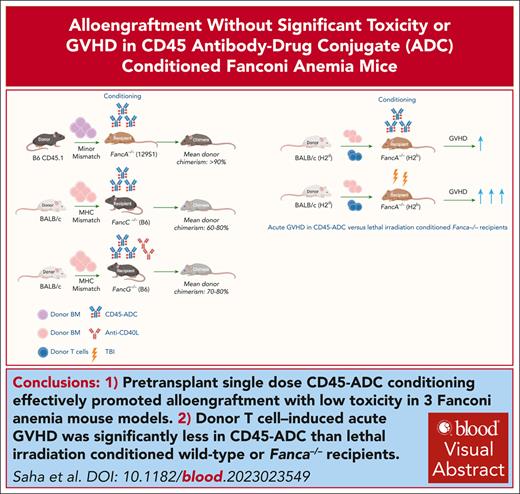
Pretransplant single dose CD45-ADC conditioning effectively promoted alloengraftment with low toxicity in 3 FA mouse models.
Acute GVHD induced by donor T cells was significantly less in CD45-ADC vs lethal irradiation conditioned wild-type or Fanca−/− recipients.
Visual Abstract
Fanconi anemia (FA) is an inherited DNA repair disorder characterized by bone marrow (BM) failure, developmental abnormalities, myelodysplasia, leukemia, and solid tumor predisposition. Allogeneic hematopoietic stem cell transplantation (allo-HSCT), a mainstay treatment, is limited by conditioning regimen–related toxicity and graft-versus-host disease (GVHD). Antibody-drug conjugates (ADCs) targeting hematopoietic stem cells (HSCs) can open marrow niches permitting donor stem cell alloengraftment. Here, we report that single dose anti-mouse CD45–targeted ADC (CD45-ADC) facilitated stable, multilineage chimerism in 3 distinct FA mouse models representing 90% of FA complementation groups. CD45-ADC profoundly depleted host stem cell enriched Lineage−Sca1+cKit+ cells within 48 hours. Fanca−/− recipients of minor-mismatched BM and single dose CD45-ADC had peripheral blood (PB) mean donor chimerism >90%; donor HSCs alloengraftment was verified in secondary recipients. In Fancc−/− and Fancg−/− recipients of fully allogeneic grafts, PB mean donor chimerism was 60% to 80% and 70% to 80%, respectively. The mean percent donor chimerism in BM and spleen mirrored PB results. CD45-ADC–conditioned mice did not have clinical toxicity. A transient <2.5-fold increase in hepatocellular enzymes and mild-to-moderate histopathological changes were seen. Under GVHD allo-HSCT conditions, wild-type and Fanca−/− recipients of CD45-ADC had markedly reduced GVHD lethality compared with lethal irradiation. Moreover, single dose anti–human CD45-ADC given to rhesus macaque nonhuman primates on days −6 or −10 was at least as myeloablative as lethal irradiation. These data suggest that CD45-ADC can potently promote donor alloengraftment and hematopoiesis without significant toxicity or severe GVHD, as seen with lethal irradiation, providing strong support for clinical trial considerations in highly vulnerable patients with FA.
Introduction
Fanconi anemia (FA) is characterized by progressive bone marrow (BM) failure, congenital malformations, chromosomal instability, and predisposition to malignancies.1-4 FA gene products are key regulators counteracting replication stress and promoting accurate DNA replication.1,5,6 Twenty-three FA complementation (FANC) groups have been identified, each with hypersensitivity to DNA interstrand crosslinking agents. FANCA (60% frequency) and FANCC (12% frequency) are most common, with 90% of patients with FA have FANCA, FANCC, or FANCG mutations.7,8 Therapies for FA–associated BM failure include allogeneic hematopoietic stem cell transplantation (allo-HSCT) and gene therapy.7-11
The primary cause of FA early morbidity and mortality is BM failure.12 FA hematopoietic stem cells (HSCs) are reduced in number, function poorly, and are progressively eliminated due to unrepaired DNA damage,13,14 stress-induced apoptosis, and reduced repopulation ability.15,16 FA proteins assist in maintaining homeostasis and in oxidative stress signaling including in hematopoietic cells.17-19
Allo-HSCT can be complicated by transplant-related toxicities.20,21 Higher rates of graft failure, graft-versus-host disease (GVHD), infections, and ionizing radiation or alkylating agent hypersensitivity are seen in patients with FA than other patients with hematological malignancies.22 Cyclophosphamide (Cy), total body irradiation (TBI), antithymocyte globulin, and fludarabine–based reduced intensity conditioning in patients with FA have been shown lower graft rejection and GVHD rates, thereby improving survival.23,24 However, conditioning regimens toxicity and the risk of secondary malignancy remain high.24-26 Patients with FA have a high risk (≥500-fold) of developing myeloid leukemia and head and neck, skin, gastrointestinal, and genitourinary malignancies.12,27,28
There is a considerable interest in exploring less toxic conditioning, especially for patients with FA. Monoclonal antibodies (mAbs) may augment alloengraftment without the need of chemotherapy or radiotherapy and expected to have less off-target and off-tumor toxicity with lower toxicity.29 Recently we demonstrated the efficacy of pyrrolobenzodiazepine (PBD)–based CD45–targeted antibody-drug conjugate (CD45-ADC) conditioning to obtain stable, multilineage donor engraftment in wild-type (WT) mouse transplant models.30 Here, we report the safety and efficacy of single dose murine CD45-ADC monotherapy as a pretransplant conditioning regimen in 3 well-established FA mouse models, simulating >90% of FANC mutations, for achieving high chimerism levels with minor histocompatibility antigen (miH)-disparate or combined with costimulatory blockade, and full major histocompatibility complex (MHC)–disparate donor alloengraftment. In contrast to TBI, CD45-ADC did not facilitate GVHD under GVHD-permissive conditions.
Methods
BM transplantation (BMT)
Conditioning
Female or male recipients were injected via intraperitoneal or IV route, as indicated, with isotype-ADC or murine CD45-ADC day (d) 2 at 0.5, 1.5, or 3 mg/kg doses, as indicated, and female donor BM (IV d0).
miH–mismatch BMT model and mAb–based conditioning
Female or male Fanca−/− (129S1/SvlmJ, CD45.2) mice31,32 (10- to 16-week-old) received single dose isotype-ADC, or CD45.2-ADC or multiple dose (d6 to d3) of unconjugated CD45.2 mAb (1 μg/g per day,33 clone 104; BioLegend, San Diego, CA) daily, followed by B6.SJL-PtprcaPepcb/BoyJ (H-2b, CD45.1) donor BM cells (40 × 106) on d0. Where indicated, 200 μg anti-CD40L mAb (clone MR1; Bio X Cell, Lebanon, NH) was administered intraperitoneal daily (d1 until d+5) then 2×/week until d+14.
Secondary transplant
At 26 weeks, B6-CD45.1+ donor BM was isolated from CD45.2-ADC (1.5 or 3 mg/kg) conditioned primary Fanca−/− recipients; BM (10 × 106 cells) was given IV into lethal TBI (900 cGy) conditioned WT B6-CD45.2 secondary recipients. CD45.1+ BM (10 × 106 cells) was infused into lethally irradiated WT B6 recipients as controls.
MHC–disparate BMT models
Female or male Fancc−/− (B6, H-2b)34-36 or Fancg−/− (B6, H-2b)37,38 mice (10- to 16-week-old) received isotype-ADC or CD45-ADC (0.5, 1.5, or 3 mg/kg; d2). BALB/c (H-2d) donor BM (40 × 106 cells) was infused IV on d0 with/without 200 μg anti-CD40L mAb. Mice were weighed weekly, observed daily for toxicity and graft failure, and euthanized if moribund per IACUC approved protocol.
Supplemental methods
Descriptions of mice and rhesus macaques, ADC preparation, BM culture, GVHD studies, plasma drug concentrations, flow cytometry, cytokine Luminex assay, liver function tests, histopathology, γ-H2AX immunofluorescence, and statistical analyses are provided.
Experiments were performed in accordance with approved protocols by the institutional animal care and use committee at the University of Minnesota and Massachusetts General Hospital.
Results
Single dose CD45-ADC conditioning results in complete donor chimerism in Fanca−/− recipients of miH–mismatched donor BM
We previously demonstrated that CD45-ADC but not isotype-ADC (3 mg/kg; d2) effectively depleted host BM–derived hematopoietic stem and progenitor cells (HSPCs) in C57BL/6 (B6)–CD45.2 immunocompetent mice by d0.30 In recipients of miH–mismatched B6-CD45.1 BM, peripheral blood (PB) percentage of donor engraftment was 79% (week 4), rising to 92% to 94% (weeks 12-20) in CD45-ADC and 0% in isotype-ADC–treated controls; survival ranged from 60% to 80%.30
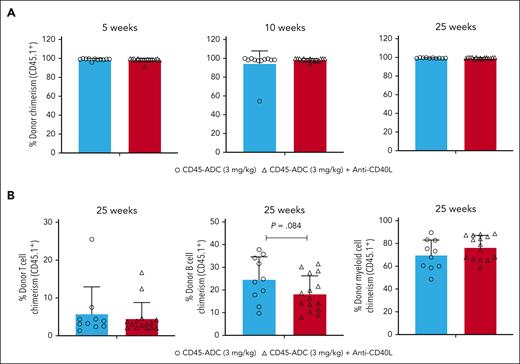
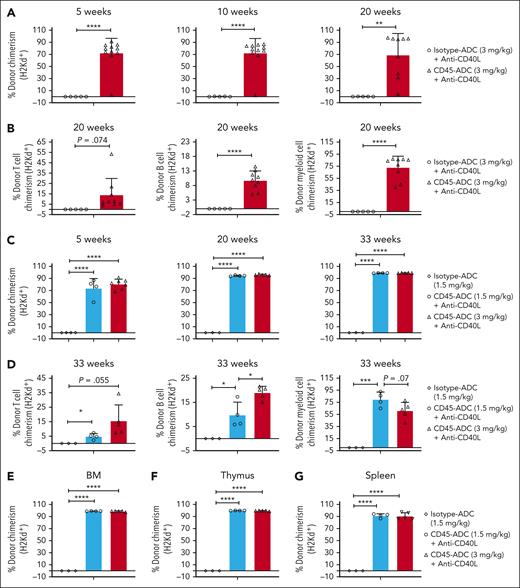
Despite the DNA repair defect, CD45+, lineage depleted (Lin−), and HSC (LSK: Lin−Sca1+cKit+) WT and Fanca−/− BM populations were equally sensitive to CD45-ADC killing over a wide dose-range (supplemental Figure 1, available on the Blood website). The same CD45-ADC dosing (3 mg/kg; d2) was tested in Fanca−/− (CD45.2) murine recipients of miH–mismatched B6-CD45.1 donor BM (40 × 106 cells; d0). Since our and previous study showed that blocking CD40/CD40L costimulatory pathway can effectively induce tolerance in solid organ transplantation models and augment donor BM alloengraftment in MHC–disparate recipients,39-43 1 cohort also received anti-CD40L mAb (d1 to +14) to speed alloengraftment. From 5 to 25 weeks of observation, PB mean percentage donor chimerism was 97.8% to 99.9% regardless of anti-CD40L mAb. No significant differences between CD45-ADC and no mAb vs anti-CD40L mAb were seen in multilineage chimerism (Figure 1A-B) or survival (71% vs 83%, respectively; P = .376) (supplemental Figure 2A).
Single dose CD45-ADC (3 mg/kg, d2) monotherapy conditioning results in complete donor PB chimerism in a minor–mismatch transplant model. (A-B) Female or male Fanca−/− (129S1/SvlmJ, CD45.2) mice (10- to 16-week-old) were conditioned with 3 mg/kg CD45-ADC (n = 14) on d2 and underwent transplantation with 40 × 106 B6.SJL-PtprcaPepcb/BoyJ (H-2b, CD45.1) donor BM cells on d0. A cohort of CD45-ADC–conditioned Fanca−/− recipients (n = 18) were also treated with anti-CD40L mAb (200 μg) daily from d1 through d+5, then 2×/week until d+14 post-BMT. (A) Engraftment of donor cells (CD45.1) in the PB of mice who underwent transplantation were analyzed at 5, 10, and 25 weeks post-BMT by flow cytometry, and multilineage PB donor chimerism was analyzed at 25 weeks post-BMT (B). For panels A-B, data represent mean ± standard error of the mean (SEM). Pooled data from 2 experiments are shown. P value was determined using the 2-tailed Student t test.
Single dose CD45-ADC (3 mg/kg, d2) monotherapy conditioning results in complete donor PB chimerism in a minor–mismatch transplant model. (A-B) Female or male Fanca−/− (129S1/SvlmJ, CD45.2) mice (10- to 16-week-old) were conditioned with 3 mg/kg CD45-ADC (n = 14) on d2 and underwent transplantation with 40 × 106 B6.SJL-PtprcaPepcb/BoyJ (H-2b, CD45.1) donor BM cells on d0. A cohort of CD45-ADC–conditioned Fanca−/− recipients (n = 18) were also treated with anti-CD40L mAb (200 μg) daily from d1 through d+5, then 2×/week until d+14 post-BMT. (A) Engraftment of donor cells (CD45.1) in the PB of mice who underwent transplantation were analyzed at 5, 10, and 25 weeks post-BMT by flow cytometry, and multilineage PB donor chimerism was analyzed at 25 weeks post-BMT (B). For panels A-B, data represent mean ± standard error of the mean (SEM). Pooled data from 2 experiments are shown. P value was determined using the 2-tailed Student t test.
To determine specificity of CD45–ADC alloengraftment effects, Fanca−/− were treated with isotype- or CD45-ADC (3 mg/kg; d2) and miH-disparate B6-CD45.1 BM (d0). Isotype-ADC–treated mice had poor survival (25%) with most deaths within 2 to 3 weeks (supplemental Figure 2B). At 25 weeks, 2 of 3 mice had 0% chimerism; high chimerism in 1 of 3 surviving mice may be due to free PBD at this apparently toxic dose (not shown). In contrast, CD45-ADC–treated mice had 64% survival and mean percentage donor chimerism was ≥98% at all time points (5-25 weeks; not shown).
Because isotype-ADC survival was poor, we investigated plasma drug concentration of IV-administered ADCs. Because CD45+ cell mass would be lower in Fanca−/− than WT recipients, we tested CD45-ADC at reduced dose (1.5 mg/kg). Plasma CD45-ADC levels were detectable immediately postinfusion but declined rapidly. When isotype-ADC was adjusted to 0.5 mg/kg, half-life was longer (2.2 times) than CD45-ADC due to lack of target-mediated drug disposition but at these adjusted doses, 4-hour clearance was equivalent (supplemental Figure 3). This adjusted dose did not result in nonspecific effects (see below).
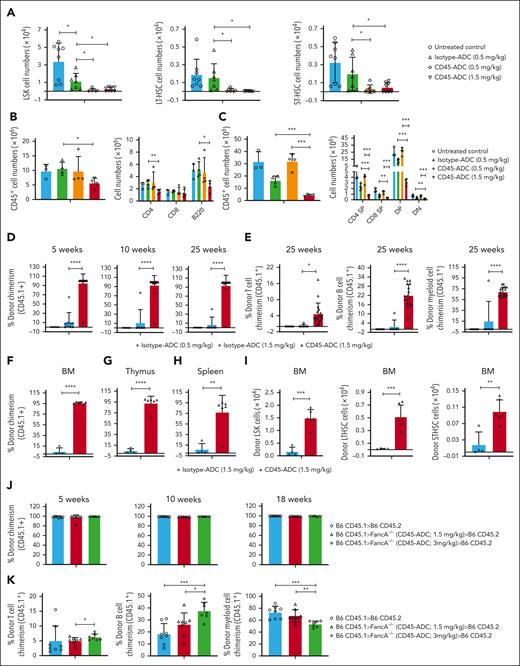
To assess whether this intermediate dose CD45-ADC (1.5 mg/kg; d2) conditioning could effectively deplete BM HSPCs and peripheral organ effector cells, Fanca−/− recipients were given CD45-ADC or isotype-ADC (0.5 or 1.5 mg/kg). Two days post–CD45-ADC (0.5 mg/kg), BM LSK, long-term HSCs (LT-HSC: LSK,CD150+CD48−), and short-term HSCs (ST-HSC: LSK,CD150+CD48+) were reduced by >88%, >90%, and >85%, respectively, equivalent to CD45-ADC 1.5 mg/kg and significantly higher than pharmacokinetic/pharmacodynamic dose-adjusted isotype-ADC 0.5 mg/kg (P < .05; Figure 2A). Splenic CD45+ hematopoietic cells, CD4 T cells, B cells (Figure 2B), and total and differentiated thymocytes (Figure 2C) were significantly lower in CD45-ADC (1.5 mg/kg) than isotype-ADC (0.5 mg/kg) conditioned mice; isotype-ADC conditioning did not differ from untreated controls. Although only modest thymocyte and lymph node but not splenic T-cell depletion was observed with CD45-ADC (1.5 mg/kg), in previous studies, we found the remaining T cells to be dysfunctional.30
CD45-ADC conditioning at lower dose (1.5 mg/kg, d2) facilitated complete donor chimerism in a minor–mismatch transplant model. (A-C) Female or male Fanca−/− mice (10- to 12-week-old) were conditioned with isotype-ADC (0.5 mg/kg) or CD45-ADC (0.5 or 1.5 mg/kg) on d2. Depletion of HSPCs in BM (A), and hematopoietic cells and adaptive immune cells in spleen (B) and thymus (C) were assessed by flow cytometry on day 0. Untreated mice served as control. (A) Pooled data from 2 experiments are shown (n = 7-8 mice per group). For panels B-C, experiments were performed twice, and data from 1 representative experiment are shown (n = 3-4 mice per group). For panels A-C, data represent mean ± SEM. ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001 using the 2-tailed Student t test. (D-I) Female or male Fanca−/− mice (10- to 16-week-old) were conditioned with isotype-ADC (0.5 mg/kg, n = 7; or 1.5 mg/kg, n = 12) or CD45-ADC (1.5 mg/kg, n = 20) on d2 and received 40 × 106 donor (H-2b, CD45.1) BM cells on d0. (D) Engraftment of donor cells (CD45.1) in the PB of mice who underwent transplantation were analyzed at 5, 10, and 25 weeks post-BMT by flow cytometry, and multilineage PB donor chimerism was analyzed at 25 weeks post-BMT (E). For panels D-E, data represent mean ± SEM. ∗P < .05 and ∗∗∗∗P < .0001 using the 2-tailed Student t test. Pooled data from 2 experiments are shown. (F-I) Recipients were killed (n = 4-6 mice per group) at 30 weeks post-BMT and donor (CD45.1+) chimerism in BM (F,I), thymus (G), and spleen (H) were analyzed by flow cytometry. Experiments were performed twice, and data from 1 representative experiment are shown. Data represent mean ± SEM. ∗∗P < .01, ∗∗∗P < .001, and ∗∗∗∗P < .0001 using the 2-tailed Student t test. (J-K) WT B6 (H-2b, CD45.2) mice received lethal TBI (900 cGy) on d1. CD45.1+ (B6) donor BM cells were isolated at 26 weeks post-BMT from primary Fanca−/− recipients conditioned with CD45-ADC (1.5 or 3 mg/kg). For adoptive transfer into secondary recipients, BM cells (10 × 106) from primary recipients were infused into lethally irradiated WT B6 recipients (n = 7-8 mice per group) on d0. CD45.1+ (B6) donor BM cells isolated from naïve mice and infused (10 × 106) into lethally irradiated WT B6 recipients (n = 8 mice) served as control. (J) Engraftment of donor cells (CD45.1) in PB of secondary WT recipients were analyzed at 5, 10, and 18 weeks post-BMT and multilineage PB donor chimerism was analyzed at 18 weeks post-BMT (K). For panels J-K, data represent mean ± SEM. ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001 using the 2-tailed Student t test.
CD45-ADC conditioning at lower dose (1.5 mg/kg, d2) facilitated complete donor chimerism in a minor–mismatch transplant model. (A-C) Female or male Fanca−/− mice (10- to 12-week-old) were conditioned with isotype-ADC (0.5 mg/kg) or CD45-ADC (0.5 or 1.5 mg/kg) on d2. Depletion of HSPCs in BM (A), and hematopoietic cells and adaptive immune cells in spleen (B) and thymus (C) were assessed by flow cytometry on day 0. Untreated mice served as control. (A) Pooled data from 2 experiments are shown (n = 7-8 mice per group). For panels B-C, experiments were performed twice, and data from 1 representative experiment are shown (n = 3-4 mice per group). For panels A-C, data represent mean ± SEM. ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001 using the 2-tailed Student t test. (D-I) Female or male Fanca−/− mice (10- to 16-week-old) were conditioned with isotype-ADC (0.5 mg/kg, n = 7; or 1.5 mg/kg, n = 12) or CD45-ADC (1.5 mg/kg, n = 20) on d2 and received 40 × 106 donor (H-2b, CD45.1) BM cells on d0. (D) Engraftment of donor cells (CD45.1) in the PB of mice who underwent transplantation were analyzed at 5, 10, and 25 weeks post-BMT by flow cytometry, and multilineage PB donor chimerism was analyzed at 25 weeks post-BMT (E). For panels D-E, data represent mean ± SEM. ∗P < .05 and ∗∗∗∗P < .0001 using the 2-tailed Student t test. Pooled data from 2 experiments are shown. (F-I) Recipients were killed (n = 4-6 mice per group) at 30 weeks post-BMT and donor (CD45.1+) chimerism in BM (F,I), thymus (G), and spleen (H) were analyzed by flow cytometry. Experiments were performed twice, and data from 1 representative experiment are shown. Data represent mean ± SEM. ∗∗P < .01, ∗∗∗P < .001, and ∗∗∗∗P < .0001 using the 2-tailed Student t test. (J-K) WT B6 (H-2b, CD45.2) mice received lethal TBI (900 cGy) on d1. CD45.1+ (B6) donor BM cells were isolated at 26 weeks post-BMT from primary Fanca−/− recipients conditioned with CD45-ADC (1.5 or 3 mg/kg). For adoptive transfer into secondary recipients, BM cells (10 × 106) from primary recipients were infused into lethally irradiated WT B6 recipients (n = 7-8 mice per group) on d0. CD45.1+ (B6) donor BM cells isolated from naïve mice and infused (10 × 106) into lethally irradiated WT B6 recipients (n = 8 mice) served as control. (J) Engraftment of donor cells (CD45.1) in PB of secondary WT recipients were analyzed at 5, 10, and 18 weeks post-BMT and multilineage PB donor chimerism was analyzed at 18 weeks post-BMT (K). For panels J-K, data represent mean ± SEM. ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001 using the 2-tailed Student t test.
To determine whether HSC depletion by CD45-ADC would permit durable multilineage engraftment, Fanca−/− recipients were given CD45-ADC (1.5 mg/kg) or isotype-ADC (0.5 or 1.5 mg/kg; d2) and miH-disparate CD45.1+ B6 donor BM (d0). At 5 weeks, PB analysis showed 0% or 11% mean donor chimerism in isotype-ADC (0.5 or 1.5 mg/kg, respectively). In contrast, at 5 to 25 weeks posttransplant, >90% chimerism was seen in PB of 95% of engrafted CD45-ADC (1.5 mg/kg) treated recipients (Figure 2D); chimerism was multilineage (Figure 2E). At 30 weeks, BM and thymus mean donor chimerism was <10% vs >92% in isotype-ADC vs CD45-ADC (1.5 mg/kg) conditioned recipients (Figure 2F-G). CD45-ADC recipients had >77% splenic chimerism (Figure 2H); absolute donor LSK, LT-HSC, and ST-HSC BM numbers were significantly higher in CD45-ADC vs isotype-ADC–conditioned recipients (P < .01; Figure 2I).
To confirm long-term HSPC engraftment, CD45.1+ donor BM cells isolated at 26 weeks post-BMT from primary miH-disparate Fanca−/− BMT recipients conditioned with CD45-ADC (1.5 or 3 mg/kg) were infused into lethally irradiated WT B6 (CD45.2) secondary recipients. PB analysis showed >96% donor chimerism in B6-CD45.2 secondary recipients at 5 weeks post-BMT, equivalent to primary recipients (Figure 2J). At 18 weeks, PB showed increased donor T and B cells and reduced myeloid-cell chimerism in secondary recipients that received BM from primary recipients conditioned with the higher vs lower CD45-ADC dose (3 vs 1.5 mg/kg; Figure 2K).
Previous studies showed conditioning with unconjugated anti-CD45 mAb (clone 30F11) plus TBI but not mAb alone, were required for syngeneic or allogeneic donor engraftment.33 Our findings confirmed that unconjugated anti-CD45.2 mAb (clone 104) given repetitively (d6 to d3) did not deplete BM LSK, LT-HSC, or ST-HSC populations on d0 along with no PB chimerism or, at 13 weeks, BM, thymus, or spleen chimerism in striking contrast to CD45-ADC (1.5 mg/kg; d2) (Figure 3A; supplemental Figure 4A-D).
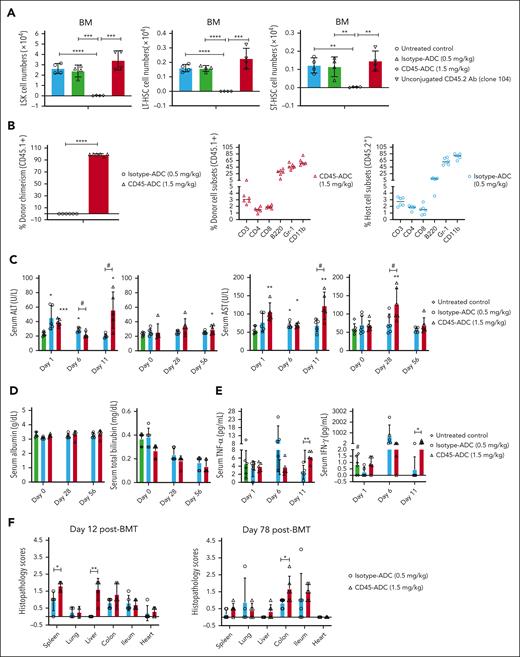
Conditioning with lower dose CD45-ADC (1.5 mg/kg) is associated with mild, transient hepatotoxicity in Fanca−/− recipients. (A) Unconjugated CD45.2 antibody conditioning could not deplete HSPCs in BM. Female WT B6 mice (10 weeks old) were conditioned with isotype-ADC (0.5 mg/kg) or CD45-ADC (1.5 mg/kg) on d2. A cohort of B6 mice were treated with unconjugated CD45.2 antibody (1 μg/gm per day, clone 104, mouse IgG2a) daily from d6 through d3. Depletion of HSPCs in BM were assessed on d0 by flow cytometry. Untreated B6 mice served as control. Data from 1 experiment are shown (n = 4 mice per group). Data represent mean ± SEM. ∗∗P < .01, ∗∗∗P < .001, and ∗∗∗∗P < .0001 using the 2-tailed Student t test. (B-F) Conditioning with lower dose of CD45-ADC (1.5 mg/kg) caused mild, transient hepatotoxicity in Fanca−/− recipients. Female or male Fanca−/− mice (10- to 12-week-old) were conditioned with isotype-ADC (0.5 mg/kg) or CD45-ADC (1.5 mg/kg) on d2 and then received 40 × 106 donor (H-2b, CD45.1) BM cells on d0. (B) Engraftment of donor cells (CD45.1) in PB of mice that underwent transplant, and PB donor and host cell subsets were analyzed by flow cytometry at 10 weeks post-BMT. (C-D) Blood samples were collected at d1, d6, and d11 post-BMT. In a second experiment, blood samples were also collected at d0 before BM infusion and at d28 and d56 post-BMT. The levels of ALT, AST, albumin, and bilirubin in serum samples were measured. Untreated mice served as control. ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001 vs untreated mice. #; isotype-ADC vs CD45-ADC, P < .05. (E) Blood samples were collected at d1, d6, and d11 post-BMT and levels of tumor necrosis factor-α or IFN-γ in serum samples were measured. Untreated mice served as control. ∗P < .05 and ∗∗P < .01, isotype-ADC vs CD45–ADC-conditioned mice. #; IFN-γ (d11): untreated control vs CD45-ADC, P < .05. (F) Representative mice were euthanized at d12 or d78 post-BMT and harvested organs were processed for hematoxylin and eosin staining and histopathological assessment was performed. For panels B-F, data represent mean ± SEM (n = 5-6 mice per group per time point). ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, and ∗∗∗∗P < .0001 using the 2-tailed Student t test.
Conditioning with lower dose CD45-ADC (1.5 mg/kg) is associated with mild, transient hepatotoxicity in Fanca−/− recipients. (A) Unconjugated CD45.2 antibody conditioning could not deplete HSPCs in BM. Female WT B6 mice (10 weeks old) were conditioned with isotype-ADC (0.5 mg/kg) or CD45-ADC (1.5 mg/kg) on d2. A cohort of B6 mice were treated with unconjugated CD45.2 antibody (1 μg/gm per day, clone 104, mouse IgG2a) daily from d6 through d3. Depletion of HSPCs in BM were assessed on d0 by flow cytometry. Untreated B6 mice served as control. Data from 1 experiment are shown (n = 4 mice per group). Data represent mean ± SEM. ∗∗P < .01, ∗∗∗P < .001, and ∗∗∗∗P < .0001 using the 2-tailed Student t test. (B-F) Conditioning with lower dose of CD45-ADC (1.5 mg/kg) caused mild, transient hepatotoxicity in Fanca−/− recipients. Female or male Fanca−/− mice (10- to 12-week-old) were conditioned with isotype-ADC (0.5 mg/kg) or CD45-ADC (1.5 mg/kg) on d2 and then received 40 × 106 donor (H-2b, CD45.1) BM cells on d0. (B) Engraftment of donor cells (CD45.1) in PB of mice that underwent transplant, and PB donor and host cell subsets were analyzed by flow cytometry at 10 weeks post-BMT. (C-D) Blood samples were collected at d1, d6, and d11 post-BMT. In a second experiment, blood samples were also collected at d0 before BM infusion and at d28 and d56 post-BMT. The levels of ALT, AST, albumin, and bilirubin in serum samples were measured. Untreated mice served as control. ∗P < .05, ∗∗P < .01, and ∗∗∗P < .001 vs untreated mice. #; isotype-ADC vs CD45-ADC, P < .05. (E) Blood samples were collected at d1, d6, and d11 post-BMT and levels of tumor necrosis factor-α or IFN-γ in serum samples were measured. Untreated mice served as control. ∗P < .05 and ∗∗P < .01, isotype-ADC vs CD45–ADC-conditioned mice. #; IFN-γ (d11): untreated control vs CD45-ADC, P < .05. (F) Representative mice were euthanized at d12 or d78 post-BMT and harvested organs were processed for hematoxylin and eosin staining and histopathological assessment was performed. For panels B-F, data represent mean ± SEM (n = 5-6 mice per group per time point). ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, and ∗∗∗∗P < .0001 using the 2-tailed Student t test.
Fanca−/− recipients conditioned with an intermediate CD45-ADC dose experienced only mild, transient hepatotoxicity
As mentioned earlier, Fanca−/− recipients given isotype-ADC vs CD45-ADC (3 mg/kg; d2) had 25% vs 64% long-term survival (supplemental Figure 2B; P = .0343), consistent with high-dose isotype-ADC toxicity. With intermediate dose (1.5 mg/kg) isotype-ADC, long-term survival was 50% and improved to 100% with lower dose (0.5 mg/kg) isotype-ADC (P = .032), whereas mice receiving CD45-ADC vs isotype-ADC had 90% vs 50% survival (P = .007) (supplemental Figure 2C); mean weights reflected survival (not shown). A second experiment confirmed >90% mean PB donor chimerism in all CD45-ADC (1.5 mg/kg) conditioned recipients at 10 weeks post-BMT with comparable subset distribution between CD45-ADC (1.5 mg/kg) for donor cells and isotype-ADC (0.5 mg/kg) conditioned recipients for host cells, respectively (Figure 3B); both groups had 100% survival (supplemental Figure 2D).
To assess ADC-mediated toxicity, isotype-ADC or CD45-ADC–conditioned Fanca−/− recipients received miH-disparate donor BM (d0). Serum samples were collected on d0 before BM infusion, and at designated time points post-BMT. Compared with untreated controls, isotype-ADC conditioning resulted in ≤2-fold increased serum alanine aminotransferase (ALT) (d1,6), and 1.2-fold increased serum aspartate aminotransferase (AST) (d6) (Figure 3C), whereas CD45-ADC conditioning resulted <2.5-fold increased ALT (d1, d11, and d56), and ≤2.2-fold increased AST (d1, d6, d11, and d28). CD45-ADC vs isotype-ADC conditioning revealed increased ALT (d11), and increased AST (d11,28). ADC-treated and untreated recipients had comparable albumin and bilirubin with no significant difference between groups (Figure 3D). Serum proinflammatory cytokines revealed increased tumor necrosis factor-α and interferon gamma (IFN-γ) levels on d11 with CD45-ADC vs isotype-ADC conditioning (Figure 3E). In CD45-ADC vs isotype-ADC–conditioned recipients, multiorgan histopathology revealed mild-moderate liver toxicity on d12 (P = .002) but not d78, d12 spleen scores (P = .012) and d78 colon scores (P = .029) without histopathological evidence of GVHD in other organs or cardiac toxicity (Figure 3F). Taken together, our data suggest that conditioning with CD45-ADC at 1.5 mg/kg as a single agent was a reasonably well-tolerated strategy for achieving stable multilineage donor chimerism in Fanca−/− recipients of miH–mismatched donor graft.
Anti-CD40L mAb has modest effects on CD45-ADC facilitated alloengraftment in MHC–mismatched transplant models
Previously, we demonstrated CD45-ADC (3 mg/kg) and anti-CD40L mAb–treated WT recipients of full MHC–disparate donor cells did not engraft.30 Because anti-CD40L mAb can both promote alloengraftment43,44 and reduce Th1 proinflammatory cytokine release under inflammatory conditions (eg, acute GVHD [aGVHD]),41 we hypothesized that anti-CD40L mAb might permit high CD45-ADC dosing and optimize alloengraftment. Fancc−/− (B6, H-2b) recipients were given isotype-ADC or CD45-ADC (3 mg/kg; d2), fully allogeneic BALB/c (H-2d) donor BM (d0), and anti-CD40L mAb (d1 to d+14). PB had ≥10% donor cells in 91% of CD45-ADC recipients (mean 72%); isotype-ADC and anti-CD40L failed to engraft. Overall PB alloengraftment was stable through 20 weeks observation period (Figure 4A). PB B- and myeloid-cell donor chimerism were significantly higher in CD45-ADC vs isotype-ADC–treated recipients with a trend toward higher T-cell chimerism (P = .074) (Figure 4B). Recipients conditioned with isotype-ADC or CD45-ADC and anti-CD40L mAb had 71% vs 57% long-term survival (supplemental Figure 5A).
CD45-ADC (1.5 or 3 mg/kg) conditioning facilitates alloengraftment in an MHC–mismatched transplant model. (A-B) Female or male Fancc−/− (B6, H-2b) mice (10- to 16-weeks-old) were conditioned with 3 mg/kg isotype-ADC (n = 7) or CD45-ADC (n = 14) on d2 and received 40 × 106 BALB/c (H-2d) donor BM cells on d0. ADC–conditioned Fancc−/− recipients were also treated with anti-CD40L mAb (200 μg) from d1 through d+5, then 2× per week until d+14 post-BMT. (A) Engraftment of donor cells (H-2d) in PB of mice who underwent transplant were analyzed at 5, 10, and 20 weeks post-BMT by flow cytometry, and PB multilineage donor chimerism was analyzed at 20 weeks post-BMT (B). Data represent mean ± SEM. ∗∗P < .01 and ∗∗∗∗P < .0001 using the 2-tailed Student t test. Pooled data from 2 experiments are shown. (C-G) Female or male Fancc−/− mice (10- to 16-weeks-old) were conditioned with isotype-ADC (1.5 mg/kg) or CD45-ADC (1.5 or 3 mg/kg) on d2 and then received 40 × 106 donor (H-2d) BM cells on d0. CD45-ADC–conditioned recipients were also treated with anti-CD40L mAb as mentioned above. (C) Engraftment of donor cells (H-2d) in PB of mice who underwent transplant were analyzed at 5, 20, and 33 weeks post-BMT, and PB multilineage donor chimerism was analyzed at 33 weeks post-BMT (D). (E-G) Recipients were killed at 34 weeks post-BMT and donor (H-2d) chimerism in BM (E), thymus (F), and spleen (G) were analyzed. For panels C-G, data represent mean ± SEM (n = 4-7 mice per group). ∗P < .05, ∗∗∗P < .001, and ∗∗∗∗P < .0001 using the 2-tailed Student t test.
CD45-ADC (1.5 or 3 mg/kg) conditioning facilitates alloengraftment in an MHC–mismatched transplant model. (A-B) Female or male Fancc−/− (B6, H-2b) mice (10- to 16-weeks-old) were conditioned with 3 mg/kg isotype-ADC (n = 7) or CD45-ADC (n = 14) on d2 and received 40 × 106 BALB/c (H-2d) donor BM cells on d0. ADC–conditioned Fancc−/− recipients were also treated with anti-CD40L mAb (200 μg) from d1 through d+5, then 2× per week until d+14 post-BMT. (A) Engraftment of donor cells (H-2d) in PB of mice who underwent transplant were analyzed at 5, 10, and 20 weeks post-BMT by flow cytometry, and PB multilineage donor chimerism was analyzed at 20 weeks post-BMT (B). Data represent mean ± SEM. ∗∗P < .01 and ∗∗∗∗P < .0001 using the 2-tailed Student t test. Pooled data from 2 experiments are shown. (C-G) Female or male Fancc−/− mice (10- to 16-weeks-old) were conditioned with isotype-ADC (1.5 mg/kg) or CD45-ADC (1.5 or 3 mg/kg) on d2 and then received 40 × 106 donor (H-2d) BM cells on d0. CD45-ADC–conditioned recipients were also treated with anti-CD40L mAb as mentioned above. (C) Engraftment of donor cells (H-2d) in PB of mice who underwent transplant were analyzed at 5, 20, and 33 weeks post-BMT, and PB multilineage donor chimerism was analyzed at 33 weeks post-BMT (D). (E-G) Recipients were killed at 34 weeks post-BMT and donor (H-2d) chimerism in BM (E), thymus (F), and spleen (G) were analyzed. For panels C-G, data represent mean ± SEM (n = 4-7 mice per group). ∗P < .05, ∗∗∗P < .001, and ∗∗∗∗P < .0001 using the 2-tailed Student t test.
To determine if anti-CD40L mAb would permit use of a lower CD45-ADC dose, CD45-ADC (3 vs 1.5 mg/kg) and anti-CD40L mAb–treated recipients of fully allogeneic BM were analyzed for chimerism; ≥10% PB alloengraftment was seen at 20 weeks (mean donor chimerism >94% irrespective of dose) (Figure 4C). At 33 weeks, mean donor T- and B-cell chimerism increased 2-3-fold with CD45-ADC (3 vs 1.5 mg/kg) and anti-CD40L mAb (Figure 4D); 71% and 100% of mice survived long-term (supplemental Figure 5B). At 34 weeks, BM, thymus, and spleen confirmed ≥90% mean donor chimerism at both CD45-ADC doses and no engraftment with isotype-ADC (Figure 4E-G).
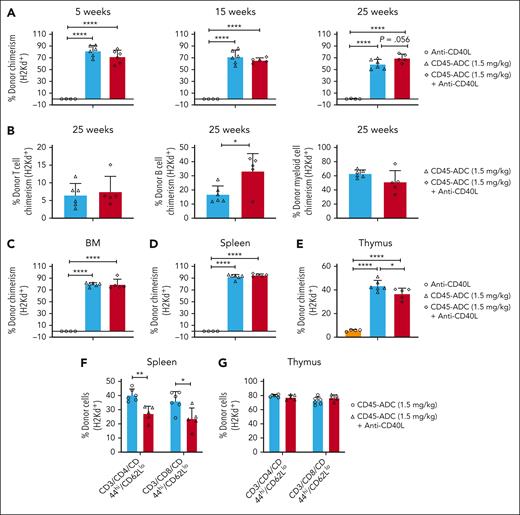
To assess whether anti-CD40L mAb was required for high donor alloengraftment, Fancc−/−recipients conditioned with CD45-ADC (1.5 mg/kg) with/without anti-CD40L mAb or anti-CD40L mAb alone were given BALB/c BM. Long-term survival ranged from 83% to 100% (supplemental Figure 5C). From 5 to 30 weeks, overall and multilineage PB, BM, and spleen chimerism was virtually equivalent with CD45-ADC (1.5 mg/kg) regardless of anti-CD40L mAb (Figure 5A-E) with the exception of lower thymus engraftment with mAb that resulted in reduced splenic effector/memory (CD44hiCD62Llo) T cells (Figure 5F-G).
Conditioning with CD45-ADC (1.5 mg/kg) monotherapy was sufficient for achieving alloengraftment in Fancc−/− recipients. (A-G) Female or male Fancc−/− mice (10- to 16-weeks-old) were conditioned with CD45-ADC (1.5 mg/kg) ± anti-CD40L mAb, or anti-CD40L mAb alone and then received 40 × 106 BALB/c (H-2d) donor BM cells. (A) Engraftment of donor cells (H-2d) in the PB of mice who underwent transplant were analyzed at 5, 15, and 25 weeks post-BMT by flow cytometry, and multilineage peripheral donor chimerism was analyzed at 25 weeks post-BMT (B). (C-G) Recipients were killed at 30 weeks post-BMT, and donor cell (H-2d) chimerism in BM (C), spleen (D), and thymus (E) were analyzed. The frequencies of donor (H-2d) effector/memory (CD44hiCD62Llo) T cells in spleen (F) and thymus (G) were also analyzed at 30 weeks post-BMT. For panels A-G, data represent mean ± SEM (n = 4-6 mice per group). ∗P < .05, ∗∗P < .01, and ∗∗∗∗P < .0001 using the 2-tailed Student t test.
Conditioning with CD45-ADC (1.5 mg/kg) monotherapy was sufficient for achieving alloengraftment in Fancc−/− recipients. (A-G) Female or male Fancc−/− mice (10- to 16-weeks-old) were conditioned with CD45-ADC (1.5 mg/kg) ± anti-CD40L mAb, or anti-CD40L mAb alone and then received 40 × 106 BALB/c (H-2d) donor BM cells. (A) Engraftment of donor cells (H-2d) in the PB of mice who underwent transplant were analyzed at 5, 15, and 25 weeks post-BMT by flow cytometry, and multilineage peripheral donor chimerism was analyzed at 25 weeks post-BMT (B). (C-G) Recipients were killed at 30 weeks post-BMT, and donor cell (H-2d) chimerism in BM (C), spleen (D), and thymus (E) were analyzed. The frequencies of donor (H-2d) effector/memory (CD44hiCD62Llo) T cells in spleen (F) and thymus (G) were also analyzed at 30 weeks post-BMT. For panels A-G, data represent mean ± SEM (n = 4-6 mice per group). ∗P < .05, ∗∗P < .01, and ∗∗∗∗P < .0001 using the 2-tailed Student t test.
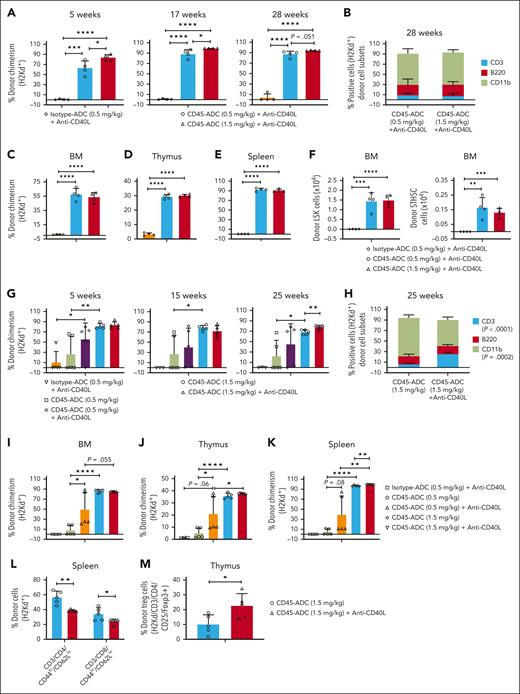
These findings were extended into Fancg−/− (B6, H-2b) recipients given isotype-ADC (0.5 mg/kg) or CD45-ADC (0.5 or 1.5 mg/kg), BALB/c (H-2d) BM, and anti-CD40L mAb. Long-term survival ranged from 80% to 100% (supplemental Figure 6A). PB analysis from 5 weeks showed 84% vs 63% mean donor chimerism in recipients conditioned with CD45-ADC (1.5 vs 0.5 mg/kg) and anti-CD40L mAb, respectively (P < .05). Donor chimerism improved by 17 weeks then remained stable thereafter, with significantly higher chimerism for CD45-ADC (1.5 vs 0.5 mg/kg) and anti-CD40L mAb (mean 99.15% vs 88.55%) (Figure 6A). At 28 to 29 weeks, PB multilineage donor T-, B-, and myeloid-cell chimerism (Figure 6B) and BM, thymus, and spleen chimerism (Figure 6C-E) were equivalent, and donor LSK and ST-HSCs (Figure 6F) were comparable.
Adding anti-CD40L mAb has variable efficacy in promoting fully allogeneic donor engraftment in Fancg−/− recipients. A−F, Female or male Fancg−/− (B6, H-2b) mice (10- to 16-weeks-old) were conditioned with isotype-ADC (0.5 mg/kg) or CD45-ADC (0.5 or 1.5 mg/kg) on d2 and received 40 × 106 BALB/c (H-2d) donor BM cells on d0. ADC–conditioned Fancg−/− recipients were also treated with anti-CD40L mAb per Figure 5. (A) Engraftment of donor cells (H-2d) in the PB of mice who underwent transplant were analyzed at 5, 17, and 28 weeks post-BMT by flow cytometry, and multilineage peripheral donor chimerism was analyzed at 28 weeks post-BMT (B). (C-F) Recipients were killed at 29 weeks post-BMT, and donor (H-2d) chimerism in BM (C,F), thymus (D), and spleen (E) were analyzed. (G-M) Female or male Fancg−/− mice (10- to 16-weeks-old) were conditioned with isotype-ADC (0.5 mg/kg) plus anti-CD40L mAb or CD45-ADC (0.5 or 1.5 mg/kg) ± anti-CD40L mAb and received 40 × 106 donor (H-2d) BM cells as above. (G) Engraftment of donor cells (H-2d) in the PB of mice who underwent transplant were analyzed at 5, 15, and 25 weeks post-BMT, and multilineage peripheral donor chimerism was analyzed (H) at 25 weeks post-BMT. (H) CD45-ADC (1.5 mg/kg) vs CD45-ADC (1.5 mg/kg) + anti-CD40L mAb; CD3 (P < .0001), CD11b (P = .0002). (I-M) Recipients were killed at 30 weeks post-BMT, and donor (H-2d) chimerism in BM (I), thymus (J), and spleen (K) were analyzed. The frequencies of donor (H-2d) effector/memory (CD44hiCD62Llo) T cells in spleen (L) and donor (H-2d) Treg cells in thymus (M) were also analyzed at 30 weeks post-BMT by flow cytometry. For panels A-M, data represent mean ± SEM (n = 4-5 mice per group). ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, and ∗∗∗∗P < .0001 using the 2-tailed Student t test.
Adding anti-CD40L mAb has variable efficacy in promoting fully allogeneic donor engraftment in Fancg−/− recipients. A−F, Female or male Fancg−/− (B6, H-2b) mice (10- to 16-weeks-old) were conditioned with isotype-ADC (0.5 mg/kg) or CD45-ADC (0.5 or 1.5 mg/kg) on d2 and received 40 × 106 BALB/c (H-2d) donor BM cells on d0. ADC–conditioned Fancg−/− recipients were also treated with anti-CD40L mAb per Figure 5. (A) Engraftment of donor cells (H-2d) in the PB of mice who underwent transplant were analyzed at 5, 17, and 28 weeks post-BMT by flow cytometry, and multilineage peripheral donor chimerism was analyzed at 28 weeks post-BMT (B). (C-F) Recipients were killed at 29 weeks post-BMT, and donor (H-2d) chimerism in BM (C,F), thymus (D), and spleen (E) were analyzed. (G-M) Female or male Fancg−/− mice (10- to 16-weeks-old) were conditioned with isotype-ADC (0.5 mg/kg) plus anti-CD40L mAb or CD45-ADC (0.5 or 1.5 mg/kg) ± anti-CD40L mAb and received 40 × 106 donor (H-2d) BM cells as above. (G) Engraftment of donor cells (H-2d) in the PB of mice who underwent transplant were analyzed at 5, 15, and 25 weeks post-BMT, and multilineage peripheral donor chimerism was analyzed (H) at 25 weeks post-BMT. (H) CD45-ADC (1.5 mg/kg) vs CD45-ADC (1.5 mg/kg) + anti-CD40L mAb; CD3 (P < .0001), CD11b (P = .0002). (I-M) Recipients were killed at 30 weeks post-BMT, and donor (H-2d) chimerism in BM (I), thymus (J), and spleen (K) were analyzed. The frequencies of donor (H-2d) effector/memory (CD44hiCD62Llo) T cells in spleen (L) and donor (H-2d) Treg cells in thymus (M) were also analyzed at 30 weeks post-BMT by flow cytometry. For panels A-M, data represent mean ± SEM (n = 4-5 mice per group). ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, and ∗∗∗∗P < .0001 using the 2-tailed Student t test.
To assess whether anti-CD40L mAb was needed to improve CD45-ADC facilitated alloengraftment in Fancg−/− mice, recipients were conditioned with isotype-ADC (0.5 mg/kg) or CD45-ADC (0.5 or 1.5 mg/kg) with/without anti-CD40L mAb and given BALB/c BM. At 25 weeks, PB chimerism was undetectable in isotype-ADC + anti-CD40L mAb cohort; recipients given CD45-ADC (1.5 mg/kg) and anti-CD40L mAb had modestly higher chimerism than CD45-ADC alone (mean 78% vs 68%, respectively; P = .0082). No significant differences were seen for CD45-ADC (0.5 mg/kg) with/without anti-CD40L mAb (Figure 6G-H). CD45-ADC (0.5 mg/kg) and anti-CD40L mAb did not significantly improve PB donor chimerism over isotype-ADC and anti-CD40L mAb, although CD45-ADC (0.5 mg/kg) with vs without anti-CD40L mAb significantly increased mean donor chimerism in BM and thymus with a trend in the spleen (Figure 6I-K). Significantly lower splenic donor effector/memory T cells and higher regulatory T-cell frequency were seen with CD45-ADC (1.5 mg/kg) and anti-CD40L mAb (Figure 6L-M). CD45-ADC–conditioned recipients remained healthy with 100% long-term survival (supplemental Figure 6B).
To search for possible organ toxicity, Fancg−/− mice conditioned with CD45-ADC (1.5 mg/kg) vs isotype-ADC (0.5 mg/kg) and given BALB/c BM had >76% vs 0% mean donor chimerism at 9 weeks post-BMT with all mice surviving (supplemental Figure 6C) and a similar multilineage distribution of donor vs host cell subsets, respectively (supplemental Figure 7A). Liver function tests in serum samples (d0, d27, and d57) revealed no liver toxicity (supplemental Figure 7B) reflective of its variable but at most modest effects on liver function. Of the GVHD organs, only d67 colon histopathology was increased (P < .034) in CD45-ADC–conditioned recipients (supplemental Figure 7C). Engraftment and survival data are summarized in supplemental Table 1.
In WT or Fanca−/− recipients, allogeneic donor T-cell induced aGVHD lethality was significantly lower with CD45-ADC vs lethal TBI conditioning
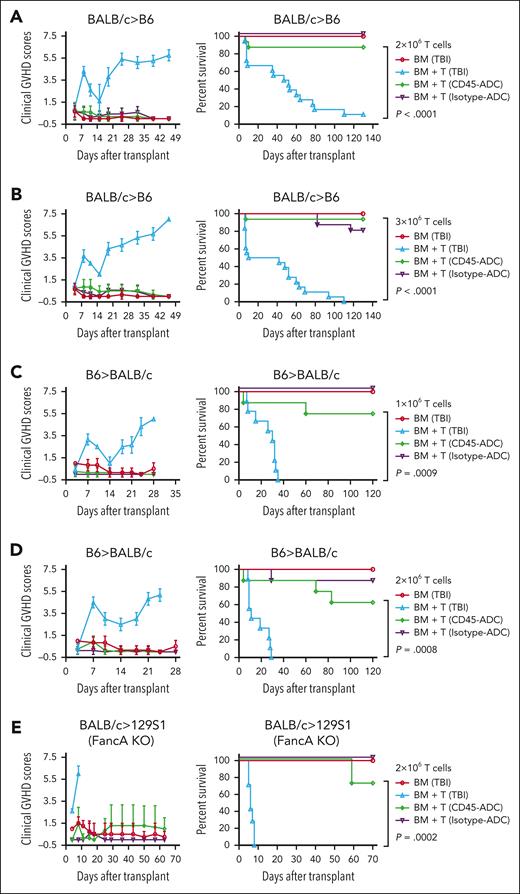
Alloreactive donor T-cell expansion in a proinflammatory environment induced by conditioning regimen and deregulated immune mechanisms causes aGVHD. To determine the physiological significance of CD45-ADC vs TBI (900 cGy) conditioning in aGVHD, B6 recipients were given allogeneic BALB/c BM ± purified T cells (2 × 106) (Figure 7A). GVHD–induced lethality was significantly lower (P < .0001) in WT recipients conditioned with CD45-ADC vs lethal TBI (median survival time [MST]: 49.5d). Specifically, 88% of CD45-ADC–conditioned recipients had long-term survival and significantly reduced clinical GVHD scores. At a higher T-cell dose (3 × 106), TBI-conditioned recipients experienced rapidly lethal GVHD (MST: 26d), whereas 94% of CD45-ADC–conditioned recipients receiving the same donor graft had long-term survival (P < .0001) and no GVHD clinical signs (Figure 7B). Day 7 splenic CD4+ and CD8+ T cells analyzed for IFN-γ production by intracellular cytokine staining; each showed significantly lower IFN-γ production in CD45-ADC vs TBI-conditioned recipients (supplemental Figure 8A). Day 25 PB analysis confirmed equivalent donor engraftment in both CD45-ADC and TBI-conditioned recipients (Figure 7F). PB differentials were comparable between both conditioning groups (supplemental Figure 8B-C). Notably, isotype-ADC conditioning led to 100% survival but with no donor engraftment (Figure 7A-B,F).
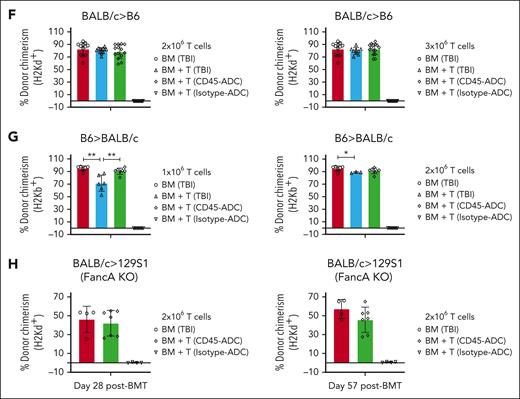
Donor T cells induced aGVHD in allogeneic settings were significantly less in CD45-ADC vs lethal TBI conditioning. (A-B,F) Female WT B6 mice (10- to 12-weeks-old) conditioned with lethal TBI (900 cGy), or 5 mg/kg CD45-ADC or isotype-ADC were infused with 10 × 106 WT BALB/c BM cells alone or with BALB/c purified T cells (2 × 106 or 3 × 106 cells, as indicated). (A) Mice who underwent transplant were evaluated for clinical GVHD (n = 6-9/group). BM + T cells: TBI vs CD45-ADC–conditioned recipients, P < .0001 on d8, d19, d25, d32, d39, and d46; P = .0004 on d11. Data are representative of 2 independent experiments. Kaplan-Meier survival plot represents pooled data (n = 12-18 mice per group) from 2 independent experiments (BM + T cells: TBI vs CD45–ADC-conditioned recipients, P < .0001). (B) Mice who underwent transplant were evaluated for clinical GVHD (n = 6-9 per group). BM + T cells: TBI vs CD45-ADC–conditioned recipients, P = .0003 on d8; P = .0008 on d11; P = .0012 on d15; P < .0001 on d19, d25, d32, and d39. Data are representative of 2 independent experiments. Kaplan-Meier survival plot represents pooled data (n = 12-18 mice per group) from 2 independent experiments (BM + T cells: TBI vs CD45-ADC–conditioned recipients, P < .0001). (C-D,G) Female WT BALB/c mice (10- to 12-weeks-old) conditioned with lethal TBI (600 cGy), or 5 mg/kg CD45-ADC or isotype-ADC were infused with 10 × 106 WT B6 BM cells alone or with 1 × 106 to 2 × 106 B6 purified T cells as indicated. (C) Mice who underwent transplant were evaluated for clinical GVHD (n = 6-9 per group). BM + T cells: TBI vs CD45-ADC–conditioned recipients, P < .0001 on d7, d10, d18, d21, and d24; P = .0132 on d14. Data were obtained from 1 experiment. Kaplan-Meier survival plot represents data (n = 6-9 mice per group) from 1 experiment (BM + T cells: TBI vs CD45-ADC–conditioned recipients, P = .0009). (D) Mice who underwent transplant were evaluated for clinical GVHD (n = 6-9 per group). BM + T cells: TBI vs CD45-ADC–conditioned recipients, P < .0001 on d7, d10, d14, d18, d21, and d24. Data were obtained from 1 experiment. Kaplan-Meier survival plot represents data (n = 6-9 mice per group) from 1 experiment (BM + T cells: TBI vs CD45-ADC–conditioned recipients, P = .0008). (E,H) Female or male Fanca−/− (H-2b) mice (10- to 12-weeks-old) were conditioned with lethal TBI (900 cGy), isotype-ADC (0.5 mg/kg), or CD45-ADC (1.5 mg/kg) and infused with 10 × 106 WT BALB/c BM cells alone or with 2 × 106 BALB/c purified T cells. (E) Mice who underwent transplant were evaluated for clinical GVHD (n = 4-7 per group). BM + T cells: TBI vs CD45-ADC–conditioned recipients, P < .0001 on d4; P = .0052 on d8. Data were obtained from 1 experiment. Kaplan-Meier survival plot represents data (n = 4-7 mice per group) from 1 experiment (BM + T cells: TBI vs CD45-ADC–conditioned recipients, P = .0002). (F) Engraftment of donor cells (H-2d) in PB of mice who underwent transplant were analyzed at d25 post-BMT by flow cytometry. Data represent mean ± SEM. Pooled data from 2 independent experiments are shown. 2 × 106 T cells: BM only (n = 12), BM + T (TBI, n = 12), BM + T (CD45-ADC, n = 14), BM + T (Isotype-ADC, n = 16); 3 × 106 T cells: BM only (n = 12), BM + T (TBI, n = 9), BM + T (CD45-ADC, n = 15), BM + T (Isotype-ADC, n = 16). (G) Engraftment of donor cells (H-2b) in the PB of mice who underwent transplant were analyzed at d25 post-BMT. Data represent mean ± SEM. ∗P < .05, ∗∗P < .01 using the 2-tailed Student t test. Data were obtained from 1 experiment. 1 × 106 T cells: BM only (n = 6), BM + T (TBI, n = 6), BM + T (CD45-ADC, n = 7), BM + T (Isotype-ADC, n = 8); 2 × 106 T cells: BM only (n = 6), BM + T (TBI, n = 3), BM + T (CD45-ADC, n = 7), BM + T (Isotype-ADC, n = 8). (H) Engraftment of donor cells (H-2d) in the PB of mice who underwent transplant were analyzed at d28 and d57 post-BMT. Data represent mean ± SEM and were obtained from 1 experiment. BM only (n = 4), BM + T (CD45-ADC, n = 7), BM + T (isotype-ADC, n = 4).
Donor T cells induced aGVHD in allogeneic settings were significantly less in CD45-ADC vs lethal TBI conditioning. (A-B,F) Female WT B6 mice (10- to 12-weeks-old) conditioned with lethal TBI (900 cGy), or 5 mg/kg CD45-ADC or isotype-ADC were infused with 10 × 106 WT BALB/c BM cells alone or with BALB/c purified T cells (2 × 106 or 3 × 106 cells, as indicated). (A) Mice who underwent transplant were evaluated for clinical GVHD (n = 6-9/group). BM + T cells: TBI vs CD45-ADC–conditioned recipients, P < .0001 on d8, d19, d25, d32, d39, and d46; P = .0004 on d11. Data are representative of 2 independent experiments. Kaplan-Meier survival plot represents pooled data (n = 12-18 mice per group) from 2 independent experiments (BM + T cells: TBI vs CD45–ADC-conditioned recipients, P < .0001). (B) Mice who underwent transplant were evaluated for clinical GVHD (n = 6-9 per group). BM + T cells: TBI vs CD45-ADC–conditioned recipients, P = .0003 on d8; P = .0008 on d11; P = .0012 on d15; P < .0001 on d19, d25, d32, and d39. Data are representative of 2 independent experiments. Kaplan-Meier survival plot represents pooled data (n = 12-18 mice per group) from 2 independent experiments (BM + T cells: TBI vs CD45-ADC–conditioned recipients, P < .0001). (C-D,G) Female WT BALB/c mice (10- to 12-weeks-old) conditioned with lethal TBI (600 cGy), or 5 mg/kg CD45-ADC or isotype-ADC were infused with 10 × 106 WT B6 BM cells alone or with 1 × 106 to 2 × 106 B6 purified T cells as indicated. (C) Mice who underwent transplant were evaluated for clinical GVHD (n = 6-9 per group). BM + T cells: TBI vs CD45-ADC–conditioned recipients, P < .0001 on d7, d10, d18, d21, and d24; P = .0132 on d14. Data were obtained from 1 experiment. Kaplan-Meier survival plot represents data (n = 6-9 mice per group) from 1 experiment (BM + T cells: TBI vs CD45-ADC–conditioned recipients, P = .0009). (D) Mice who underwent transplant were evaluated for clinical GVHD (n = 6-9 per group). BM + T cells: TBI vs CD45-ADC–conditioned recipients, P < .0001 on d7, d10, d14, d18, d21, and d24. Data were obtained from 1 experiment. Kaplan-Meier survival plot represents data (n = 6-9 mice per group) from 1 experiment (BM + T cells: TBI vs CD45-ADC–conditioned recipients, P = .0008). (E,H) Female or male Fanca−/− (H-2b) mice (10- to 12-weeks-old) were conditioned with lethal TBI (900 cGy), isotype-ADC (0.5 mg/kg), or CD45-ADC (1.5 mg/kg) and infused with 10 × 106 WT BALB/c BM cells alone or with 2 × 106 BALB/c purified T cells. (E) Mice who underwent transplant were evaluated for clinical GVHD (n = 4-7 per group). BM + T cells: TBI vs CD45-ADC–conditioned recipients, P < .0001 on d4; P = .0052 on d8. Data were obtained from 1 experiment. Kaplan-Meier survival plot represents data (n = 4-7 mice per group) from 1 experiment (BM + T cells: TBI vs CD45-ADC–conditioned recipients, P = .0002). (F) Engraftment of donor cells (H-2d) in PB of mice who underwent transplant were analyzed at d25 post-BMT by flow cytometry. Data represent mean ± SEM. Pooled data from 2 independent experiments are shown. 2 × 106 T cells: BM only (n = 12), BM + T (TBI, n = 12), BM + T (CD45-ADC, n = 14), BM + T (Isotype-ADC, n = 16); 3 × 106 T cells: BM only (n = 12), BM + T (TBI, n = 9), BM + T (CD45-ADC, n = 15), BM + T (Isotype-ADC, n = 16). (G) Engraftment of donor cells (H-2b) in the PB of mice who underwent transplant were analyzed at d25 post-BMT. Data represent mean ± SEM. ∗P < .05, ∗∗P < .01 using the 2-tailed Student t test. Data were obtained from 1 experiment. 1 × 106 T cells: BM only (n = 6), BM + T (TBI, n = 6), BM + T (CD45-ADC, n = 7), BM + T (Isotype-ADC, n = 8); 2 × 106 T cells: BM only (n = 6), BM + T (TBI, n = 3), BM + T (CD45-ADC, n = 7), BM + T (Isotype-ADC, n = 8). (H) Engraftment of donor cells (H-2d) in the PB of mice who underwent transplant were analyzed at d28 and d57 post-BMT. Data represent mean ± SEM and were obtained from 1 experiment. BM only (n = 4), BM + T (CD45-ADC, n = 7), BM + T (isotype-ADC, n = 4).
GVHD effects of CD45-ADC vs lethal TBI conditioning were tested in a second model, B6→BALB/c. At a reduced T-cell dose (1 × 106), GVHD–induced lethality was significantly lower (P = .0009) in CD45-ADC vs TBI (MST: 30d) conditioned recipients; 75% of CD45-ADC–conditioned recipients had long-term survival with no sign of GVHD (Figure 7C). At a higher T-cell dose (2 × 106), TBI-conditioned recipients uniformly experienced GVHD lethality (MST: 11d), whereas 63% of CD45-ADC–conditioned recipients had long-term survival (P = .0008; Figure 7D). PB analysis on d25 confirmed high donor alloengraftment in both CD45-ADC and TBI-conditioned recipients (Figure 7G). Increased donor B-cell chimerism in CD45-ADC vs TBI-conditioned recipients of allogeneic donor T cells is consistent with absence of GVHD because B cells are highly sensitive to GVHD–induced depletion (supplemental Figure 8B-E). Isotype-ADC again had 100% survival but no donor alloengraftment.
Finally, we tested donor T-cell alloreactivity in an FA mouse model. Isotype-ADC, CD45-ADC, or TBI–conditioned Fanca−/− (H-2b) recipients were given allogeneic BALB/c BM ± purified T cells (2 × 106). CD45-ADC vs TBI-conditioned recipients had significantly reduced GVHD (P = .0002) with 71% recipients surviving long-term, in contrast to 8d uniform lethality seen in TBI-conditioned recipients (Figure 7E). PB analyses (d28 and d57) confirmed donor engraftment in both CD45-ADC and TBI-conditioned recipients (Figure 7H) along with donor T-, B-, and myeloid-cell chimerism (supplemental Figure 8F). Taken together, these data suggest TBI vs CD45-ADC conditioning induced more proinflammatory responses and an environment more conducive to GVHD lethality.
Rhesus macaques given single dose human (h)CD45-ADC or lethal TBI are fully myeloablated
Toward clinical translation, rhesus macaques were conditioned with a different mAb and payload CD45-ADC than the murine CD45-ADC described above. Recipients received CD45-ADC at 0.2 mg/kg, d6 or 0.3 mg/kg, d10, or lethal TBI (1040 cGy: 260 cGy/fraction twice daily; d2, d1). BM CD34+ and CD34+CD90+CD45RA− HSCs were enumerated pretransplant and on d0 (supplemental Table 2). These preliminary studies indicated that CD45-ADC was at least as effective in eliminating CD34+ cells and the more primitive HSCs as lethal TBI.
Discussion
Here, we demonstrated the efficacy of a hematopoietic–specific conditioning strategy utilizing CD45-ADC for multilineage donor chimerism in 3 distinct FA mouse transplant models. Single agent CD45-ADC–mediated BM HSPCs depletion, and peripheral organ depletion of hematopoietic cells and mature lymphocytes resulted in robust donor engraftment (mean, >90%) in an miH–mismatch transplant model. CD45-ADC alone ± anti-CD40L mAb resulted long-term stable mixed chimerism (mean, 70% to 90%) of MHC–disparate donor grafts in Fancc−/− or Fancg−/− recipients, respectively. Strikingly, allogeneic donor grafts containing T cells did not induce GVHD in CD45-ADC–conditioned WT or Fanca−/− recipients.
CD45 is widely expressed in the hematopoietic system and participates in the regulation of lymphocyte activation and maturation, as well as thymic selection.45 Because mature leukocytes including tissue resident lymphocytes and BM precursor cells express CD45,45,46 mAb-based approaches targeting CD45 for recipient HSC depletion have been explored and shown promise for hematopoietic cell specific allo-HSCT conditioning. In a recent study, Fanca−/− mice were conditioned with a saporin (SAP)47–based anti–CD45-ADC or Cy followed by transplantation with unmanipulated, heterozygous, healthy donor BM. ADC conditioning resulted in HSC depletion and facilitated donor alloengraftment comparable with Cy treatment but with less toxicity.48
Fanca−/− recipients conditioned with isotype-ADC or CD45-ADC (3 mg/kg) plus anti-CD40L mAb and infused with B6 CD45.1+ donor BM cells (20 × 106) had poor survival (16% each), whereas 67% long-term survival (P = .052) was observed in CD45-ADC plus anti-CD40L mAb conditioned recipients receiving a higher BM cell dose (40 × 106) (supplemental Table 1); these data suggest the need for sufficient HSPCs to fill niches of CD45-depleted Fanca−/− recipients. MHC-disparate donor BM (40 × 106 cells) engraftment was undetectable in WT, Fancc−/−, and Fancg−/− recipients who underwent transplant without conditioning (supplemental Table 1). Unconjugated anti-CD45.2 mAb conditioning was insufficient for donor alloengraftment in miH-mismatch settings indicating antibody conjugation was critical for targeted delivery and efficient host HSPC depletion pretransplant.
Consistent with the known DNA repair defect and susceptibility to toxicity, isotype-ADC conditioning at higher dose (3 mg/kg) compromised survival and led to measurable nonspecific alloengraftment effects in 1 of 3 surviving Fanca−/− recipients, which may be attributable to high free PBD supported in part by pharmacokinetic/pharmacodynamic measurements (supplemental Figure 3). Compared with CD45-ADC (1.5 mg/kg), isotype-ADC (1.5 mg/kg) significantly compromised survival (90% vs 50%, respectively) but without nonspecific engraftment effects. Compared with CD45-ADC (1.5 mg/kg), isotype-ADC dose-adjusted to a one-third dose (0.5 mg/kg) level had a longer half-life (2.2 times) due to lack of target–mediated drug disposition but equivalent clearance at 4 hours without nonspecific alloengraftment effects. These data are consistent with our previous studies in WT mice demonstrating that isotype-ADC vs CD45-ADC at 3 mg/kg have a longer half-life (15.3 hours) and higher ADC exposure (3.8-fold).30 Due to a broad CD45 expression profile, CD45-ADC at 3 mg/kg had the desired rapid half-life (2.05 hours) suitable for conditioning that must have active drug clearance before hematopoietic cell infusion.30
In the FA models studied, transient ALT, AST, and serum cytokine increases were observed early post-BMT along with mild-to-moderate increases in liver and spleen (d12), and colon histopathology (d67 and d78). The transient hepatotoxicity may also reflect on-target depletion of CD45+ cells in the liver (eg, Kupffer cells; recruited monocyte–derived macrophages and capsular macrophages), clearance of dead/dying cells by the reticuloendothelial system and/or free PBD levels. Despite potential ADC–mediated cell and organ toxicity that might be exaggerated in FA mice, CD45-ADC–conditioned recipients survived long-term post-BMT with no clinical GVHD signs. Histone H2AX phosphorylation on a serine 4 residues from the carboxyl terminus results in the production of γ-H2AX, a sensitive marker for DNA double-strand breaks induced by endogenous or exogenous (eg, cytotoxic drugs; radiation) injury and the first step in DNA repair protein recruitment. Fanca−/− mice given dose-adjusted isotype-ADC (0.5 mg/kg) or CD45-ADC (1.5 mg/kg) and miH–mismatched donor grafts had 90% to 100% survival rates (supplemental Figure 2C-D), without increasing γ-H2AX expression in BM or any of the GVHD organs studied on d12 or d78 post-BMT (supplemental Figure 9A-D).
Conditioning pretransplant can cause host tissue damage resulting in innate immune cell activation that amplifies inflammatory cytokine secretion, increases antigen presenting cell function, and drives donor T-cell priming causing lethal aGVHD.49 CD45-ADC vs lethal TBI conditioning and donor BM + T cells resulted in significantly reduced aGVHD in 2 distinct MHC-disparate WT donor-recipient pairs and, more importantly, in an FA model. The low inflammatory and tissue injury responses observed may be the direct consequences of CD45-ADC vs lethal TBI conditioning on tissue injury, thereby permitting infusion of donor grafts containing T cells that could provide immune surveillance against infections and neoplasms. Alternatively, pretransplant CD45-ADC may have depleted host antigen presenting cells that can play a key role in driving GVHD.50,51 Therefore, CD45-ADC conditioning represents a viable replacement of TBI for allo-HSCT, especially relevant in patients with FA to minimize regimen-related toxicity, GVHD, and secondary malignancies. Further, significant healthy donor cell engraftment after CD45-ADC conditioning could eliminate DNA repair defective host hematopoietic cells, resulting in a proportional decrease in the likelihood for leukemia and head and neck cancers. CD45-SAP vs sublethal TBI conditioning improved survival in presence of allogeneic splenocytes in a B6→CB6F1 model.52 Preliminary studies in rhesus macaques indicated that hCD45-ADC is at least as effective as lethal TBI in eliminating HSCs. In aggregate, these data point to translating CD45 mAb–based conditioning for allo-HSCT.
Other approaches have targeted c-kit ligand that binds to CD117 (KIT), a dimeric transmembrane receptor tyrosine kinase expressed by HSCs and their progenitors,53 essential for HSC homing, proliferation, adhesion, maintenance, and survival.54,55 ACK2, a blocking anti-mouse c-kit mAb administration reduced host HSCs of sufficient magnitude to allow donor BM engraftment in Fanca−/− recipients. CD4 depletion along with c-kit mAb further improved donor engraftment in this miH–mismatch transplant model compared with c-kit mAb alone.56 Recent findings in an FA mouse model using CD117-SAP proved to be more effective in achieving donor engraftment than anti-c-kit mAb alone.48 Since c-kit is not expressed on lymphocytes, anti-CD117 mAb–based conditioning approaches may not deplete host mature lymphoid cells, setting up the possibility of donor graft rejection and host niche and cytokine competition with newly developed donor lymphocytes, in contrast to CD45 mAb–based conditioning. CD117 expression on gastrointestinal cells, neuronal cells, mast cells, and cardiac progenitors also raise concerns about potential off-target toxicity.57
In a different approach, investigators leveraged the fact that murine (Fancc−/−) and human (FANCC) hematopoietic cells and FANCA progenitors are hypersensitive to proinflammatory cytokines including IFN-γ through increase in apoptosis.58-60 Si et al exploited the hypersensitivity of FA HSCs to IFN-γ as a hematopoietic–specific conditioning strategy for engraftment of congenic donor BM in FA mouse models. Recipients treated with IFN-γ for 7 days followed by BM infusion resulted in significant peripheral donor chimerism in Fanca−/− and Fancg−/− but not WT recipients as detected at 4 months post-BMT.61 Although effective, the clinical translation of this conditioning strategy remains limited likely due to the findings of leukemic clonal evolution of murine stem cells after exposure to inflammatory cytokines in FA.62
Taken together, we have shown that CD45-ADC can effectively deplete host HSPCs and lymphoid cells and allow robust donor engraftment and lymphohematopoietic recovery without a propensity to GVHD in 3 distinct preclinical FA mouse models. Because FANCA, FANCC, and FANCG account for 90% of all patients with FA, these data provide support for further development of CD45-ADC conditioning in patients with FA with the desired goal of reducing regimen-related toxicity while achieving alloengraftment and reducing the risk of GVHD and secondary malignancies.
Acknowledgments
The authors thank the members of the Blazar laboratory and Magenta Therapeutics for technical assistance.
This work was supported in part by a grants from National Institutes of Health (NIH), National Heart, Lung, and Blood Institute (NHLBI) (R01 HL147324), and NIH, National Institute of Allergy and Infectious Diseases (NIAID) (R37 AI34495) and Kidz 1st Fund (to B.R.B.); NIH/NIAID grants (U19 Al1051731 and U19 AI174967), NIH/NHLBI (grants R01 HL095791, P01 HL158504, P01 HL158505), and a Leukemia and Lymphoma Society Translational Research Program grant (to L.S.K.); and NIH/NHLBI grants (U19 HL156247 and P01 HL142494) (to D.T.S.).
Authorship
Contribution: A.S. designed and performed experiments, analyzed data, and wrote the manuscript; R.P., L.L., and S.H. discussed data, and edited the manuscript; R.P. and L.M.O. provided antibody-drug conjugate reagents and edited the manuscript; M.J.R., J.P., L.G., V.T., U.G., F.A.C., and E.R.P. provided data and edited the manuscript; A.P.-M. provided data and edited the manuscript; C.R.E., J.T., M.L.M., J.E.W., L.S.K., M.J.O., H.-P.K., D.T.S., and L.M.O. discussed data and edited the manuscript; and B.R.B. designed experiments, discussed data, and edited the manuscript.
Conflict-of-interest disclosure: B.R.B., J.E.W., and D.T.S. served as advisers to Magenta Therapeutics. R.P., L.L., S.H., and L.M.O. were employees and equity holders of Magenta Therapeutics at the time this work was performed. D.T.S. was a cofounder and equity holder of Magenta Therapeutics. L.S.K. is a member of scientific advisory board for Mammoth Biosciences and HiFiBiO Therapeutics; reports research funding from Magenta Therapeutics, Vor Bio, Tessera Therapeutics, Novartis, EMD Serono, Gilead Sciences, and Regeneron Pharmaceuticals; reports consulting fees from Vertex, as well as grants and personal fees from Bristol Myers Squibb. L.S.K. has a conflict-of-interest with Bristol Myers Squibb, which is managed under an agreement with Harvard Medical School.
Correspondence: Bruce R. Blazar, Division of Blood and Marrow Transplant & Cellular Therapy, Masonic Cancer Center and Department of Pediatrics, University of Minnesota, 420 Delaware St SE, Minneapolis, MN 55455; email: blaza001@umn.edu.
References
Author notes
Data are available on request from the corresponding author, Bruce R. Blazar (blaza001@umn.edu).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal