In this issue of Blood, Batty and colleagues1 report on hepatic adeno-associated virus vector–factor III (AAV-FIII) integration and fate more than 10 years after AAV gene delivery in dogs with hemophilia A (HA).
Reports that a single gene therapy treatment with an AAV vector essentially eliminated bleeding in the majority of adult HA patients have raised hopes that AAV-based gene therapy could provide a safe, affordable, lifelong correction of this disease.2-4
Currently, there are several ongoing clinical trials using different serotypes of AAV vectors and/or optimized FVIII transgenes, and, less than 1 year ago, one AAV5-FVIII (valoctocogene roxaparvovec-rvox; BioMarin, San Rafael, CA) gene therapy product was approved by the US Food and Drug Administration. Although the results of these clinical trials have been extremely promising, with some demonstrating durable FVIII expression, they have also identified unanticipated risks, including hepatic inflammation.
A wealth of preclinical work has also underscored the possibility that AAV, which was long assumed to remain episomal, integrates into the genome of hepatocytes, raising concern of the potential for genotoxicity. For example, injection of AAV vectors in mice with mucopolysaccharidosis type VII5 or Krabbe disease6 resulted in the development of hepatocellular carcinoma (HCC), and an increase in hepatocyte turnover and/or inflammation has also been reported to increase the risk of HCC in mice receiving AAV vectors.7
In contrast to these troubling findings in the murine system, one report on the 10-year follow-up of AAV-mediated FVIII expression in adult HA dogs detected >1700 integration events and indications of clonal expansion in liver tissue, yet there was no evidence of tumorigenesis.8 Similarly, molecular characterization of an HCC excised from a patient with hemophilia B who received AAV-FIX gene therapy demonstrated that the malignancy was unrelated to the therapy.9 Given these conflicting outcomes reported thus far, the potential for AAV vectors to induce genotoxicity remains one of the most important and pertinent areas of research to ascertain safety and predictable outcomes for people receiving AAV gene therapy.
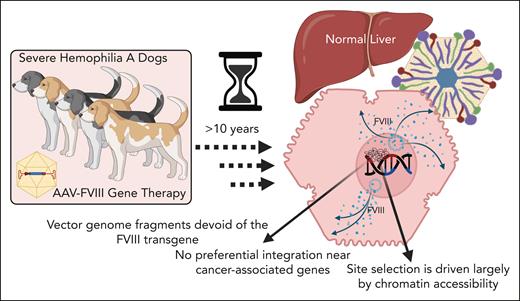
Batty et al provide important new insights regarding the fate of AAV vector genomes in the liver in a cohort of HA dogs over a decade after AAV-FVIII gene therapy administered via the portal vein (see figure). Using multiple complementary molecular techniques, the authors show that, at least in the dog model, long-term FVIII expression after AAV gene therapy is derived from long-lived episomal vector genomes, not from genomic integration. Moreover, and in agreement with previous reports, numerous genomic integration events were found in each AAV-treated dog, but not a single integration event analyzed consisted of an intact vector genome; that is, only fragments of the vector genome were present, which were often devoid of the FVIII transgene. Importantly, after analyzing thousands of integration sites and mapping these to both the canine and human genomes (as cancer-associated gene databases are not available for the canine genome), there was also no evidence of preferential integration near cancer-associated genes, nor for the emergence of clonal dominance over time. Interestingly, most integrations occurred in intergenic regions, and those integrations that resided within gene bodies were most often in genes that are highly expressed in the liver, such as albumin. Importantly, evidence of dysregulated expression of adjacent genes compared with normal or untreated hemophilia dogs were found.
Mechanisms of AAV-FVIII persistence in the liver of dogs with severe HA more than 10 years after therapy. Nonintegrated episomal forms of the vector correlated with long-term FVIII expression. Random integration was frequent, but no full-length integrated vector genomes were detected. Integrations were not associated with oncogene upregulation and no histopathological evidence of tumorigenesis was found.
Mechanisms of AAV-FVIII persistence in the liver of dogs with severe HA more than 10 years after therapy. Nonintegrated episomal forms of the vector correlated with long-term FVIII expression. Random integration was frequent, but no full-length integrated vector genomes were detected. Integrations were not associated with oncogene upregulation and no histopathological evidence of tumorigenesis was found.
The authors also performed an assay for transposase-accessible chromatin sequencing and bisulfite sequencing/mapping, the results of which revealed that integration site selection is driven largely by chromatin accessibility and is unrelated to methylation status of the locus in question. Intriguingly, they also showed that one of the more common sites for genomic integration was within the native canine FVIII gene, most often within intronic regions. The authors postulate that these integrations were likely driven by homologous recombination.
The study by Batty and colleagues adds greatly to our understanding of AAV-mediated gene therapy for HA and the risks/safety of such a treatment approach. The rigor and thoroughness of the methodology showing that genomic integration is frequent, but that long-term FVIII expression after AAV gene therapy is derived from long-lived episomal vector genomes at over a decade after gene therapy, makes their work a unique and important addition to this field of study.
Conflict-of-interest disclosure: G.A.-P. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal