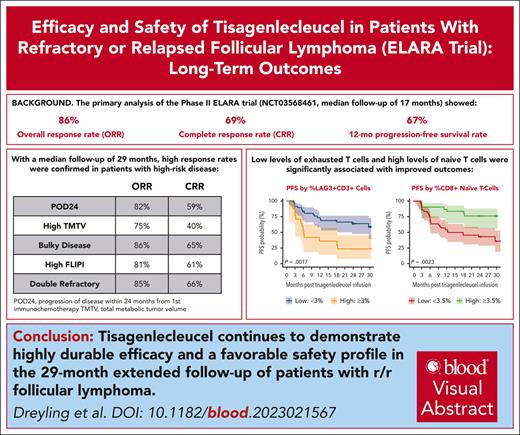
Tisagenlecleucel responses in patients with r/r FL remain highly durable a year after primary analysis; no new safety signals were observed.
Low levels of LAG3+CD3+ exhausted T cells and higher levels of naïve CD8+ T cells were significantly associated with improved outcomes.
Visual Abstract
Tisagenlecleucel is approved for adults with relapsed/refractory (r/r) follicular lymphoma (FL) in the third- or later-line setting. The primary analysis (median follow-up, 17 months) of the phase 2 ELARA trial reported high response rates and excellent safety profile in patients with extensively pretreated r/r FL. Here, we report longer-term efficacy, safety, pharmacokinetic, and exploratory biomarker analyses after median follow-up of 29 months (interquartile range, 22.2-37.7). As of 29 March 2022, 97 patients with r/r FL (grades 1-3A) received tisagenlecleucel infusion (0.6 × 108-6 × 108 chimeric antigen receptor–positive viable T cells). Bridging chemotherapy was allowed. Baseline clinical factors, tumor microenvironment, blood soluble factors, and circulating blood cells were correlated with clinical response. Cellular kinetics were assessed by quantitative polymerase chain reaction. Median progression-free survival (PFS), duration of response (DOR), and overall survival (OS) were not reached. Estimated 24-month PFS, DOR, and OS rates in all patients were 57.4% (95% confidence interval [CI], 46.2-67), 66.4% (95% CI, 54.3-76), and 87.7% (95% CI, 78.3-93.2), respectively. Complete response rate and overall response rate were 68.1% (95% CI, 57.7-77.3) and 86.2% (95% CI, 77.5-92.4), respectively. No new safety signals or treatment-related deaths were reported. Low levels of tumor-infiltrating LAG3+CD3+ exhausted T cells and higher baseline levels of naïve CD8+ T cells were associated with improved outcomes. Tisagenlecleucel continued to demonstrate highly durable efficacy and a favorable safety profile in this extended follow-up of 29 months in patients with r/r FL enrolled in ELARA. This trial was registered at www.clinicaltrials.gov as #NCT03568461.
Introduction
Follicular lymphoma (FL) is considered an indolent form of non-Hodgkin lymphoma with a relapsing and remitting course.1,2 There is no clear standard of care (SOC) for patients with relapsed/refractory (r/r) disease, and immunochemotherapy is repeatedly used from first- to later-line settings with diminishing efficacy and the potential for accumulation of toxicities.3-5 Patients with high-risk disease, such as progression of disease within 24 months from first immunochemotherapy (POD24) and high baseline tumor burden have a poor prognosis and an increased risk of death.2 Tisagenlecleucel is approved in the United States, the European Union, and Japan for r/r FL in the third-line setting.
In the primary analysis of the single-arm, open-label, phase 2 ELARA trial (median follow-up, 17 months; ClinicalTrials.gov identifier: NCT03568461), tisagenlecleucel demonstrated a high overall response rate (ORR; 86%), complete response rate (CRR; 69%), and durable responses (12-month progression-free survival [PFS] rate of 67%) in adult patients with high-risk r/r FL, including patients with POD24 and high tumor burden. Grade ≥3 cytokine release syndrome and immune effector cell–associated neurotoxicity syndrome occurred in ≤1% of patients.6 This report presents the continued durability of response, longer-term safety, as well as correlative biomarker and pharmacokinetic analyses based on >2-year follow-up data from the ELARA trial of patients treated with tisagenlecleucel.
Methods
Trial design
ELARA (ClinicalTrials.gov identifier: NCT03568461) is a phase 2, single-arm, global, multicenter, open-label trial investigating the efficacy and safety outcomes of tisagenlecleucel in adults with r/r FL after ≥2 treatment lines or who relapsed after autologous stem cell transplantation (auto-SCT).6 Detailed study protocol and outcome measures are described in previous reports.6,7 Eligible patients were aged ≥18 years with r/r FL (grades 1-3A) after ≥2 lines of prior therapy including an anti-CD20 antibody and an alkylating agent or after auto-SCT. Bridging chemotherapy was permitted. After lymphodepleting (LD) chemotherapy, patients received a single dose of tisagenlecleucel (0.6 × 108-6 × 108 CAR+ viable T cells). Trial protocols were reviewed and approved by local institutional review boards; all enrolled patients provided written informed consent.
Biomarker analyses
Baseline clinical and disease factors, blood soluble biomarkers, the tumor microenvironment (TME), and circulating blood cells were explored for association with clinical response and PFS. Quantitative tumor burden (total metabolic tumor volume [TMTV]) was assessed by fluorodeoxyglucose positron emission tomography/computed tomography. Expression of exhaustion markers in the TME on tumor-infiltrating T cells (lymphocyte activation gene 3 [LAG3], programmed cell death protein-1 [PD-1], and T-cell immunoglobulin and mucin-domain containing-3), monocytes, and myeloid-derived suppressor cells (MDSCs) were measured by fluorescence immunohistochemistry. Baseline tumor tissues were available for 96 of 97 patients who received infusion, 67 of them had quantified values for LAG3+CD3+ biomarker, which were obtained <1 year (in 57 of 67 patients) or >1 year (in 10 of 67 patients) before tisagenlecleucel infusion. Further details, including the timing of archival patient tumor biopsies, are provided in the Methods section of the supplemental Data, available on the Blood website. Peripheral blood samples were obtained before LD chemotherapy and before infusion, and circulating T, B, and natural killer cells were quantified (see “Methods” in the supplemental Data).
Pharmacokinetics
Statistical analyses
As previously described, the Kaplan-Meier method was used to estimate PFS, duration of response (DOR), overall survival (OS), and time to next antilymphoma therapy (TTNT).6 Post hoc subgroup analyses of response were also completed based on key baseline subgroups. Univariate and multivariate Cox model analyses were used to explore associations of biomarker and clinical/disease characteristics with PFS. Variables included in the analyses were tumor burden and other clinical factors; TME characteristics; blood T, B, and natural killer cell counts; cytokines and other soluble clinical laboratory measurements; as well as peripheral blood T-cell immunophenotypes. Correlations between baseline tumor burden and pre-LD serum cytokine levels were quantified using Spearman correlation coefficient. Chimeric antigen receptor (CAR) T-cell in vivo exposure parameters (cellular kinetics) were estimated using noncompartmental methods with Phoenix WinNonlin, version 6.4 (Pharsight Corp, St Louis, MO). All data analyses were performed by SAS (version 9.4) and R Studio (2022).9
Results
Baseline characteristics
As of 29 March 2022, 119 patients were screened and 98 patients were enrolled, of whom 97 received infusion. Median time from first line of therapy to ELARA enrollment was 59.5 months. The median follow-up time from infusion to data cutoff was 28.9 months (interquartile range [IQR], 22.2-37.7). The median time from enrollment (ie, leukapheresis product accepted) to infusion was 46 days (IQR, 38-57) and the median time from manufacturing process start to final product release from the facility for all enrolled patients (n = 98) was 24 days (IQR, 21-30). The efficacy analysis set included 94 patients who had measurable disease per independent review committee at the time of infusion. Safety analysis was conducted on all 97 patients infused with tisagenlecleucel. Patient demographics and disease characteristics at baseline are shown in supplemental Table 1. At study entry, the median number of prior lines of therapy was 4 (range, 2-13), including prior auto-SCT in 35 of 97 (36%); 76 of 97 (78%) of patients were refractory to the last prior antineoplastic treatment (69 of 97 [71%] ≥2 prior regimens) and 61 of 97 (63%) had disease progression within 2 years of initial anti-CD20–containing treatment (POD24 group). Furthermore, 63 of 97 (65%) of patients had bulky disease and 58 of 97 (60%) had a Follicular Lymphoma International Prognostic Index (FLIPI) score of ≥3. Overall, 44 of 97 (45%) of patients received bridging therapy. Baseline and disease characteristics more commonly found in patients who required bridging therapy included bulky disease, high FLIPI score, and stage III/IV disease. Tisagenlecleucel was administered in the outpatient setting for 18% of patients.
Efficacy
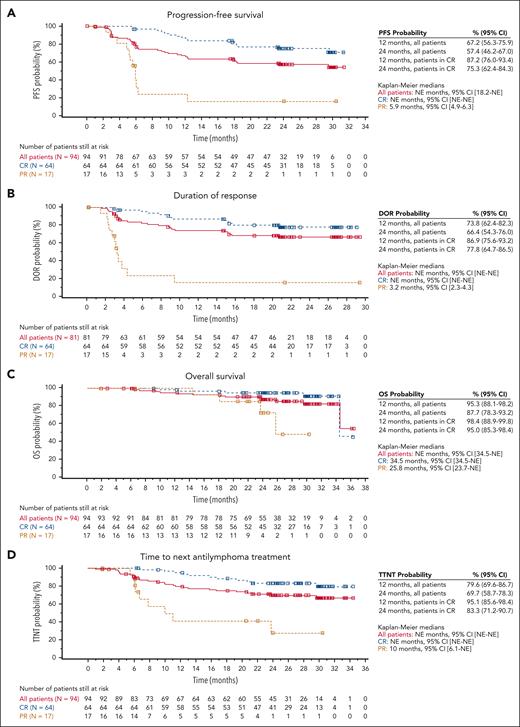
In this updated follow-up, median PFS, DOR, and OS were not reached (Figure 1A-C). The estimated 24-month PFS rate for all patients was 57.4% (95% confidence interval [CI], 46.2-67.0). Estimated 24-month DOR of patients in complete response (CR) was 77.8% (95% CI, 64.7, 86.5). Estimated 24-month OS of patients in CR was 87.7% (95% CI, 78.3-93.2). The median TTNT for all patients was not reached and the estimated 24-month TTNT was 69.7% (95% CI, 58.7-78.3; Figure 1D). Patients in CR demonstrated better efficacy than the overall ELARA population for each of these efficacy measures. Relapse occurred in 25 (31%) responders. Median time to relapse among responders was 121.5 days (range, 43-635 days). For all patients, the ORR (best overall response of CR or partial response [PR]) was 86.2% (81 of 94; 95% CI, 77.5-92.4) and CRR was 68.1% (64 of 94; 95% CI, 57.7-77.3; supplemental Table 2). Of the 31 patients who had an initial PR, 14 converted to a CR within 6 months after infusion. One patient in CR was downgraded to a PR because of a determination that their confirmatory bone marrow test was performed outside of the strict 14-day testing window, per protocol (supplemental Table 2).
Kaplan-Meier curves for secondary end points for patients with r/r FL who received tisagenlecleucel infusion. (A) PFS, (B) DOR, (C) OS, and (D) TTNT in the EAS (n = 94). PFS, DOR, and OS by best overall response (BOR) curves are per IRC assessment. TTNT curve is per local assessment. Censoring times are shown as squares. EAS, efficacy analysis set; IRC, independent review committee; NE, not estimable.
Kaplan-Meier curves for secondary end points for patients with r/r FL who received tisagenlecleucel infusion. (A) PFS, (B) DOR, (C) OS, and (D) TTNT in the EAS (n = 94). PFS, DOR, and OS by best overall response (BOR) curves are per IRC assessment. TTNT curve is per local assessment. Censoring times are shown as squares. EAS, efficacy analysis set; IRC, independent review committee; NE, not estimable.
Tisagenlecleucel induced high rates of durable responses in all patients including those with high-risk baseline disease characteristics, such as POD24 (ORR, 82%; CRR, 59%), high tumor burden by TMTV (ORR, 75%; CRR 40%), bulky disease (ORR 85.5%, CRR 64.5%), high FLIPI score (ORR 80.7%, CRR, 61.4%), and double refractoriness (ORR, 84.6%; CRR, 66.2%). A homogeneous treatment effect was observed across all subgroups with no change to response rates (CRR and ORR) when analyzed by risk subgroups (supplemental Figure 1). The estimated 24-month PFS and DOR rates after censoring for new anticancer therapy for patients in CR with high (n = 20) vs low (n = 72) tumor burden were 42.9% (95% CI, 9.8-73.4) vs 78.8% (95% CI, 64.9-87.7) and 42.9% (95% CI, 9.8-73.4) vs 81.8% (95% CI, 67.7-90.1), respectively, per independent review committee assessment; because of the low number of patients with high tumor burden, the results should be interpreted with caution.
Safety
The safety profile of tisagenlecleucel observed in this long-term follow-up analysis was consistent with published reports.6,10 No new safety signals or treatment-related mortality were observed. Any-grade infection at any time after infusion occurred in 16 (16.5%) patients, with 9 (9.3%) experiencing grade ≥3; COVID-19–related infections any time after infusion are summarized in supplemental Table 3. By month 6, the probability of resolution (defined as achieving laboratory results of grade ≤2) of any cytopenia was 82.0% and for all the cytopenias (leukopenia, anemia, thrombocytopenia, neutropenia, and lymphopenia) it ranged from 70% to 100%. At month 24, the probability of resolution was 96.7% for any cytopenia. Any-grade hypogammaglobulinemia was experienced in 11 (11.3%) patients, with 1 (1%) reporting grade ≥3 (supplemental Table 4). Additionally, 2 patients developed serious neurological events >8 weeks after tisagenlecleucel infusion (1 possible progressive multifocal leukoencephalopathy [onset, study day 238] in a patient who had prior grade 4 immune effector cell–associated neurotoxicity syndrome ongoing at the time of death, which was due to euthanasia, and 1 encephalopathy [onset, study day 345] ongoing at the time of death due to hemophagocytic lymphohistiocytosis). All other neurological events had resolved at the time of data cutoff.
Twenty-two patients (22.7%) received ≥1 new antineoplastic medication after tisagenlecleucel, primarily because of stable disease or progressive disease. Two patients experienced a secondary malignancy during this longer-term follow-up (squamous cell carcinoma and bladder transitional cell carcinoma, respectively). Additionally, 3 new deaths occurred during this updated 2-year follow-up period (progressive disease, n = 1; and serious adverse events, n = 2 [urothelial bladder carcinoma and graft-versus-host disease after allogeneic SCT, respectively]). None of the malignancies or deaths were considered related to study treatment. Further details concerning deaths are included in the Results section of the supplemental Data.
Biomarker analysis
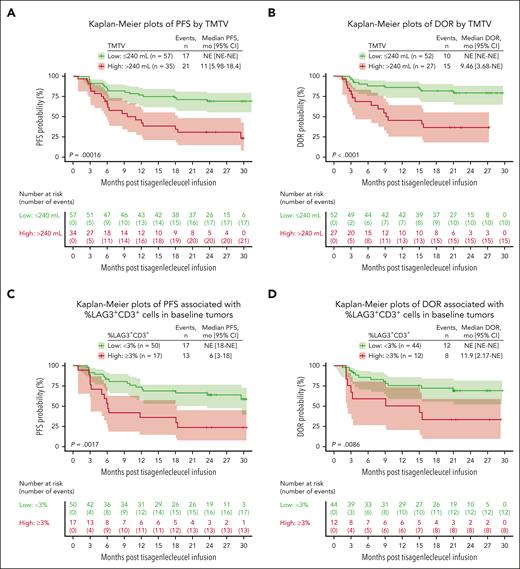
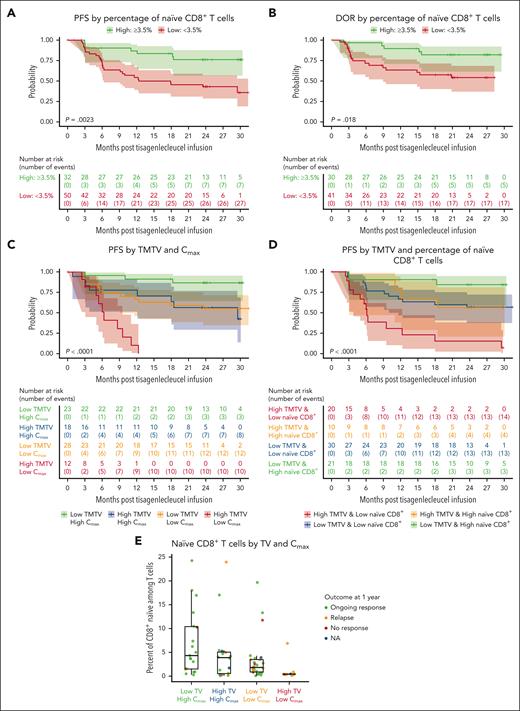
Exploratory analyses were performed on long-term efficacy outcomes for patients with several known high-risk disease characteristics. Higher tumor burden at baseline (pre-LD chemotherapy, TMTV of >240 mL) was associated with shorter PFS (P = .00016; Figure 2A) and DOR in responders (P < .0001; Figure 2B). Lower levels of tumor-infiltrating LAG3+ exhausted T cells (<3% of total CD3+ T cells) in the TME were significantly associated with longer PFS (Figure 2C) and DOR (Figure 2D). No (meaningful/significant) difference was observed for other exhaustion markers such as PD-1 and T-cell immunoglobulin and mucin-domain containing-3; similarly, no differences were observed for monocyte and MDSCs in the TME (data not shown). Lower tumor necrosis factor α (TNF-α) and interleukin-10 (IL-10) levels were associated with prolonged PFS (Figure 3A-B). Assessment of T and B cells and various cytokines in blood samples showed that baseline tumor burden strongly correlated with pre-LD levels of TNF-α and IL-10 (Spearman correlation = 0.86 and 0.8, respectively; both P < .001; Figure 3C-D). A higher proportion of circulating naïve CD8+ T cells (≥3.5% of T cells) at baseline was associated with longer PFS (Figure 4A) and DOR (Figure 4B). Lower baseline tumor volume (≤240 mL) and higher maximum transgene level (Cmax; >4000 copies per μg) were associated with longer PFS (Figure 4C). Patients with high baseline tumor volume (>240 mL) and high Cmax (>4000 copies per μg) had PFS that was comparable with that of patients with low baseline tumor volume (≤240 mL) and low Cmax (<4000 copies per μg; Figure 4C). Patients with lower tumor volume (≤240 mL) and higher naïve CD8+ T cells (≥3.5% of T cells) at baseline had longer PFS than other subgroups (Figure 4D). Higher levels (%) of CD8+ naïve T cells at baseline were associated with ongoing response at 1 year and were observed among the tumor volume and Cmax subgroups that experienced prolonged PFS (Figure 4E). Furthermore, a multivariate analysis showed that nodal area involvement (>4 nodal areas), high tumor volume (>240 mL), and low percentage of naïve CD8+ T cells (<3.5% of T cells) were significantly associated with worse PFS outcomes (supplemental Figure 2).
Association between baseline TMTV and tumor-infiltrated LAG3+ T cells with PFS and DOR. (A) Kaplan-Meier plots of PFS by TMTV. (B) Kaplan-Meier plots of DOR by TMTV. (C) Kaplan-Meier plots of PFS associated with percent LAG3+CD3+ cells in baseline tumors. (D) Kaplan-Meier plots of DOR associated with percent LAG3+CD3+ cells in baseline tumors. A cutoff (240 mL) at which most separation between PFS and DOR was derived and results are shown here. CD, cluster of differentiation; NE, not estimable.
Association between baseline TMTV and tumor-infiltrated LAG3+ T cells with PFS and DOR. (A) Kaplan-Meier plots of PFS by TMTV. (B) Kaplan-Meier plots of DOR by TMTV. (C) Kaplan-Meier plots of PFS associated with percent LAG3+CD3+ cells in baseline tumors. (D) Kaplan-Meier plots of DOR associated with percent LAG3+CD3+ cells in baseline tumors. A cutoff (240 mL) at which most separation between PFS and DOR was derived and results are shown here. CD, cluster of differentiation; NE, not estimable.
Lower pre-LD serum TNF-α and IL-10 levels correlated with lower tumor volume and prolonged PFS. (A) TNF-α and PFS. (B) IL-10 and PFS. (C) TNF-α vs tumor volume. (D) IL-10 vs tumor volume. NE, not estimable.
Lower pre-LD serum TNF-α and IL-10 levels correlated with lower tumor volume and prolonged PFS. (A) TNF-α and PFS. (B) IL-10 and PFS. (C) TNF-α vs tumor volume. (D) IL-10 vs tumor volume. NE, not estimable.
Lower tumor volume, high Cmax, and high naïve CD8+ T cells at baseline were associated with prolonged PFS. (A) PFS by percentage of naïve CD8+ T cells. (B) DOR by percentage of naïve CD8+ T cells. Significant associations between naïve CD8+ cells and DOR (P = .044) and PFS (P = .014) were observed when using median (2.14%) as cutoff (data not shown). A cutoff (3.5%) at which most separation between PFS and DOR was derived, and results are shown here. (C) PFS by tumor volume and Cmax. (D) PFS by TMTV and percentage of naïve CD8+ T cells. (E) Naïve CD8+ T cells by tumor volume and Cmax. CD, cluster of differentiation; NA, not available; TV, tumor volume.
Lower tumor volume, high Cmax, and high naïve CD8+ T cells at baseline were associated with prolonged PFS. (A) PFS by percentage of naïve CD8+ T cells. (B) DOR by percentage of naïve CD8+ T cells. Significant associations between naïve CD8+ cells and DOR (P = .044) and PFS (P = .014) were observed when using median (2.14%) as cutoff (data not shown). A cutoff (3.5%) at which most separation between PFS and DOR was derived, and results are shown here. (C) PFS by tumor volume and Cmax. (D) PFS by TMTV and percentage of naïve CD8+ T cells. (E) Naïve CD8+ T cells by tumor volume and Cmax. CD, cluster of differentiation; NA, not available; TV, tumor volume.
Pharmacokinetics
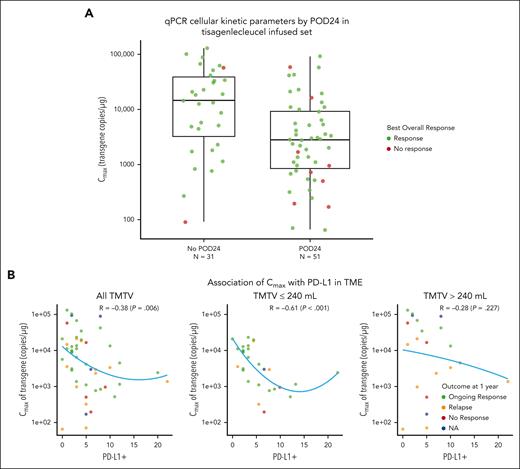
Among 97 patients evaluable, CAR transgene persistence was detectable for up to 925 days (median, 210 days; range, 13-925 days). The mean area under the concentration-time curve (AUC) from day 0 to day 84 (AUC0-84d) in responders (CR and PR) was similar to that of nonresponders (stable disease and progressive disease). The geometric mean AUC0-28d and geometric mean Cmax values in responders were nearly 2.4-fold higher than for nonresponders; however, considering the high interindividual variability, small number of nonresponders, and overlapping expansion ranges observed between responders and nonresponders, the exposure differences should be interpreted with caution (supplemental Table 5). For patients with POD24 or without POD24, the geometric mean Cmax (geometric mean coefficient of variation percent) was 2700 copies per μg (n = 51, 434%) and 9890 copies per μg (n = 31, 529%), respectively; however, responses were observed in patients with POD24 despite lower expansion than found in patients without POD24 (Figure 5A). Similar to all patients, persistence of CAR transgene in patients with POD24 was detected up to 925 days (median, 184 days; range, 13-925 days). Lastly, there was a negative association between programmed cell death ligand 1 (PD-L1) expression in the TME and expansion (Figure 5B). Although shorter PFS and DOR were observed in patients with high (median cutoff ≥4%) PD-L1 expression, this was not statistically significant (data not shown).
Pharmacokinetic analysis. (A) qPCR cellular kinetic parameters by POD24 in tisagenlecleucel infused set. (B) Association of Cmax with PD-L1 in patients based on TMTV. NA, not available; PD-L1, programmed cell death ligand 1; qPCR, quantitative polymerase chain reaction; R, correlation coefficient.
Pharmacokinetic analysis. (A) qPCR cellular kinetic parameters by POD24 in tisagenlecleucel infused set. (B) Association of Cmax with PD-L1 in patients based on TMTV. NA, not available; PD-L1, programmed cell death ligand 1; qPCR, quantitative polymerase chain reaction; R, correlation coefficient.
Dose-response relationship
Logistic regression analysis showed no strong evidence of dose-response relationship. A slight trend toward decreased response with lower doses was observed at doses <1.0 × 108 cells; however, because of the small number of nonresponders, these findings should be interpreted with caution. Favorable responses were observed across a wide dose range (Figure 6). Similarly, dose was neither associated with PFS (supplemental Figure 3A) nor DOR (supplemental Figure 3B). Baseline characteristics, such as TMTV (supplemental Figure 4A) and POD24 status (supplemental Figure 4B), did not correlate with dose response.
Discussion
Findings from this longer-term update of the ELARA trial continue to demonstrate high response rates and durable remissions with a favorable safety profile in patients with heavily pretreated r/r FL treated with tisagenlecleucel. Median DOR, PFS, OS, and TTNT were not reached after >2 years of follow-up. Durable antitumor efficacy was observed in most patients, including those with high-risk clinical characteristics (POD24, high metabolic tumor volume, bulky disease, double refractory disease, and high FLIPI score). Long-term efficacy in these patients supports use of tisagenlecleucel in a broad population of patients with r/r FL.
These updated data are bolstered by recent findings from retrospective analyses (before mosunetuzumab) comparing ELARA with real-world evidence, demonstrating improved efficacy of tisagenlecleucel over other SOC options.11,12 Patients from ELARA had an estimated 1.4-fold higher PFS rate at 12 months and an 80% reduction in risk of death compared with SOC.11 Similarly, comparison of ELARA vs the US Flatiron Health Research Database indicated improved efficacy across CRR (69% vs 18%), 12-month PFS (73% vs 42%), OS (97% vs 85%), and TTNT (86% vs 52%) for tisagenlecleucel compared with SOC.12 These benefits over SOC were observed regardless of number of previous therapies, requirement for bridging therapy, or POD24 status. Although the median time from study enrollment to infusion was 48 days, median manufacturing time was only 24 days. Numerous factors may influence the time from leukapheresis to infusion during a clinical trial (logistical, disease control, and infection) and, given the indolent nature of FL, some physicians took a slightly less aggressive approach to treatment timing. Because turnaround time was not optimized as a part of the ELARA trial, commercial production is likely to have a faster turnaround time for patients.
Exploratory biomarker analyses were implemented to provide a better understanding of long-term efficacy outcomes in patients with high-risk disease characteristics. TME composition in pretreatment biopsies collected from patients with FL was assessed by measuring the expression of several exhaustion markers in T cells, monocytes, and MDSCs (data not shown). We found that low expression of LAG3+ on T cells (<3%) was associated with favorable PFS and DOR. Similarly, increased numbers of LAG3+ T cells have been shown to correlate with poor outcomes in patients with FL, whereby LAG3 expression on intratumoral T cells identifies a functionally exhausted subset of T cells with impaired cytokine (IL-2 and interferon-γ) and granule (perforin and granzyme B) production.13 Inhibition of LAG-3 has shown the capability to restore cytotoxic activity in exhausted T cells and reduce regulating T cells’ immune suppression function, thereby enhancing the killing effect on tumors.14 Although the anti-LAG-3 antibody relatlimab has been approved in combination with nivolumab for patients with advanced melanoma,15 clinical trials are ongoing in patients with classical Hodgkin lymphoma16,17 and other indications,18 and prospective studies would be needed to evaluate the benefit of anti-LAG3 therapies in patients with FL.
We also found that higher levels of TNF-α and IL-10 (at baseline) were associated with lower PFS and DOR. Similar to these findings, high levels of TNF-α correlated with a higher tumor burden, lower CRR, and shorter PFS and OS in patients with FL19 and diffuse large B-cell lymphoma.20 TNF-α is thought to influence lymphomagenesis through upregulation of inflammatory and antiapoptotic signals, possibly via the nuclear factor kappa B pathway.21 TNF-α may also cooperate with other cytokines, including IL-10, to increase cell proliferation.22 Macrophages may be the main source of TNF-α, especially when they are exposed to IL-4 and IL-10, both inducing an M2 phenotype characterized by their ability to suppress T-cell adaptive immunity.23 Altogether, these data suggest that a preexisting immunosuppressive environment before infusion may hinder effective antitumor function of CAR T cells.
The prognostic value of high baseline tumor volume in patients with FL and its association with shorter PFS has previously been validated.24,25 Although lower tumor burden before tisagenlecleucel infusion may be associated with improved outcomes, the causality is not clear. There are various scenarios in which tumor burden cannot be reduced before infusion, which should not preclude patients with high tumor burden from considering CAR T-cell therapy. Our exploratory analysis revealed that, despite high baseline tumor volume, patients with high CAR T-cell expansion (Cmax) had PFS that was comparable with that of patients with low baseline tumor volume and low Cmax, suggesting that higher CAR T-cell expansion may be able to compensate for high baseline tumor volume. Interestingly, baseline circulating naïve CD8+ cells showed a positive association with Cmax, and the percentage of naïve CD8+ T cells was higher in patients who achieved a CR and had ongoing response ≥1 year. Our analysis has shown that the presence of a higher percentage of circulating naïve CD8+ T cells at baseline was associated with improved efficacy outcomes. Responses were also observed in patients with POD24 despite lower tisagenlecleucel expansion than in patients without POD24. No strong evidence suggesting a relationship between dose and overall response was observed. Cellular kinetic analyses showed CAR transgene persistence for up to 925 days in ELARA; however, the relationship between prolonged persistence and long-term clinical efficacy remains unclear, and longer follow-up is needed.
Updated findings from the phase 2 ZUMA-5 trial of axicabtagene ciloleucel in patients with r/r indolent non-Hodgkin lymphoma (median follow-up of 40.5 months) demonstrated efficacy comparable with that observed in ELARA,26 despite fewer patients who were heavily pretreated enrolled in ZUMA-5 than in ELARA (3 vs 4 median lines of prior therapy).6,27 These findings are consistent with the homogeneous treatment effect seen in ELARA regardless of the number of prior lines of therapy, and demonstrates that patient outcomes were still superior to the current non–CAR T-cell therapy SOC.11,28-34 Although difficulties with cross-trial comparisons and variation in follow-up duration between the studies limit definitive conclusions, the safety profile of tisagenlecleucel continues to compare favorably with that of axicabtagene ciloleucel.26 Notably, both ZUMA-5 and the TRANSCEND-FL phase 2 trial allowed the use of bridging therapy at the discretion of the physician. In total, 4% of patients received bridging in ZUMA-5, and 38% to 41% received bridging in TRANSCEND-FL (compared with 45% in ELARA).6,27,35 The lower frequency of bridging therapy reported in ZUMA-5 may have been a result of the fast product delivery, or could reflect the higher clinical risk profile of the ELARA population. However, this hypothesis remains speculative.
The comparable efficacy and lower rates of severe cytokine release syndrome and neurological events reported in ELARA vs ZUMA-5 reported in a matching-adjusted indirect comparison36 support the feasibility of tisagenlecleucel outpatient administration. Of the 30 clinical trial sites, only 4 in the United States and 1 in Australia allowed outpatient infusions. In total, 18% of patients in ELARA received infusion in the outpatient setting, 12 of 17 patients who received infusion in the outpatient setting were in the United States. Patients who received infusion in the outpatient setting generally had favorable Eastern Cooperative Oncology Group performance status scores, favorable FLIPI scores, and less bulky disease at baseline. Findings from a recent report of the ELARA trial after a median follow-up of 20 months showed that, compared with inpatients, outpatients had higher proportions of patients with grade 3A FL, primary refractory disease, and >5 lines of prior therapy; 41% of patients treated in the outpatient setting did not require hospitalization within 30 days; and no patients required intensive care unit admission.37
Our biomarker findings provide evidence for prognostic markers of PFS; however, the criteria used to select patients who are likely to benefit from tisagenlecleucel encompasses a broad range of factors that cannot be fully addressed with these data. Nonetheless, these findings suggest improved tisagenlecleucel efficacy with a favorable TME (lower LAG3+ exhausted T cells) and decreased inflammatory status (lower TNF-α and IL-10). Additional correlative analyses are needed to understand how these findings could inform clinical decision making.
As more treatment options become available for patients with r/r FL, the question of how to individualize the sequencing of therapy to achieve the best possible outcomes remains, especially in patients who relapse after CAR T-cell therapy. Long-term follow-up and real-world data are needed to help to identify the optimal sequencing for these patients and to understand the influence previous treatment regimens and patient-related criteria may have on available treatment options. Overall, extended follow-up of >2 years from ELARA demonstrates that tisagenlecleucel continues to provide substantial clinical benefit with a remarkable safety profile in adult patients with r/r FL (including patients with high-risk characteristics for whom effective therapeutic options are currently unavailable), suggesting a potential new SOC.
Acknowledgments
Fluorescence immunohistochemistry images were provided by Navigate BioPharma Services, a subsidiary of Novartis Pharmaceuticals Corporation. Medical writing support was provided by Nitya Venkataraman of Healthcare Consultancy Group, and was funded by Novartis Pharmaceuticals Corporation.
The study was sponsored and designed by Novartis Pharmaceuticals Corporation and was approved by the institutional review board at each participating institution. Data were analyzed and interpreted by the sponsor and authors.
Authorship
Contribution: N.H.F., M. Dreyling, M. Dickinson, S.J.S., and C.T. were responsible for study conception and design; M. Dreyling, M. Dickinson, J.M.-L., A.K., J.B., M.G., L.P., J.C.C., E.B., K.K., H.H., M.J.K., C.A., P.A.R., P.J.H., J.A.P.-S., A.I.C., L.J.N., B.v.T., A.J.M.F., T.T., P.E.M.P., J.P.M., A.L.P., F.O., A.V., P.L.Z., R.M., C.T., N.H.F., and S.J.S. recruited patients and provided study materials; M. Dreyling, M. Dickinson, J.M.-L., A.K., J.B., M.G., L.P., J.C.C., E.B., K.K., H.H., M.J.K., C.A., P.A.R., P.J.H., J.A.P.-S., A.I.C., L.J.N., B.v.T., A.J.M.F., T.T., P.E.M.P., J.P.M., A.L.P., F.O., A.V., P.L.Z., R.M., C.T., N.H.F., and S.J.S. collected and assembled data; all authors contributed to data analysis and had access to primary clinical trial data; all authors contributed to writing the manuscript, and reviewed the manuscript and approved of the final version before submission; and all authors vouch for the data, analyses, and adherence of the study to the protocol.
Conflicts-of-interest disclosure: M. Dreyling reports consulting for, or advisory role with, AbbVie, AstraZeneca, Bayer, BeiGene, Bristol Myers Squibb/Celgene, Genmab, Gilead Kite, Janssen, Lilly, Novartis, and Roche; is a member of the speakers’ bureau of AstraZeneca, Gilead Kite, Janssen, Lilly, Novartis, and Roche; and reports research funding from AbbVie, Bayer, Bristol Myers Squibb/Celgene, Gilead Kite, Janssen, and Roche. N.H.F. reports grants from Novartis and TG Therapeutics; and reports consulting for, or advisory role with, Bristol Myers Squibb, Gilead, and Roche. M. Dickinson received honoraria from Amgen, Bristol Myers Squibb, Gilead Sciences, Janssen, Novartis, Merck Sharp & Dohme, and Roche; reports consulting for, or advisory role with, Bristol Myers Squibb, Gilead Sciences, Janssen, Merck Sharp & Dohme, Novartis, and Roche; is a member of the speakers’ bureau of Gilead Sciences, Novartis, Merck Sharp & Dohme, and Roche; received research funding from Celgene, Novartis, Merck Sharp & Dohme, Roche, and Takeda; and received funds for travel, accommodations, and expenses from Roche. J.M.-L. reports consulting for, or advisory role with, Astellas, Bristol Myers Squibb, Incyte, Janssen, Novartis, and Roche; is a member of the speakers’ bureau of Astellas, Bristol Myers Squibb, Incyte, Janssen, Novartis, and Roche; and received research funding from Astellas, Bristol Myers Squibb, Incyte, and Roche. A.K. reports consulting for, or advisory role with, Nordic Nanovector; and received research funding from Nordic Nanovector. J.B. received honoraria from Janssen and Novartis; reports consulting for, or advisory role with, Gilead Sciences, Janssen, and Novartis; and is a member of the speakers’ bureau of Gilead Sciences, Novartis, Janssen, Roche, and Takeda. M.G. received research funding from Bristol Myers Squibb, Kite, Takeda, Fate, Atara, Incyte, and Miltenyi. L.P. received honoraria from Pfizer and Roche; and received funds for travel, accommodations, and expenses from Novartis. J.C.C. reports consulting for, or advisory role with, Bayer, Bristol Myers Squibb, Karyopharm Therapeutics, Kite/Gilead, MorphoSys, Novartis, Pfizer, Teneobio, Adicet Bio, ADC Therapeutics, and BeiGene; is a member of the speakers’ bureau of BeiGene; and received research funding from Merck, AstraZeneca, and Adaptive. E.B. reports grants from Amgen and Takeda; reports personal fees from Celgene, Gilead, Janssen, Novartis, and Roche; reports nonfinancial support from AbbVie, BeiGene, Celgene, and Roche; and reports consulting for, or advisory role with, Incyte and Roche. K.K. received honoraria from Bristol Myers Squibb, Celgene, Dainippon-Sumitomo, Janssen, Kyowa Kirin, Merck Sharp & Dohme, Mundi, and Ono; reports consulting for, or advisory role with, AbbVie, AstraZeneca, Celgene, Chugai Pharmaceutical, Daiichi Sankyo, Eisai, Janssen, and Novartis; and received research funding from AbbVie, Bristol Myers Squibb, Celgene, Chugai, Daiichi Sankyo, Eisai, Janssen, Kyowa Kirin, Novartis, and Ono. H.H. received honoraria from Bristol Myers Squibb, Chugai, Novartis, and Sanofi; and received research funding from Asahi Kasei Pharma, Chugai, Eisai, Kyowa Kirin, and Takeda. M.J.K. received honoraria from Bristol Myers Squibb/Celgene, Kite/Gilead, Novartis, and Roche; reports consulting for, or advisory role with, Bristol Myers Squibb/Celgene, Kite/Gilead, Miltenyi Biotech, Novartis, Takeda, Adicet Bio, and Miltenyi Biomedicine; and received research funding from Kite/Gilead. C.A. reports consulting for, or advisory role with, Atara Biotherapeutics, Epizyme, Gilead Sciences, Incyte, Karyopharm Therapeutics, Kite Pharma, and TG Therapeutics; received research funding from Amgen, Celgene, GlaxoSmithKline, Juno Therapeutics, Merck, and Novartis; and received funds for travel, accommodations, and expenses from Gilead Sciences and Kite Pharma. P.A.R. received honoraria from Novartis; reports consulting for, or advisory role with, AbbVie, Genmab, ADC Therapeutics, Pharmacyclics, Novartis, Bristol Myers Squibb, Kite/Gilead, Nurix Therapeutics, Nektar Therapeutics, Takeda, Intellia Therapeutics, Sana Biotechnology, BeiGene, Janssen, and CVS Caremark; is a member of the speakers’ bureau of Kite/Gilead; and received research funding from Bristol Myers Squibb, Kite/Gilead, Novartis, MorphoSys, CRISPR Therapeutics, Calibr, Xencor, Fate Therapeutics, and Tessa Therapeutics. J.A.P.-S. received honoraria from Alexion, Kite/Gilead, Novartis, Janssen, and Jazz; and is a member of the speakers’ bureau of, and/or received research funding from, Alexion, Kite/Gilead, Novartis, Bristol Myers Squibb, Amgen, Jazz, Takeda, Pfizer, and AbbVie. A.I.C. received honoraria from MorphoSys and Mesoblast. L.J.N. received honoraria from ADC Therapeutics, Bayer, Bristol Myers Squibb/Celgene, Denovo Pharma, Epizyme, Genentech, Gilead/Kite, Janssen, MorphoSys, Novartis, Pfizer, Takeda, and TG Therapeutics; and received research funding from Bristol Myers Squibb/Celgene, Caribou Biosciences, Epizyme, Genentech, Gilead/Kite, IGM Biosciences, Janssen, Novartis, Pfizer, Takeda, and TG Therapeutics. B.v.T. received honoraria from AstraZeneca, Bristol Myers Squibb, Incyte, Novartis, Roche Pharma AG, Takeda, and Merck Sharp & Dohme; reports consulting for, or advisory role with, Allogene, Bristol Myers Squibb/Celgene, Cerus, Incyte, IQVIA, Gilead Kite, Miltenyi, Novartis, Noscendo, PentixaPharm, Roche, Amgen, Pfizer, Takeda, Merck Sharp & Dohme, and Gilead Kite; received research funding from Merck Sharp & Dohme, Novartis, and Takeda; and received funds for travel, accommodations, and expenses from AbbVie, AstraZeneca, Gilead Kite, Merck Sharp & Dohme, Roche, Takeda, and Novartis. A.J.M.F. reports consulting for, or advisory role with, Gilead, Incyte, Novartis, PentixaPharm, and Roche; is a member of the speakers’ bureau of Adienne; received research funding from ADC Therapeutics, Amgen, BeiGene, Bristol Myers Squibb, Genmab, Gilead, Hutchison Medipharma, Novartis, Pharmacyclics, PentixaPharm, Pfizer, and Roche; and reports patents with Ospedale San Raffaele SRL. T.T. received honoraria from Bristol Myers Squibb, Kyowa Kirin, Merck Sharp & Dohme, Pfizer, and Takeda; reports consulting for, or advisory role with, Merck Sharp & Dohme, Novartis, and Takeda; received research funding from Astellas, Chugai Pharmaceutical, Fuji Pharma, Kyowa Kirin, Nippon Shinyaku, Novartis, Sanofi, and Teijin Pharma; and reports other manuscript support from Janssen and Novartis. P.E.M.P. received honoraria from AbbVie, AstraZeneca, BeiGene, Gilead Sciences, Janssen, and Novartis; and received research funding from Gilead Sciences and Roche; and reports consulting for, or advisory role with, AbbVie, BeiGene, and Novartis. J.P.M. is employed by the University of Kansas Medical Center; received honoraria from Juno Therapeutics, Kite Pharmaceuticals, and Magenta Therapeutics; reports consulting for, or advisory role with, AlloVir, EcoR1 CAPITAL, Juno Therapeutics, Kite Pharmaceuticals, Magenta Therapeutics, and Novartis; and received research funding from Juno Therapeutics and Kite Pharmaceuticals. A.L.P. received honoraria from Amgen, Celgene, Janssen, Kite/Gilead, and Novartis; reports consulting for, or advisory role with, Amgen, Celgene, Kite/Gilead, and Novartis; and is a member of the speakers’ bureau of Janssen. A.V. received honoraria from Amgen, Bristol Myers Squibb, Kite/Gilead, Novartis, and Roche; and reports consulting for, or advisory role with, Amgen, Bristol Myers Squibb, Kite/Gilead, Novartis, Roche, and AbbVie. P.L.Z. reports consulting for, or advisory role with, ADC Therapeutics, BeiGene, Bristol Myers Squibb, Celltrion, EUSA Pharma, Gilead, Incyte, Janssen-Cilag, Kyowa Kirin, Merck Sharp & Dohme, Novartis, Roche, Sandoz, Servier, TG Therapeutics, Takeda, and Verastem; and is a member of the speakers’ bureau of BeiGene, Bristol Myers Squibb, Celltrion, EUSA Pharma, Gilead, Incyte, Janssen-Cilag, Kyowa Kirin, Merck Sharp & Dohme, Novartis, Roche, Servier, TG Therapeutics, Takeda, and Verastem. R.M. received honoraria from Novartis; and reports consulting for, or advisory role with, Novartis. I.P., A.Z., R.A., X.H., D.G., and D.O. are employed by Novartis. R.R., H.J.M., and A.M. are employed by Novartis; and reports stock and other ownership interests in Novartis. C.T. received honoraria from ADC Therapeutics, BeiGene, Gilead, Novartis, Roche, and Takeda; reports consulting for, or advisory role with, BeiGene, Gilead, Incyte, Novartis, and Roche; and received funds for travel, accommodations, and expenses from Novartis. S.J.S. received honoraria from Incyte, Novartis, and Takeda; reports consulting for, or advisory role with, AstraZeneca, BeiGene, Celgene/Bristol Myers Squibb/Juno Therapeutics, Fate Therapeutics, Genentech/Roche, Genmab, Incyte, Janssen, Legend Biotech, MorphoSys, Mustang Biotech, Nordic Nanovector, Novartis, Regeneron, and Vittoria; reports grants from AbbVie, Acerta, AstraZeneca, Celgene/Bristol Myers Squibb/Juno Therapeutics, Genentech/Roche, Merck, Novartis, and TG Therapeutics; and reports patents with Novartis. P.J.H. and F.O. report no competing financial interests.
Correspondence: Martin Dreyling, Medizinische Klinik III, Klinikum der Universität, LMU München, Marchioninistrasse 15, 81377 Munich, Germany; email: martin.dreyling@med.uni-muenchen.de.
References
Author notes
Long-term clinical outcomes were presented at the 64th American Society of Hematology Annual Meeting & Exposition, 10-13 December 2022, New Orleans, LA (abstract 608).
Novartis is committed to sharing with qualified external researchers access to patient-level data and supporting clinical documents from eligible studies. These requests are reviewed and approved by an independent review panel on the basis of scientific merit. All data provided is anonymized to respect the privacy of patients who have participated in the trial in line with applicable laws and regulations. The data availability of these trials is according to the criteria and process described on www.clinicalstudydatarequest.com.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal