CD19 occupancy with CD19 monoclonal antibody leads to less activation of CART19, which reduces CAR T apoptosis and tumor cell pyroptosis.
Sequential therapy with tafasitamab followed by CART19 results in enhanced antitumor activity of CART19 and lower CRS severity after CART19.
Visual Abstract
In the development of various strategies of anti-CD19 immunotherapy for the treatment of B-cell malignancies, it remains unclear whether CD19 monoclonal antibody therapy impairs subsequent CD19-targeted chimeric antigen receptor T-cell (CART19) therapy. We evaluated the potential interference between the CD19-targeting monoclonal antibody tafasitamab and CART19 treatment in preclinical models. Concomitant treatment with tafasitamab and CART19 showed major CD19 binding competition, which led to CART19 functional impairment. However, when CD19+ cell lines were pretreated with tafasitamab overnight and the unbound antibody was subsequently removed from the culture, CART19 function was not affected. In preclinical in vivo models, tafasitamab pretreatment demonstrated reduced incidence and severity of cytokine release syndrome and exhibited superior antitumor effects and overall survival compared with CART19 alone. This was associated with transient CD19 occupancy with tafasitamab, which in turn resulted in the inhibition of CART19 overactivation, leading to diminished CAR T apoptosis and pyroptosis of tumor cells.
Introduction
Over the last 3 decades, the development of monoclonal antibodies (mAbs) has transformed the landscape of cancer treatment.1,2 Many breakthrough immunotherapies have arisen from this revolutionary concept, including chimeric antigen receptor T (CAR T) cell therapy.3,4 CD19-targeted CAR T-cell (CART19) therapy has demonstrated unprecedented outcomes in patients with relapsed/refractory (R/R) B-cell neoplasms, which has led to the recent approval of multiple CART19 products, such as tisagenlecleucel, axicabtagene ciloleucel, lisocabtagene maraleucel, and brexucabtagene autoleucel, by the Food and Drug Administration (FDA) and other regulatory agencies.5-11 Despite the clinical success of CAR T-cell therapy, it often mediates limited responses in some hematologic malignancies,12-15 and its efficacy in solid tumors is limited.16 Furthermore, the widespread use of CAR T-cell therapy is limited owing to life-threatening toxicities, including cytokine release syndrome (CRS) and neurotoxicity.17-19
Tafasitamab is an immunotherapeutic drug comprising a CD19-targeting Fc-enhanced humanized mAb that mediates antibody-dependent cellular cytotoxicity, antibody-dependent cellular phagocytosis, and direct cytotoxicity.20,21 It was studied in a pivotal phase 2 clinical trial for R/R diffuse large B-cell lymphoma (DLBCL) in combination with the immunomodulatory agent lenalidomide (L-MIND, NCT02399085).22,23 Tafasitamab was recently approved in combination with lenalidomide for the treatment of R/R DLBCL not otherwise specified, including DLBCL arising from low-grade lymphoma, and for patients who are not eligible for autologous stem cell transplant.24
In the development of various strategies of anti-CD19 immunotherapy for the treatment of B-cell malignancies, important questions have arisen regarding the sequence of different CD19-directed therapies in the clinic and whether a CD19 mAb, such as tafasitamab, affects subsequent CART19 cell therapy. Relevant to the context of this study, all approved CART19 cell therapies utilize a CD19-targeting single-chain variable fragment (scFv) derived from clone FMC63, and its CD19 epitope overlaps with the epitope of another anti-CD19 antibody clone, 4G7.25 4G7 was used for antibody humanization to generate tafasitamab,21 raising concerns about potential CD19 binding competition and interference between the 2 therapeutic strategies (CART19 and tafasitamab). Another important aspect to consider is the observation that CD19-negative relapse after anti-CD19 therapy is a common phenomenon26-28 in B-acute lymphoblastic leukemia (ALL), which precludes subsequent therapy with another anti-CD19 agent. A recently published report by Duell et al, demonstrated in a small cohort of 6 patients that CD19 expression is maintained in patients with DLBCL after treatment with tafasitamab.29
In this study, we evaluated the potential interaction between tafasitamab and CART19 treatment in preclinical lymphoma and leukemia models, using a CAR construct similar to an FDA-approved therapy (FMC63-41BBζ).30 Specifically, we focused on whether there was any impairment in antitumor activity when tafasitamab was used either in combination with or before CART19 cell therapy.
Material and methods
Tafasitamab and HD37
Tafasitamab was obtained from Morphosys (Planegg, Germany), and HD37 was purchased from Absolute Antibody (Boston, MA). For HD37, Pierce Protein Concentrators (ThermoFisher Scientific, Waltham, MA) were used to remove the preservative before the assay. For in vitro experiments, tumor cells were pretreated or treated with different doses of tafasitamab (10-400 μg/mL) or human immunoglobulin G1 (IgG1) isotype control, as outlined in the specific sections. In some experiments, tafasitamab was washed and removed from the culture before in vitro experiments, as outlined in the specific sections of our study. For in vivo experiments, 5 to 10 mg/kg of tafasitamab was intraperitoneally injected as indicated.
The other materials and methods used in this study are described in supplemental Material and methods, available on the Blood website.
Results
Tafasitamab competes with the FMC63 binding domain of CART19 for binding to CD19
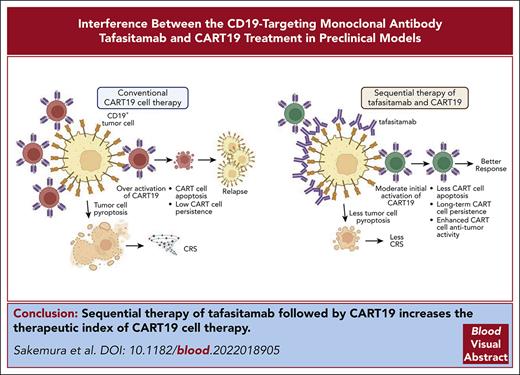
To study the potential CD19 competition between tafasitamab and CART19, we performed flow cytometry experiments using the CD19+ cell line Nalm6. Different treatment sequences of tafasitamab and FMC63 Fab were tested on Nalm6, and the final detection of CD19-targeted molecules was performed using different PE-labeled antibodies. Preincubation with tafasitamab completely abolished FMC63 binding to CD19 (Figure 1A, left panel), whereas analysis of the opposite sequence showed that preincubation with FMC63 only partially prevented tafasitamab binding (Figure 1A, right panel). These data demonstrated that both antibody-binding domains compete for an overlapping CD19 epitope. The reduction in FMC63 binding was more prominent when the cells were first treated with tafasitamab than in the reverse setting. This can be explained by the higher affinity of bivalent binding of tafasitamab compared with the relatively weak binding of monovalent FMC63 Fab to the common CD19 epitope.31
Binding competition of tafasitamab and CART19 to the B-cell surface marker CD19. (A) Detection of FMC63 using anti-His-PE (left) and tafasitamab using anti-Fcγ PE (right). First, Nalm6 was incubated with either FMC63-based His-tagged Fab (3.3 μg/mL = 66 nM), tafasitamab (10 μg/mL = 66.67 nM), or no antibody as a negative control. Second, either FMC63-based anti-CD19 Fab, tafasitamab, or no antibody was added and further incubation was performed. Third, a washing step, incubation with PE-labeled detection antibodies binding to FMC63-Fab (anti-His) or tafasitamab (anti-IgG, Fcγ-specific), another washing step, and flow cytometric analysis were performed (mean ± standard deviation [SD], ∗∗∗∗P < .0001, 1-way analysis of variance [ANOVA]; 2 independent experiments, 3 replicates). (B) Cytotoxicity assay of CART19 against CD19+ luciferase+ JeKo-1 in the presence of increasing doses of tafasitamab (10-400 μg/mL). Isotype IgG antibody was used as a control. At 24 hours, cytotoxicity was assessed by luminescence relative to controls (mean ± standard error of the mean [SEM], ∗P < .05, ∗∗P = .01, ∗∗∗P < .001, ∗∗∗∗P < .0001, 2-way ANOVA; n = 3, 2 replicates). (C) CART19 cell antigen-specific proliferation assay in the presence of increasing doses of tafasitamab (10-400 μg/mL). Isotype IgG antibody was used as a control. Lethally irradiated JeKo-1 were used for antigen stimulation. On day 5 of coculture, the absolute number of CD3+ T cells were quantified using volumetric flow cytometry (mean ± SEM, ∗∗P < .01, 1-way ANOVA; n = 3, 2 replicates). (D) CART19 cell antigen-specific CD107a degranulation assay in the presence of increasing doses of tafasitamab (10-400 μg/mL). Isotype IgG antibody was used as a control (mean ± SEM, ∗∗∗∗P < .0001, 2-way ANOVA; n = 3, 2 replicates). n.s., not significant; UTD, untransduced T cells.
Binding competition of tafasitamab and CART19 to the B-cell surface marker CD19. (A) Detection of FMC63 using anti-His-PE (left) and tafasitamab using anti-Fcγ PE (right). First, Nalm6 was incubated with either FMC63-based His-tagged Fab (3.3 μg/mL = 66 nM), tafasitamab (10 μg/mL = 66.67 nM), or no antibody as a negative control. Second, either FMC63-based anti-CD19 Fab, tafasitamab, or no antibody was added and further incubation was performed. Third, a washing step, incubation with PE-labeled detection antibodies binding to FMC63-Fab (anti-His) or tafasitamab (anti-IgG, Fcγ-specific), another washing step, and flow cytometric analysis were performed (mean ± standard deviation [SD], ∗∗∗∗P < .0001, 1-way analysis of variance [ANOVA]; 2 independent experiments, 3 replicates). (B) Cytotoxicity assay of CART19 against CD19+ luciferase+ JeKo-1 in the presence of increasing doses of tafasitamab (10-400 μg/mL). Isotype IgG antibody was used as a control. At 24 hours, cytotoxicity was assessed by luminescence relative to controls (mean ± standard error of the mean [SEM], ∗P < .05, ∗∗P = .01, ∗∗∗P < .001, ∗∗∗∗P < .0001, 2-way ANOVA; n = 3, 2 replicates). (C) CART19 cell antigen-specific proliferation assay in the presence of increasing doses of tafasitamab (10-400 μg/mL). Isotype IgG antibody was used as a control. Lethally irradiated JeKo-1 were used for antigen stimulation. On day 5 of coculture, the absolute number of CD3+ T cells were quantified using volumetric flow cytometry (mean ± SEM, ∗∗P < .01, 1-way ANOVA; n = 3, 2 replicates). (D) CART19 cell antigen-specific CD107a degranulation assay in the presence of increasing doses of tafasitamab (10-400 μg/mL). Isotype IgG antibody was used as a control (mean ± SEM, ∗∗∗∗P < .0001, 2-way ANOVA; n = 3, 2 replicates). n.s., not significant; UTD, untransduced T cells.
To better understand the functional consequences of binding competition between tafasitamab and CART19, we measured CART19 effector functions in the presence or absence of tafasitamab (10-400 μg/mL). A significant reduction in CART19 cytotoxicity (Figure 1B; supplemental Figure 1A) and proliferation (Figure 1C; supplemental Figure 1B) was observed in the presence of a supraphysiological concentration of tafasitamab (400 μg/mL). Similarly, CART19 degranulation was significantly reduced in the presence of higher doses of tafasitamab (Figure 1D; supplemental Figure 1C), suggesting dose-dependent impairment of CART19 function.
Tafasitamab treatment demonstrates potent in vitro and in vivo activity against CD19+ tumors
To confirm the antitumor activity of tafasitamab, an antibody-dependent cellular cytotoxicity assay was performed. Natural killer cells were isolated from healthy donors and cocultured with the CD19+ tumor cell lines JeKo-1, Nalm6, or OCI-Ly7 in the presence of tafasitamab (0.1-100 μg/mL). Tafasitamab induced potent cytotoxicity against all tested target cell lines (supplemental Figure 2A). Next, we determined the antitumor activity of tafasitamab in vivo. Here, tumor xenograft models were generated by inoculating 1.0 × 106 luciferase+ JeKo-1, Nalm6, or OCI-Ly7 into NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice via tail vein injection. One week after the injection, engraftment of tumor cells was confirmed by bioluminescence imaging (BLI), and mice were randomized according to tumor burden to receive either 10 mg/kg of tafasitamab or phosphate-buffered saline (PBS).32 All mice were treated with peripheral blood mononuclear cells derived from healthy donors before treatment with 10 mg/kg tafasitamab or PBS (supplemental Figure 2B). Tafasitamab showed a significant antitumor effect compared with PBS in the 3 different tumor xenograft models (supplemental Figure 2C). Notably, tafasitamab treatment of these xenografts in NSG mice did not induce antitumor activity in the absence of peripheral blood mononuclear cells.21,31 This indicates the importance of effector cells in the antitumor effects of tafasitamab (supplemental Figure 4A-B).
Prior treatment with tafasitamab does not impact CART19 functions in vitro
To determine whether prior tafasitamab treatment impacts subsequent CART19 cell activity, CD19+ cell lines were first treated with different doses of tafasitamab overnight. Before the cell lines were cocultured with CART19, tafasitamab was removed from the cell lines by simple washing/centrifugation. Then, tafasitamab-pretreated CD19+ cell lines were subsequently cocultured with CART19 immediately after washing out tafasitamab, and the CART19 effector functions were measured. There was no difference in cytotoxicity (Figure 2A; supplemental Figure 3A), proliferation (Figure 2B; supplemental Figure 3B), degranulation and intracellular cytokine levels (Figure 2C; supplemental Figure 3C) after coculture with tafasitamab pretreatment compared with isotype-control-pretreated JeKo-1, Nalm6, or OCI-Ly7.
Pretreating tumor cells with tafasitamab does not affect the antitumor activity of CART19. (A) Cytotoxicity assay of CART19 with tafasitamab-pretreated CD19+ cell line JeKo-1. JeKo-1 were treated with either the isotype IgG control or increasing doses of tafasitamab (10-400 μg/mL) overnight. The next day, tafasitamab was removed from the JeKo-1 by centrifugation. CART19 were cocultured at different effector-to-target ratios (E:T) with tafasitamab-pretreated luciferase+ JeKo-1. At 24 hours, cell death was assessed by luminescence, relative to that of the control. CART19 were able to induce cell death in all CD19+ cell lines, regardless of tafasitamab treatment (mean ± SEM, 2-way ANOVA; n = 3, 2 replicates). (B) CART19 cell antigen-specific proliferation assay after stimulation with tafasitamab-pretreated JeKo-1. The CD19+ cell line JeKo-1 was treated with different doses of tafasitamab (10-400 μg/mL) or an isotype IgG control overnight. Before coculture, tafasitamab or the isotype control was removed from JeKo-1 by centrifugation. On day 5 of culture, the absolute number of CD3+ T cells was quantified by volumetric flow cytometry (1-way ANOVA; n = 3, 2 replicates). (C) CART19 CD107a degranulation and intracellular cytokine assays after stimulation with tafasitamab-pretreated JeKo-1. CD19+ cell line JeKo-1 was treated with different doses of tafasitamab (10-400 μg/mL) or isotype IgG control overnight. Before coculture, tafasitamab or isotype control was removed from JeKo-1 by simple centrifugation. The levels of CD107a and intracellular cytokines were assessed by flow cytometry. The negative gate was determined based on the fluorescence minus one (FMO) control (mean ± SEM, 1-way ANOVA; n = 3, 2 replicates).
Pretreating tumor cells with tafasitamab does not affect the antitumor activity of CART19. (A) Cytotoxicity assay of CART19 with tafasitamab-pretreated CD19+ cell line JeKo-1. JeKo-1 were treated with either the isotype IgG control or increasing doses of tafasitamab (10-400 μg/mL) overnight. The next day, tafasitamab was removed from the JeKo-1 by centrifugation. CART19 were cocultured at different effector-to-target ratios (E:T) with tafasitamab-pretreated luciferase+ JeKo-1. At 24 hours, cell death was assessed by luminescence, relative to that of the control. CART19 were able to induce cell death in all CD19+ cell lines, regardless of tafasitamab treatment (mean ± SEM, 2-way ANOVA; n = 3, 2 replicates). (B) CART19 cell antigen-specific proliferation assay after stimulation with tafasitamab-pretreated JeKo-1. The CD19+ cell line JeKo-1 was treated with different doses of tafasitamab (10-400 μg/mL) or an isotype IgG control overnight. Before coculture, tafasitamab or the isotype control was removed from JeKo-1 by centrifugation. On day 5 of culture, the absolute number of CD3+ T cells was quantified by volumetric flow cytometry (1-way ANOVA; n = 3, 2 replicates). (C) CART19 CD107a degranulation and intracellular cytokine assays after stimulation with tafasitamab-pretreated JeKo-1. CD19+ cell line JeKo-1 was treated with different doses of tafasitamab (10-400 μg/mL) or isotype IgG control overnight. Before coculture, tafasitamab or isotype control was removed from JeKo-1 by simple centrifugation. The levels of CD107a and intracellular cytokines were assessed by flow cytometry. The negative gate was determined based on the fluorescence minus one (FMO) control (mean ± SEM, 1-way ANOVA; n = 3, 2 replicates).
Sequential therapy with tafasitamab and CART19 improves the efficacy of CART19 cell therapy in lymphoma xenograft models
Based on the in vitro studies highlighted above, we aimed to study the impact of tafasitamab treatment on sequential or concomitant CART19 cell therapy in vivo. First, we used a two-step JeKo-1 lymphoma xenograft strategy to simulate patients treated first with tafasitamab, followed by CART19 cell therapy at a later time point. This model was generated through the IV injection of luciferase+ JeKo-1 into NSG mice. One week later, the tumor burden was assessed by BLI, and the mice were randomized to receive either 10 mg/kg of tafasitamab32 or PBS (Figure 3A). Treatment with tafasitamab did not induce discernable antitumor effects in this model (supplemental Figure 4A-B), most likely due to a lack of effector cells. Flow cytometric analysis of splenic cells isolated from JeKo-1 xenografts demonstrated that CD19 was not detectable in tumor cells in mice treated with tafasitamab compared with mice treated with PBS (supplemental Figure 4C), suggesting that CD19 was masked with the anti-CD19 antibody in tafasitamab-treated mice.
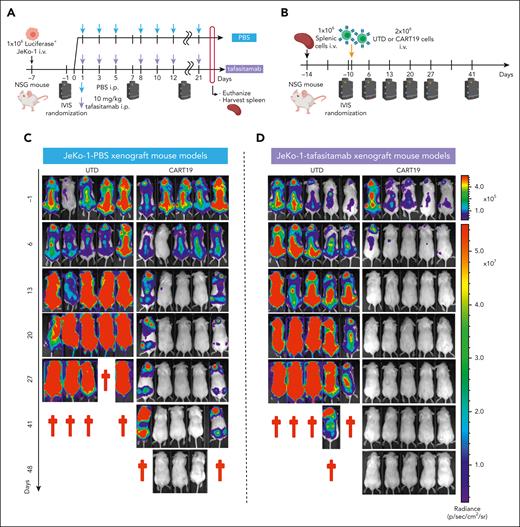
Pretreating mice with tafasitamab does not affect CART19 cell therapy. (A-B) The schemes of “two-step” JeKo-1 xenograft mouse model. (A) Immunocompromised NSG mice were inoculated with 1 × 106 of luciferase+JeKo-1 via IV injection. On day −1, the mice were imaged and randomized based on the tumor burden to receive either 10 mg/kg per day of tafasitamab or PBS. Treatment was performed three times per week until the end point. The mice were euthanized when they reached the end point, and their spleens were harvested and cryopreserved. (B) NSG mice were inoculated with 1 × 106 splenocytes (IV) obtained from panel A. On day −1, the mice were imaged and randomized based on the tumor burden to receive UTD or CART19. (C-D) BLI of mice engrafted with splenocytes derived from PBS (C) or tafasitamab (D) pretreated xenograft mice. (E) BLI curve for PBS-pretreated JeKo-1 xenograft model (mean ± SEM, ∗∗P < .01 at days 13, 20, and 27, t test); n = 5 in each group. (F) Kaplan-Meier curve for the PBS-pretreated JeKo-1 xenograft model (∗∗P < .01, log-rank test; hazard ratio (HR), 7.0; 95% confidence interval [CI], 1.381-35.48). (G) BLI curve for tafasitamab-pretreated xenograft mice (mean ± SEM, ∗P < .01 at days 13, 20, and 27, t test); n = 5 in each group. (H) Kaplan-Meier curve for the tafasitamab-pretreated JeKo-1 xenograft model. Mice treated with CART19 showed significantly better survival than those in the UTD group (∗∗P < .01, log-rank test; HR, 3.689; 95% CI, 0.8209-16.58). (I) BLI curves comparing CART19-administered mice engrafted with splenocytes derived from PBS- or tafasitamab-pretreated xenograft mice (mean ± SEM, ∗P < .05 at day 41, t test). (J) Kaplan-Meier curves comparing CART19-administered mice engrafted with splenocytes derived from PBS- or tafasitamab-pretreated xenograft mice (P = .05, log-rank test; HR, 5.96; 95% CI, 0.9971-35.63).
Pretreating mice with tafasitamab does not affect CART19 cell therapy. (A-B) The schemes of “two-step” JeKo-1 xenograft mouse model. (A) Immunocompromised NSG mice were inoculated with 1 × 106 of luciferase+JeKo-1 via IV injection. On day −1, the mice were imaged and randomized based on the tumor burden to receive either 10 mg/kg per day of tafasitamab or PBS. Treatment was performed three times per week until the end point. The mice were euthanized when they reached the end point, and their spleens were harvested and cryopreserved. (B) NSG mice were inoculated with 1 × 106 splenocytes (IV) obtained from panel A. On day −1, the mice were imaged and randomized based on the tumor burden to receive UTD or CART19. (C-D) BLI of mice engrafted with splenocytes derived from PBS (C) or tafasitamab (D) pretreated xenograft mice. (E) BLI curve for PBS-pretreated JeKo-1 xenograft model (mean ± SEM, ∗∗P < .01 at days 13, 20, and 27, t test); n = 5 in each group. (F) Kaplan-Meier curve for the PBS-pretreated JeKo-1 xenograft model (∗∗P < .01, log-rank test; hazard ratio (HR), 7.0; 95% confidence interval [CI], 1.381-35.48). (G) BLI curve for tafasitamab-pretreated xenograft mice (mean ± SEM, ∗P < .01 at days 13, 20, and 27, t test); n = 5 in each group. (H) Kaplan-Meier curve for the tafasitamab-pretreated JeKo-1 xenograft model. Mice treated with CART19 showed significantly better survival than those in the UTD group (∗∗P < .01, log-rank test; HR, 3.689; 95% CI, 0.8209-16.58). (I) BLI curves comparing CART19-administered mice engrafted with splenocytes derived from PBS- or tafasitamab-pretreated xenograft mice (mean ± SEM, ∗P < .05 at day 41, t test). (J) Kaplan-Meier curves comparing CART19-administered mice engrafted with splenocytes derived from PBS- or tafasitamab-pretreated xenograft mice (P = .05, log-rank test; HR, 5.96; 95% CI, 0.9971-35.63).
To ensure that tafasitamab treatment did not result in CD19 mutations, we further assessed tumor cells harvested after treatment, as shown in Figure 3A. CD20 microbeads were used to isolate JeKo-1 cells within splenic cells. Flow cytometric analysis after isolation revealed a pure population composed of >98% human CD45+CD20+ cells (supplemental Figure 5A-B). Flow cytometric analysis also demonstrated that CD19 recovered to baseline levels within 3 days of stopping tafasitamab (supplemental Figure 5C).
To further validate that prior tafasitamab treatment did not result in CD19 mutations, we performed whole-exome sequencing of isolated JeKo-1 cells. We observed no copy number variations or focal deletions at the CD19 locus (supplemental Figure 6A). A point mutation was observed in exome 3, but this occurred regardless of the CD19 antibody treatment (supplemental Figure 6B).
After confirming that tafasitamab did not result in CD19 mutations, we tested whether CART19 was efficacious in tafasitamab-pretreated JeKo-1 xenograft mouse models to mimic the clinical setting of CART19 cell therapy after tafasitamab treatment. Splenic cells were harvested (Figure 3A) and injected into naive NSG mice via the tail vein (Figure 3B). Tumor progression was assessed by BLI, and the mice were randomized to receive either untransduced T cells or CART19 (Figure 3B). CART19 treatment of PBS- or tafasitamab-pretreated JeKo-1 xenografts demonstrated potent antitumor effects (Figure 3C-E,G) and prolonged survival compared with untransduced T-cells treatment (Figure 3F,H). Interestingly, CART19 treatment of tafasitamab-pretreated JeKo-1 xenografts resulted in superior antitumor activity and overall survival compared with CART19 treatment of PBS-pretreated JeKo-1 xenografts (Figure 3I-J).
We aimed to expand these findings in an alternative experimental in vivo model that tested the immediate use of CART19 following tafasitamab discontinuation. To investigate tafasitamab and concomitant or subsequent CART19 treatment in the same mouse, a two-phase model was established. Here, we generated JeKo-1 xenografts by inoculating 1.0 × 106 cells of luciferase+ JeKo-1 into NSG mice via tail vein injection on day −14 (Figure 4A). One week after the injection of JeKo-1, on day −7, tumor burden was assessed using BLI. Mice were then randomized to receive (1) PBS (group 1, 3 times per week) or (2) tafasitamab (groups 2 and 3, 10 mg/kg intraperitoneal [IP] injection, 3 times per week). One week later, the tumor burden was reassessed using BLI. Mice treated with tafasitamab were rerandomized to either stop (group 2) or continue tafasitamab (group 3, 10 mg/kg IP, 3 times per week). All mice (groups 1, 2, and 3) were treated with 2.0 × 106 CART19 via tail vein injection on day 0, 14 days after JeKo-1 injection. Serial BLI and peripheral blood (PB) sampling were performed to monitor disease burden and CART19 expansion in vivo. The tafasitamab-continuation group (group 3) showed inferior antitumor control, less CART19 expansion, and reduced survival compared with either group 1 or 2 (Figure 4B-E). In the tafasitamab-discontinuation group (group 2), CART19 expansion was initially reduced compared with that in mice treated with CART19 alone (group 1) (Figure 4D, day 10). However, these mice (group 2) exhibited robust delayed CART19 expansion 23 days after infusion, which was significantly higher than CART19 expansion in the mice treated with PBS followed by CART19 (group 1). This was associated with significantly improved antitumor activity of CART19 (P < .05, at day 23) and overall survival in group 2 (tafasitamab followed by CART19) compared with group 1 (mice treated with PBS followed by CART19 alone, P = .04) and group 3 (tafasitamab continuation) (Figure 4C-E). Tafasitamab pharmacokinetics demonstrated a distinct drop in concentration following tafasitamab discontinuation in group 2, whereas in group 3, high tafasitamab levels were sustained throughout the experiment (Figure 4F).
CART19 in tafasitamab-pretreated mice shows a better antitumor response. (A) Treatment schema. NSG mice were inoculated with luciferase+ JeKo-1 on day −14, and tumor burden was analyzed using BLI on day −6. Mice were randomized according to their tumor burden to receive 10 mg/kg per day tafasitamab (IP, 10 mice) or PBS vehicle control (IP, 5 mice, group 1). Tumor burden was reassessed by BLI on day −1, and tafasitamab-treated mice were randomized to tafasitamab discontinuous (5 mice, group 2) or continuous (5 mice, group 3) groups. (B-C) BLI analysis. Group 2, mice pretreated with tafasitamab before CART19 cell injection, demonstrated better tumor control than those in groups 1 or 3 (∗P < .05, 2-way ANOVA). (D) CART19 cell expansion in vivo. Peripheral blood (PB) was collected on days 11 and 23 after the CART19 infusion to analyze the expansion of CART19. Group 2 showed late-onset CART19 expansion (∗∗P < .01, 2-way ANOVA). (E) Overall survival curve. Group 2 showed superior overall survival compared with groups 1 or 3 (∗P < .05, ∗∗P < .01, log-rank test, group 2 vs group 3; HR, 0.166; 95% CI, 0.04991-0.5515, group 2 vs 1 HR, 0.294; 95% CI, 0.08938-0.9671). (F) Pharmacokinetic analysis. Serial PB sampling was performed 17, 22, 28, 32, and 47 days after the first tafasitamab administration. The serum levels of tafasitamab were measured using an electrochemiluminescence (ECLA) assay.
CART19 in tafasitamab-pretreated mice shows a better antitumor response. (A) Treatment schema. NSG mice were inoculated with luciferase+ JeKo-1 on day −14, and tumor burden was analyzed using BLI on day −6. Mice were randomized according to their tumor burden to receive 10 mg/kg per day tafasitamab (IP, 10 mice) or PBS vehicle control (IP, 5 mice, group 1). Tumor burden was reassessed by BLI on day −1, and tafasitamab-treated mice were randomized to tafasitamab discontinuous (5 mice, group 2) or continuous (5 mice, group 3) groups. (B-C) BLI analysis. Group 2, mice pretreated with tafasitamab before CART19 cell injection, demonstrated better tumor control than those in groups 1 or 3 (∗P < .05, 2-way ANOVA). (D) CART19 cell expansion in vivo. Peripheral blood (PB) was collected on days 11 and 23 after the CART19 infusion to analyze the expansion of CART19. Group 2 showed late-onset CART19 expansion (∗∗P < .01, 2-way ANOVA). (E) Overall survival curve. Group 2 showed superior overall survival compared with groups 1 or 3 (∗P < .05, ∗∗P < .01, log-rank test, group 2 vs group 3; HR, 0.166; 95% CI, 0.04991-0.5515, group 2 vs 1 HR, 0.294; 95% CI, 0.08938-0.9671). (F) Pharmacokinetic analysis. Serial PB sampling was performed 17, 22, 28, 32, and 47 days after the first tafasitamab administration. The serum levels of tafasitamab were measured using an electrochemiluminescence (ECLA) assay.
Tafasitamab pretreatment of tumor cells reduces early activation and apoptosis of CART19
Having observed that pretreatment of tumor cells with tafasitamab promotes subsequent CART19 efficacy in vivo, we aimed to determine the mechanisms of this effect. We hypothesized that the reduction in available CD19 antigen levels for CAR T binding on the cell surface by tafasitamab masking would modulate CAR T activation, thereby ameliorating early apoptosis. To test this, we analyzed CAR T activation following pretreatment with tafasitamab. Here, we cocultured JeKo-1, Nalm6, and OCI-Ly7 with different doses of tafasitamab. Twenty-four hours later, tafasitamab was washed away and the cell lines were cocultured with CART19 for an additional 24 hours. We observed significantly reduced expression levels of CAR T activation markers such as CD25, CD69, granzyme B, and HLA-DR (Figure 5A; supplemental Figure 7A), as well as PD-1, CTLA4, and LAG-3 (Figure 5B; supplemental Figure 7B). In addition, there was less CART19 apoptosis measured by annexin V when CART19 were pretreated with tafasitamab (Figure 5C; supplemental Figure 7C). To further investigate whether the observed improvements in CART19 were specifically associated with tafasitamab, we conducted a comparative analysis with HD37, a CD19 mAb clone utilized in blinatumomab scFv.33 Before initiating the assay, we evaluated the CD19 binding affinities of tafasitamab and HD37 using a surface plasmon resonance assay. The results demonstrated that tafasitamab exhibited a significantly stronger CD19 binding affinity than HD37 (supplemental Table 1) (Kristina Ilieva et al, unpublished data, August 2023). Subsequently, we compared the effects of the IgG control antibody, tafasitamab, and HD37 on the apoptosis and activation markers of CART19. Interestingly, HD37 failed to induce any significant reduction in CART19 apoptosis or early activation, in contrast to tafasitamab (supplemental Figure 8A-B).
Pretreating JeKo-1 xenograft mice with tafasitamab reduces early activation and apoptosis of CART19. (A) CD19+ JeKo-1 were cocultured with CART19 in the presence of different concentrations of tafasitamab (10-400 μg/mL) or IgG isotype for 24 hours, and CD25, CD69, granzyme B, and HLA-DR were assessed by flow cytometry (mean ± SEM, ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001, 1-way ANOVA; n = 3, 2 replicates). (B) JeKo-1 were cocultured with CART19 in the presence of different concentrations of tafasitamab (10-400 μg/mL) or isotype IgG control for 24 hours. The expression of PD-1, CTLA-4, and LAG-3 in CD3+ T cells was analyzed by flow cytometry (mean ± SEM, ∗P < .05, ∗∗∗P < .001, ∗∗∗∗P < .0001, 1-way ANOVA; n = 3, 2 replicates). (C) JeKo-1 were cocultured with CART19 in the presence of different concentrations of tafasitamab (10-400 μg/mL) or IgG isotype control for 1 hour. Apoptotic T cells were analyzed by flow cytometry. Apoptotic T cells were defined as CD3+ annexin V+ 7-AAD−. (∗P < .05, 1-way ANOVA; n = 3, 2 replicates). (D-E) Experimental schema and BLI analysis. NSG mice were inoculated with luciferase+ JeKo-1 on day −14, and tumor burden was assessed using BLI on day −6. Mice were randomized according to their tumor burden to receive PBS vehicle control (IP, 7 mice, group 1) or 10 mg/kg per day of tafasitamab (IP, 8 mice, group 2). On day 0, all mice received 1.0 × 106 CART19; 24 hours after CART19 cell infusion, 3 mice from each group were euthanized, and the spleens were harvested. There were no significant differences in tumor burden on days −8 or −1 in either group (mean ± SEM, t test). (F) Flow cytometric analysis of CD69 and HLA-DR in human CD3+ T cells in splenocytes. Human CD3+ T cells were determined using murine CD45−, human CD45+, and human CD3+ (mean ± SEM, ∗P < .05, t test; n = 3 per group). (G) Flow cytometric analysis of apoptotic CD3+ T cells in the splenocytes. Apoptotic T cells were defined as CD3+ annexin V+ 7-AAD− (mean ± SEM, ∗P < .05, t test; n = 3 per group).
Pretreating JeKo-1 xenograft mice with tafasitamab reduces early activation and apoptosis of CART19. (A) CD19+ JeKo-1 were cocultured with CART19 in the presence of different concentrations of tafasitamab (10-400 μg/mL) or IgG isotype for 24 hours, and CD25, CD69, granzyme B, and HLA-DR were assessed by flow cytometry (mean ± SEM, ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, ∗∗∗∗P < .0001, 1-way ANOVA; n = 3, 2 replicates). (B) JeKo-1 were cocultured with CART19 in the presence of different concentrations of tafasitamab (10-400 μg/mL) or isotype IgG control for 24 hours. The expression of PD-1, CTLA-4, and LAG-3 in CD3+ T cells was analyzed by flow cytometry (mean ± SEM, ∗P < .05, ∗∗∗P < .001, ∗∗∗∗P < .0001, 1-way ANOVA; n = 3, 2 replicates). (C) JeKo-1 were cocultured with CART19 in the presence of different concentrations of tafasitamab (10-400 μg/mL) or IgG isotype control for 1 hour. Apoptotic T cells were analyzed by flow cytometry. Apoptotic T cells were defined as CD3+ annexin V+ 7-AAD−. (∗P < .05, 1-way ANOVA; n = 3, 2 replicates). (D-E) Experimental schema and BLI analysis. NSG mice were inoculated with luciferase+ JeKo-1 on day −14, and tumor burden was assessed using BLI on day −6. Mice were randomized according to their tumor burden to receive PBS vehicle control (IP, 7 mice, group 1) or 10 mg/kg per day of tafasitamab (IP, 8 mice, group 2). On day 0, all mice received 1.0 × 106 CART19; 24 hours after CART19 cell infusion, 3 mice from each group were euthanized, and the spleens were harvested. There were no significant differences in tumor burden on days −8 or −1 in either group (mean ± SEM, t test). (F) Flow cytometric analysis of CD69 and HLA-DR in human CD3+ T cells in splenocytes. Human CD3+ T cells were determined using murine CD45−, human CD45+, and human CD3+ (mean ± SEM, ∗P < .05, t test; n = 3 per group). (G) Flow cytometric analysis of apoptotic CD3+ T cells in the splenocytes. Apoptotic T cells were defined as CD3+ annexin V+ 7-AAD− (mean ± SEM, ∗P < .05, t test; n = 3 per group).
Given the in vitro findings of modified CAR T activation and apoptosis, we aimed to validate these observations following tafasitamab pretreatment in vivo using a JeKo-1 xenograft model. NSG mice were inoculated with 1.0 × 106 luciferase+ JeKo-1 on day −14 via tail vein injection. On day −8, mice were imaged with BLI and randomized according to tumor burden to receive 1) PBS (IP, 3 times per week) or 2) tafasitamab (10 mg/kg IP, 3 times per week) (Figure 5D). On day −1, the mice were reimaged to confirm that there was no difference in the tumor burden between the 2 groups (Figure 5E). All mice were then treated with 1.0 × 106 of CART19 on day 0. Twenty-four hours after CART19 cell infusion, the mice were euthanized and spleens were harvested.
As expected, CD19 on JeKo-1 was occupied by tafasitamab in mice treated with tafasitamab, which blocked binding of the detection antibody (supplemental Figure 9A-B). Flow cytometric analysis of splenic cells demonstrated that mice pretreated with tafasitamab showed significantly reduced T-cell activation (as measured by CD69 and HLA-DR [Figure 5F]) and fewer apoptotic T cells (Figure 5G) than mice pretreated with PBS.
Sequential therapy with tafasitamab and CART19 decreases the severity of CRS and increased antitumor activity of CART19
Having demonstrated that transient CD19 masking with tafasitamab results in delayed CAR T-cell expansion in vivo and enhanced antitumor activity, we next hypothesized that sequential therapy with tafasitamab and CART19 reduces CRS after CART19 therapy. Therefore, we aimed to test this hypothesis in our CAR T-cell toxicity mouse model.34-36 Briefly, this model was created by inoculating leukemic blasts from patients with R/R ALL IV into NSG mice. The mice underwent serial PB sampling to measure the disease burden using flow cytometry (determined by human CD45+ cells). Once the absolute number of human CD45+ cells reached ≥10/μL, the mice were randomized based on tumor volume into three groups: (1) 5 mg/kg tafasitamab IP on day −7, (2) IgG control on day −7, and (3) untreated group (no tafasitamab and no CART19). Mice in IgG control and tafasitamab groups were treated with high-dose CART19 (3.5 × 106 cells, IV) on day 0 (Figure 6A). To enable CAR T-cell tracking and quantification, we used luciferase+ CART19, which was generated through lentiviral transduction of T cells with CAR19 and luciferase-green fluorescent protein (GFP) viral vectors (supplemental Figure 10A). The mice then underwent daily monitoring for well-being, serial PB sampling to measure disease burden and cytokines, and serial BLI to quantify and track CAR T-cell expansion kinetics (Figure 6A). CRS was defined clinically by the development of motor weakness, hunched bodies, and weight loss and was associated with the elevation of human cytokines. Flow cytometric analysis revealed significant tumor cell regression (Figure 6B) and masking of CD19 on CD20+ leukemic cells (Figure 6C) in the mice that underwent tafasitamab pretreatment compared with the mice pretreated with IgG control. Although all mice demonstrated significant weight reduction and CAR T-cell expansion soon after CART19 administration, the IgG control group developed significantly greater weight reduction than the tafasitamab-pretreated mice (Figure 6D; supplemental Figure 10B). As we expected, serial BLI revealed that the CAR T-cell expansion of mice receiving IgG control was significantly higher compared with tafasitamab on days 1 and 2 (Figure 6E). The peak of CAR T-cell expansion in mice receiving IgG was on day 4, whereas CAR T-cell expansion in mice pretreated with tafasitamab peaked on day 6 (supplemental Figure 10C-D). Cytokine analysis on day 4 revealed significantly higher levels of CRS-associated cytokines (eg, sCD40L, interleukin 1 [IL-1] receptor antagonist, interferon gamma-inducible protein 10 (IP-10), IL-8, macrophage inflammatory protein (MIP)-1β, and granulocyte-macrophage colony-stimulating factor) in mice pretreated with IgG control than in mice pretreated with tafasitamab (Figure 6F; supplemental Figure 10E). The IgG control group developed a severely hunched body (supplemental Figure 10F) and motor weakness on day 4, whereas the tafasitamab-pretreated and no-treatment groups remained relatively healthy (supplemental Figure 10F). Cytokine analysis on day 6 demonstrated a mild elevation of proinflammatory cytokines, including IL-1ra, IL-8, and granulocyte-macrophage colony-stimulating factor, in the tafasitamab group (supplemental Figure 11). Most importantly, mice pretreated with the IgG control showed significantly shorter overall survival (all mice died within a few days of CART19 administration due to the development of CRS) than mice pretreated with tafasitamab (Figure 6G), which remained alive and achieved complete remission after CART19 cell therapy. Spleens were harvested at the end point and splenocytes were analyzed by flow cytometry. Spleens harvested from the mice pretreated with IgG control were significantly heavier (Figure 6H) and larger (Figure 6I) compared with mice pretreated with tafasitamab. Flow cytometric analysis of the spleens demonstrated significant infiltration of CD20+ leukemic blasts and a lack of CD3+ T cells in mice pretreated with control IgG, compared with CD3+ T-cell predominance in the absence of CD20+ leukemic blasts in mice pretreated with tafasitamab (Figure 6I).
Sequential therapy with tafasitamab and CART19 cell decreased the severity of CRS and increased the antitumor effect of CART19. (A) Experimental scheme. NSG mice were first treated with 30 mg/kg of busulfan via IP injection. After 24 hours, the mice were injected with 5 × 106 of leukemic blasts derived from patients with R/R ALL. The mice were then monitored for tumor burden via PB sampling. Once human CD45+ cells within mouse blood reached >10 cells per μL, the mice were randomized according to the burden of human CD45+ cells to receive (1) IgG control or (2) 5 mg tafasitamab on day −7. Tafasitamab and IgG controls were administered via IP injection. On day 0, IgG control and tafasitamab groups received 3.5 × 106 of luciferase+ CART19. The expansion of CART19 was monitored via serial BLI, and CRS was monitored through the weight and well-being of the mice. (B) Mice were bled before CART19 cell infusion, and the tumor burden was reassessed. The leukemic blasts were determined by human CD45+, mouse CD45−, and human CD20+ cells (∗P < .05, t test: n = 3-6 per group). (C) CD19 expression in leukemic blasts was assessed using flow cytometry. CD19 absolute counts were determined using Quantum Simply Cellular kits (∗P < .05, t test: n = 3-5 per group). (D) The percentage reduction in the weights from baseline is shown. The first and second asterisks or “n.s.” are showing the statistical comparisons between IgG control vs 5 mg/kg tafasitamab at day −7 or IgG control vs untreated xenografts, respectively ∗∗P < .01, ∗∗∗∗P < .0001, 2-way ANOVA). (E) Analysis of CART19 cell expansion in vivo. IgG control and tafasitamab groups were imaged with bioluminescence on days 1 and 2 (∗P < .05, ∗∗P < .01, t test). (F) On day 4 of CART19 treatment, mice were bled and cytokines were analyzed with multiplex (∗∗∗P < .001, ∗∗∗∗P < .0001, t test). (G) Kaplan-Meier curve is shown. IgG control vs 5 mg/kg tafasitamab at day −7 HR, 17.81; 95% CI, 3.805 to 83.40; ∗∗P = .0003 (log-rank test), 5 mg/kg tafasitamab vs untreated xenografts, 0.04569; 95% CI, 0.008027 to 0.2601; ∗∗∗P = .0005 (log-rank test), IgG control vs untreated xenografts HR, 13.64; 95% CI, 2.855 to 65.17, ∗∗P = .0011 (log-rank test). (H) The weights of spleens are shown. At the end of the experiments, the mice were euthanized and the spleens were harvested (∗P < .05, 1-way ANOVA). (I) The sizes of spleens are shown. Splenic cells were analyzed using flow cytometry. Leukemic blasts and CART19 were defined as human CD45+, mouse CD45−, human CD20+, and human CD45+, mouse CD45−, human CD3+, respectively.
Sequential therapy with tafasitamab and CART19 cell decreased the severity of CRS and increased the antitumor effect of CART19. (A) Experimental scheme. NSG mice were first treated with 30 mg/kg of busulfan via IP injection. After 24 hours, the mice were injected with 5 × 106 of leukemic blasts derived from patients with R/R ALL. The mice were then monitored for tumor burden via PB sampling. Once human CD45+ cells within mouse blood reached >10 cells per μL, the mice were randomized according to the burden of human CD45+ cells to receive (1) IgG control or (2) 5 mg tafasitamab on day −7. Tafasitamab and IgG controls were administered via IP injection. On day 0, IgG control and tafasitamab groups received 3.5 × 106 of luciferase+ CART19. The expansion of CART19 was monitored via serial BLI, and CRS was monitored through the weight and well-being of the mice. (B) Mice were bled before CART19 cell infusion, and the tumor burden was reassessed. The leukemic blasts were determined by human CD45+, mouse CD45−, and human CD20+ cells (∗P < .05, t test: n = 3-6 per group). (C) CD19 expression in leukemic blasts was assessed using flow cytometry. CD19 absolute counts were determined using Quantum Simply Cellular kits (∗P < .05, t test: n = 3-5 per group). (D) The percentage reduction in the weights from baseline is shown. The first and second asterisks or “n.s.” are showing the statistical comparisons between IgG control vs 5 mg/kg tafasitamab at day −7 or IgG control vs untreated xenografts, respectively ∗∗P < .01, ∗∗∗∗P < .0001, 2-way ANOVA). (E) Analysis of CART19 cell expansion in vivo. IgG control and tafasitamab groups were imaged with bioluminescence on days 1 and 2 (∗P < .05, ∗∗P < .01, t test). (F) On day 4 of CART19 treatment, mice were bled and cytokines were analyzed with multiplex (∗∗∗P < .001, ∗∗∗∗P < .0001, t test). (G) Kaplan-Meier curve is shown. IgG control vs 5 mg/kg tafasitamab at day −7 HR, 17.81; 95% CI, 3.805 to 83.40; ∗∗P = .0003 (log-rank test), 5 mg/kg tafasitamab vs untreated xenografts, 0.04569; 95% CI, 0.008027 to 0.2601; ∗∗∗P = .0005 (log-rank test), IgG control vs untreated xenografts HR, 13.64; 95% CI, 2.855 to 65.17, ∗∗P = .0011 (log-rank test). (H) The weights of spleens are shown. At the end of the experiments, the mice were euthanized and the spleens were harvested (∗P < .05, 1-way ANOVA). (I) The sizes of spleens are shown. Splenic cells were analyzed using flow cytometry. Leukemic blasts and CART19 were defined as human CD45+, mouse CD45−, human CD20+, and human CD45+, mouse CD45−, human CD3+, respectively.
Transient CD19 masking with tafasitamab contributes to diminished pyroptosis in tumor cells after CAR T-cell engagement
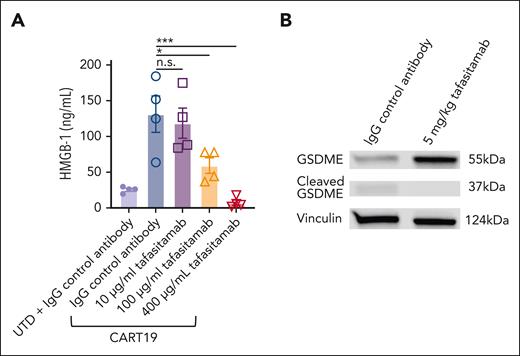
Having observed that pretreatment with tafasitamab promotes the subsequent CART19 therapeutic index in vivo, we hypothesized that transient CD19 masking of tumor cells with tafasitamab contributes to diminishing pyroptosis of tumor cells after CART19 cell therapy. Pyroptosis is a necrotic form of regulated cell death, and recent reports have indicated that it triggers CRS during CAR T-cell therapy.37,38 We first performed an in vitro high mobility group box 1 (HMGB-1) assay. CART19 and CD19+ JeKo-1 were cultured with tafasitamab (10-400 μg/mL) or IgG control for 24 hours. We then measured the level of HMGB-1 in JeKo-1 cells. The HMGB-1 assay showed that increasing doses of tafasitamab significantly reduced the level of HMGB-1 (Figure 7A), indicating reduced pyroptosis. To further investigate whether the observed reduction in HMGB-1 was specifically attributed to the binding affinity of tafasitamab, we compared its effects with those of another CD19 mAb, HD37, which has a lower binding affinity than tafasitamab (supplemental Table 1). Intriguingly, even at the highest administered dose, HD37 failed to demonstrate any significant reduction in HMGB-1 levels (supplemental Figure 12).
Transient CD19 masking with tafasitamab decreases tumor cell pyroptosis. (A) In vitro high-mobility group box 1 (HMGB-1) assay. CART19, CD19+JeKo-1, and increasing doses of tafasitamab (10-400 μg/mL) or the isotype control were cultured for 24 hours. Cells were processed with the Lumit HMGB1 immunoassay kit (mean ± SEM, ∗P < .05, ∗∗∗P < .001, 1-way ANOVA; n = 2, 2 replicates). (B) Western blot of tumor cells on day 1. Satellite mice were euthanized and their spleens were harvested. Tumor cells were then isolated with CD20 micro beads from splenic cells. The expression of GSDME was determined using western blot (n = 3 per group).
Transient CD19 masking with tafasitamab decreases tumor cell pyroptosis. (A) In vitro high-mobility group box 1 (HMGB-1) assay. CART19, CD19+JeKo-1, and increasing doses of tafasitamab (10-400 μg/mL) or the isotype control were cultured for 24 hours. Cells were processed with the Lumit HMGB1 immunoassay kit (mean ± SEM, ∗P < .05, ∗∗∗P < .001, 1-way ANOVA; n = 2, 2 replicates). (B) Western blot of tumor cells on day 1. Satellite mice were euthanized and their spleens were harvested. Tumor cells were then isolated with CD20 micro beads from splenic cells. The expression of GSDME was determined using western blot (n = 3 per group).
Given this in vitro finding, we aimed to analyze gasdermin E (GSDME), a parameter for pyroptosis in vivo. We used a CRS mouse model similar to that shown in Figure 6A. NSG mice were inoculated with 5.0 × 106 patient-derived xenograft (PDX) samples derived from patients with ALL on day −21 by tail vein injection. On day −8, the mice were bled and PB samples were analyzed by flow cytometry. Mice were randomized according to tumor burden (determined by absolute human CD45+ cells per μL) to (1) IgG control (IP, day −7), (2) tafasitamab (5 mg/kg IP, day −7), or (3) untreated (Figure 6A). IgG control and tafasitamab groups were treated with 3.5 × 106 of CART19 on day 0. Twenty-four hours after CART19 infusion, the mice were euthanized and the spleens were harvested. Tumor cells were then isolated using CD20 microbeads, and the purity of human CD45+ CD20+ cells was confirmed to be >98% by flow cytometry (supplemental Figure 13). Western blot analysis revealed that tumor cells from IgG-pretreated mice showed significantly lower expression of the apoptosis-inducing protein GSDME and higher expression of cleaved GSDME than tumors from tafasitamab-pretreated mice, indicating reduced tumor pyroptosis (Figure 7B).
Discussion
In this study, we examined the impact of prior CD19 targeting using a mAb on subsequent CART19 cell therapy in preclinical models. We demonstrated for the first time that concomitant treatment with tafasitamab and CART19 inhibits CART19 antitumor activity at high tafasitamab concentrations because of competition for CD19 binding and that prior treatment with tafasitamab does not impair the antitumor activity of subsequent CART19 cell therapy. In fact, our in vivo experiments suggest that prior tafasitamab treatment may enhance the CART19 therapeutic index, possibly through the transient occupancy of CD19 antigen availability for CAR T binding by tafasitamab-mediated CD19 epitope masking. This masking, in turn, contributes to a reduction in early T-cell activation, apoptosis, and tumor cell pyroptosis as well as the enhancement of CAR T-cell proliferation later in the course of treatment, as tafasitamab concentrations declined over time.
Our experiments address a clinically relevant question regarding the treatment options for large B-cell lymphoma: sequencing of clinically available FDA-approved CD19-directed therapies. Tafasitamab has been approved in combination with lenalidomide as a second-line therapy based on the L-MIND clinical trial in patients with large B-cell lymphoma.24 Three CART19 cell therapies (axicabtagene ciloleucel, tisagenlecleucel, and lisocabtagene maraleucel) have been approved by regulatory agencies in the United States and Europe for the treatment of large B-cell lymphoma after two or more previous therapies.10,11 Importantly, axicabtagene ciloleucel and lisocabtagene maraleucel were recently approved as second-line therapies, and tisagenlecleucel was approved as a third-line therapy.39 Ongoing clinical trials are investigating the use of tafasitamab and CART19 cell therapy in earlier lines of treatment. Given that CART19 cell therapy results in durable remission in ∼30% of patients,13 there are questions as to whether prior use of CD19-targeted therapy could influence the efficacy of subsequent CART19 cell therapy. Our preclinical studies suggest that prior tafasitamab does not affect subsequent CART19 cell therapy. These results are consistent with those of a recently published small case series that evaluated the outcomes of CART19 cell therapy in patients with R/R DLBCL previously treated with CD19-directed therapy. In one report, a total of 14 patients previously treated with loncastuximab tesirine and subsequently undergoing CART19 cell therapy (axicabtagene ciloleucel, tisagenlecleucel, lisocabtagene maraleucel, or others) were analyzed. The interval between anti-CD19 antibody treatment and CART19 treatment ranged from 22 to 600 days (median, 120 days). CR was observed in 43% of the patients, which was comparable with the overall CR rates in patients treated with CART19 cell therapy. CD19 expression was assessed by immunohistochemistry in 10 patients, all of whom were positive for CD19 expression after CD19-directed antibody-drug conjugate failure. This small study suggested that prior treatment with anti-CD19 antibody-drug conjugate in R/R DLBCL does not preclude subsequent CART19 cell therapy.40 A separate report included 12 patients treated with lisocabtagene maraleucel in the TRANSCEND study who had been previously treated with anti-CD19 therapy. Similarly, in this study, CART19 cellular kinetics and objective response rates were not impacted by prior CD19-directed therapy.41,42
Interestingly, our sequential CD19 targeting experiments in lymphoma xenograft models indicated that prior tafasitamab treatment may in fact enhance the antitumor activity of subsequent CART19 cell therapy. The improvement in CART19 antitumor activity was associated with the transient occupancy of CD19 with tafasitamab, which led to the amelioration of CAR T early activation, reduction of CAR T apoptosis, and enhanced CAR T proliferation. CAR T apoptosis is an increasingly recognized phenomenon in CAR T failure.43,44 In one study, T-cell activation and susceptibility to apoptosis were associated with a lack of response to CART19 cell therapy in patients with chronic lymphocytic leukemia.45 In another study, Fas was identified as one of the main factors leading to apoptosis of T cells in the tumor microenvironment.46 Furthermore, it has been shown in preclinical models that tuning the antigenic density of targets alters CAR T-activation and the threshold for T-cell activation.47 Fine-tuning CAR T-activation affects both antitumor activity and potential toxicity. In fact, our CRS studies showed amelioration of CAR T-mediated toxicities, while enhancing antitumor activity in preclinical models. These observations have been corroborated by early clinical trials. Ghorashian et al have reported the clinical activity of CAR T cells that are equipped with >40-fold lower affinity CD19 scFv compared with scFvs derived from FMC63. These low-affinity CAR T cells showed increased proliferation and cytotoxicity in vitro and enhanced proliferative and antitumor activity in vivo compared with the conventional FMC63 CAR T cells,48 consistent with our preclinical findings. They also tested these low-affinity CAR T cells in a clinical trial (NCT02443831) in patients with R/R pediatric B-cell ALL. Eighty-six percent of patients who were treated with low-affinity CAR T cells achieved a molecular response and demonstrated enhanced CAR T-cell expansion compared with previous studies.48 Overly potent T-cell receptor signaling or CAR stimulation is inclined to induce T-cell anergy or apoptosis. Therefore, an appropriate intensity of T-cell stimulation is critical.
Pyroptosis is a lytic form of cell death mediated by caspase-1,-4, and -5.49 It is usually accompanied by microbial infection, which leads to potent inflammation and the secretion of proinflammatory cytokines. The gasdermin (GSDM) family includes proteins that execute pyroptosis.50 GSDM perforates and disrupts the balance of the cell membrane, which results in cell swelling. Increasing evidence suggests that tumor cell pyroptosis and subsequent pyroptosis-release factors comprise a mechanism by which macrophages are activated during CRS. Liu et al have recently shown that granzyme B derived from CART19 cells enters tumor cells and leads to activation of caspase-3.37 Activated caspase-3 subsequently cleaves and activates GSDME.51 After pyroptosis, damage-associated molecular pattern molecules are released, which causes caspase-1 and GSDMD activation within macrophages and initiates CRS by producing myeloid-related cytokines/chemokines. GSDME blockade52 and caspase-3 inhibition53 have been shown to diminish pyroptosis. Liu et al successfully inhibited CRS in their CRS mouse model by knocking out GSDME, depleting macrophages, or inhibiting caspase-1.37 Our preclinical studies demonstrated a reduction in pyroptosis following CD19 masking with tafasitamab before CART19 cell therapy, which might further contribute to lowering the rates of CRS. Notably, the CAR T toxicity model used in this study has been previously reported to provide relevant similarities to the clinical parameters and time frames observed in patients with CRS and neuroinflammation after CAR T-cell administration.34 Our study suggests that using tafasitamab before CART19 cell therapy may represent a clinically viable strategy to modulate CD19 antigen density available for CAR T binding and ameliorate CAR T-cell activation and pyroptosis of tumor cells, thereby reducing the risk of CRS and enhancing CAR T activity. This warrants further investigation in phase 1 clinical studies. Future studies will assess the optimal time interval between the two treatment regimens.
It is unclear whether our findings of reduced CART19 apoptosis and enhanced antitumor activity are applicable to the sequencing of other CD19-targeted mAbs derived from different clones with CART19 therapy. It is possible that antibodies with varied affinities or CD19 binding epitopes impact CART19 differently. In this study, we showed that HD37, which has a weaker CD19 binding affinity than tafasitamab, did not contribute to diminishing apoptosis or early activation of CART19 or to the amelioration of tumor cell pyroptosis in vitro. Further investigation is warranted to precisely define the mAb characteristics responsible for favorable interactions with CART19.
Another limitation of this study is that we did not test the effect of tafasitamab on CD28-costimulated CART19 cells. Clinical trials have shown that a higher incidence or severity of CRS is seen in CD28-costimulated CAR T cells.54-56 Future studies of tafasitamab and CD28-costimulated CART19 are warranted. Additionally, to further broaden this strategy, sequential therapy with mAbs and CAR T cells that target other antigens (such as B-cell maturation antigen mAb and B-cell maturation antigen CAR T cells) will be assessed and reported in a follow-up manuscript.
In conclusion, we demonstrated that sequential treatment with tafasitamab and CART19 increased the therapeutic index of CART19 cell therapy in preclinical models.
Acknowledgments
The authors are grateful to Hong Xia, Omar L. Gutierrez Ruiz, and Charlotte Lässig for their technical assistance. Figures 3A-B, 4A, 5D, 6A, and supplemental Figure 2B were created with BioRender.com.
This work was supported by funding from Morphosys (S.S.K.), Mayo Clinic Center for Individualized Medicine (S.S.K.), Mayo Clinic Cancer Center (S.S.K.), National Institutes of Health, National Cancer Institute grants K12CA090628 (S.S.K.), R37CA266344 (S.S.K.), and K99CA273304 (R.L.S.), Predolin Foundation (R.L.S. and S.S.K.), Eagles Foundation (R.L.S.), and Gerstner Family Foundation (R.L.S.).
Authorship
Contribution: S.S.K. contributed to the conceptualization of the study; R.L.S., P.H., C.M.R., and S.S.K. contributed to the experimental design; R.L.S., P.H., C.M.R., T.N.H., and W.K.N. performed the experiments; R.L.S., P.H., and C.M.R. analyzed the data; C.A., J.S., C.H., S.S., and J.E. contributed reagents; S.S.K. supervised the study; R.L.S. and S.S.K. wrote the original draft; R.L.S., P.H., C.M.R., M.P-K., K.I., C.M.R., E.L.S., C.A., J.S., C.H., S.S., J.E., N.E.K., and S.S.K. wrote, edited, and reviewed the manuscript; and all authors edited and approved the final version of the manuscript.
Conflict-of-interest disclosure: S.S.K. is an inventor of patents in the field of chimeric antigen receptor (CAR) immunotherapy that are licensed to Novartis (through an agreement between Mayo Clinic, University of Pennsylvania, and Novartis). R.L.S., and S.S.K. are inventors of patents in the field of CAR immunotherapy that are licensed to Humanigen (through the Mayo Clinic). M.H. and S.S.K. are inventors of patents in the field of CAR immunotherapy that are licensed to Mettaforge (through the Mayo Clinic). S.S.K. receives research funding from Kite, Gilead, Juno, Bristol Myers Squibb (BMS), Novartis, Humanigen, MorphoSys, Tolero, and Lentigen. N.E.K. receives research funding from Acerta Pharma, BMS, Pharmacyclics, MEI Pharma, and Sunesis. N.E.K. has participated in the advisory board meetings of Cytomx Therapy, Janssen, Juno Therapeutics, AstraZeneca, and Oncotracker; and on DSMC for Agios and Cytomx Therapeutics. S.A.P. receives research funding from Pharmacyclics, MorphoSys, Janssen, AstraZeneca, TG Therapeutics, BMS, AbbVie, and Ascentage Pharma. S.A.P. participated in the advisory board meetings of Pharmacyclics, AstraZeneca, Genentech, Gilead, GlaxoSmithKline, Verastem Oncology, and AbbVie (he was not personally compensated for his participation). The remaining authors declare no competing financial interests.
Correspondence: Saad S. Kenderian, Division of Hematology, Mayo Clinic, 200 First St SW, Rochester, MN 55905; email: kenderian.saad@mayo.edu.
References
Author notes
∗R.L.S., C.M.R., and P.H. contributed equally.
Sequencing data are available at BioProject PRJNA974013.
The datasets generated and/or analyzed during the current study are available on reasonable request from the corresponding author, Saad S. Kenderian (Kenderian.Saad@mayo.edu).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

![Binding competition of tafasitamab and CART19 to the B-cell surface marker CD19. (A) Detection of FMC63 using anti-His-PE (left) and tafasitamab using anti-Fcγ PE (right). First, Nalm6 was incubated with either FMC63-based His-tagged Fab (3.3 μg/mL = 66 nM), tafasitamab (10 μg/mL = 66.67 nM), or no antibody as a negative control. Second, either FMC63-based anti-CD19 Fab, tafasitamab, or no antibody was added and further incubation was performed. Third, a washing step, incubation with PE-labeled detection antibodies binding to FMC63-Fab (anti-His) or tafasitamab (anti-IgG, Fcγ-specific), another washing step, and flow cytometric analysis were performed (mean ± standard deviation [SD], ∗∗∗∗P < .0001, 1-way analysis of variance [ANOVA]; 2 independent experiments, 3 replicates). (B) Cytotoxicity assay of CART19 against CD19+ luciferase+ JeKo-1 in the presence of increasing doses of tafasitamab (10-400 μg/mL). Isotype IgG antibody was used as a control. At 24 hours, cytotoxicity was assessed by luminescence relative to controls (mean ± standard error of the mean [SEM], ∗P < .05, ∗∗P = .01, ∗∗∗P < .001, ∗∗∗∗P < .0001, 2-way ANOVA; n = 3, 2 replicates). (C) CART19 cell antigen-specific proliferation assay in the presence of increasing doses of tafasitamab (10-400 μg/mL). Isotype IgG antibody was used as a control. Lethally irradiated JeKo-1 were used for antigen stimulation. On day 5 of coculture, the absolute number of CD3+ T cells were quantified using volumetric flow cytometry (mean ± SEM, ∗∗P < .01, 1-way ANOVA; n = 3, 2 replicates). (D) CART19 cell antigen-specific CD107a degranulation assay in the presence of increasing doses of tafasitamab (10-400 μg/mL). Isotype IgG antibody was used as a control (mean ± SEM, ∗∗∗∗P < .0001, 2-way ANOVA; n = 3, 2 replicates). n.s., not significant; UTD, untransduced T cells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/143/3/10.1182_blood.2022018905/3/m_blood_bld-2022-018905-gr1.jpeg?Expires=1768775785&Signature=p~uUYO2QefP71TJ1-s04-Rd~QTMpUI4ztUQ21iNrYwJasmMf~vO9r3lahtqA4rZmILG7DrVqlDGQP582VA32Z23s2TBJ8PAonvHaTy3Z8PQltieMClHcdai~bZuEetCh~wscLk~nrAK0NZuSpaXSW70LvxodGSBXz9Ah7iG-6o6oeTTHYxQ-fDr85gryLERSHE~6-q1I2PNFQJPfNnRmn2zEeLZK5fT~dx0fChDg4socUtces9QW7Vgsor9B5QCa4d0jPxPm4VKPFc0ruBx4okzGQQrGhgbpkfX7OkHpW5VmJJ5YPYZYNvl6Dd962sml7HoosKaex4gRXrTAOl4imw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Pretreating mice with tafasitamab does not affect CART19 cell therapy. (A-B) The schemes of “two-step” JeKo-1 xenograft mouse model. (A) Immunocompromised NSG mice were inoculated with 1 × 106 of luciferase+JeKo-1 via IV injection. On day −1, the mice were imaged and randomized based on the tumor burden to receive either 10 mg/kg per day of tafasitamab or PBS. Treatment was performed three times per week until the end point. The mice were euthanized when they reached the end point, and their spleens were harvested and cryopreserved. (B) NSG mice were inoculated with 1 × 106 splenocytes (IV) obtained from panel A. On day −1, the mice were imaged and randomized based on the tumor burden to receive UTD or CART19. (C-D) BLI of mice engrafted with splenocytes derived from PBS (C) or tafasitamab (D) pretreated xenograft mice. (E) BLI curve for PBS-pretreated JeKo-1 xenograft model (mean ± SEM, ∗∗P < .01 at days 13, 20, and 27, t test); n = 5 in each group. (F) Kaplan-Meier curve for the PBS-pretreated JeKo-1 xenograft model (∗∗P < .01, log-rank test; hazard ratio (HR), 7.0; 95% confidence interval [CI], 1.381-35.48). (G) BLI curve for tafasitamab-pretreated xenograft mice (mean ± SEM, ∗P < .01 at days 13, 20, and 27, t test); n = 5 in each group. (H) Kaplan-Meier curve for the tafasitamab-pretreated JeKo-1 xenograft model. Mice treated with CART19 showed significantly better survival than those in the UTD group (∗∗P < .01, log-rank test; HR, 3.689; 95% CI, 0.8209-16.58). (I) BLI curves comparing CART19-administered mice engrafted with splenocytes derived from PBS- or tafasitamab-pretreated xenograft mice (mean ± SEM, ∗P < .05 at day 41, t test). (J) Kaplan-Meier curves comparing CART19-administered mice engrafted with splenocytes derived from PBS- or tafasitamab-pretreated xenograft mice (P = .05, log-rank test; HR, 5.96; 95% CI, 0.9971-35.63).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/143/3/10.1182_blood.2022018905/3/m_blood_bld-2022-018905-gr3ej.jpeg?Expires=1768775785&Signature=R9z1Gos87e6P3or4ehto1H79riGt3uNC7fxp0hIEn26JHX-t-HPyAyam7lTJsPUFZCq1ZezII8Qd8xGCcqRL98frfiwnXkBEwXnh~osIEndYuc5lX9zshhVVkhVZiguw81NZ8rBe5D6sEEIz2dk2nNM0o6TWnWFxVhRkIHnL16DLz6w4Fkrj64WwUS1NF-OGtCXL2ynCyGZBmDrbLS2BURMIIOGxfjV-v8rq-f247P9-rd1MZmpNpcwTNuaofEbtqYYBTfi6FizAgT10cE0rhtXCC2q8tiT8u4bB96los~tTxt3p8jgqGSjpHvMwAFZX3iP4oX5iJIlS2UngGD-yrA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



Comments
CD19 Occupancy with Tafasitamab Before or After CAR-T Cell Therapy: From Bench to Bedside
In contrast, our real-world data from the DESCAR-T registry on ≥3rd line relapsed/refractory (R/R) large B-cell lymphoma (LBCL) showed that prior TAFA before CAR T-cell therapy led to very low success rates (6-month PFS: ~20%, Camus et al. Blood adv 2024). We also observed better outcome for patients starting tafasitamab beyond 6 months after CART19 cell infusion (median PFS: ~6 months vs ~2 months for early CART failure). The incidence of CRS in our patients exposed to TAFA prior to CART19 cell was ~64%, but no grade 3-4 events were observed, whereas severe CRS rates ranged from 13% to 22% in the pivotal ZUMA-1 and JULIET trials. This suggests a potential association between prior TAFA exposure and reduced CRS severity, supported by preclinical data.
A significant difference between our clinical data and preclinical models is the use of CD19 as a biomarker. In about 75% of our patients, CD19 staining was not performed, as rebiopsy or CD19 verification is not standard before CAR-T therapy, highlighting a challenge in clinical settings.
As anti-CD19 therapies become more common in LBCL treatment, optimizing their sequencing is crucial. Understanding how prior CD19 exposure affects therapy outcomes is a key focus of both our studies.