolysis parameters, and treatment initiation. Most importantly, although previously TEs prompted life-long anticoagulation, its discontinuation is possible and safe without TE recurrence if appropriate complement inhibition is prescribed. Selective application of DOACs in the early phase of acute TEs may not only lead to better TE control but also mitigate risks of prolonged anticoagulation. Perhaps, thanks to the recent expansion of the PNH therapeutic arsenal and the increased knowledge of DOACs use in a variety of clinical settings, we are getting close to taming this instance of “the most vicious acquired thrombophilic state known in medicine.”47
Acknowledgments
The authors thank the patients and their families.
This project was supported by National Institutes of Health, National Heart, Lung, and Blood Institute grant R35HL135795 (J.P.M.); Aplastic Anemia and MDS International Foundation (V.V., S.P., and J.P.M.); and Foundation For Rare Diseases and Association hémoglobinurie paroxystique nocturne (HPN) France, Aplasie Médullaire (S.P.).
Authorship
Contribution: C.G., and H.A. collected, analyzed, interpreted clinical and molecular data, and wrote the manuscript; D.D., F.U., N.K., Y.K., V.V., S.P., B.J.P., V.D., N.M., S.K.B., A.K., and T.B. participated in patient recruitment, collected clinical data, gave helpful intellectual insights, and edited the manuscript; C.C. revised clinical patient data and radiologic images; S.P. analyzed and interpreted clinical data; J.P.M. designed the study, conceptualized and sponsored the overall project, and edited the manuscript; and all authors read and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jaroslaw P. Maciejewski, Department of Translational Hematology and Oncology Research, Taussig Cancer Institute 9620 Carnegie Ave, Building NE6-314, Cleveland, OH 44106; email: maciejj@ccf.org.
References
Author notes
C.G. and H.A. contributed equally to this study.
The data sets used within this study, and additional information not presented in the manuscript or data sharing are available on request from the corresponding author, Jaroslaw P. Maciejewski (maciejj@ccf.org).
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal