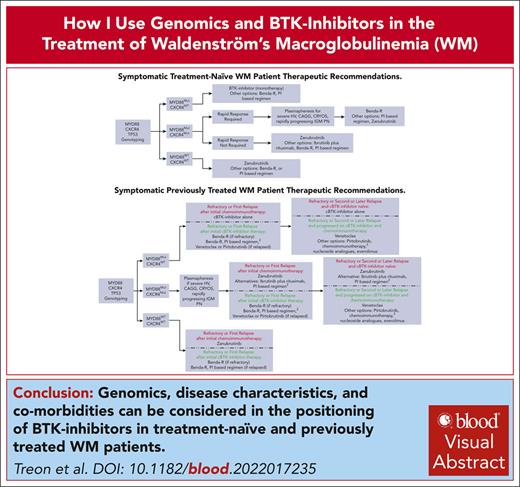
Visual Abstract
Mutations in MYD88 (95%-97%) and CXCR4 (30%-40%) are common in Waldenström macroglobulinemia (WM). TP53 is altered in 20% to 30% of patients with WM, particularly those previously treated. Mutated MYD88 activates hematopoietic cell kinase that drives Bruton tyrosine kinase (BTK) prosurvival signaling. Both nonsense and frameshift CXCR4 mutations occur in WM. Nonsense variants show greater resistance to BTK inhibitors. Covalent BTK inhibitors (cBTKi) produce major responses in 70% to 80% of patients with WM. MYD88 and CXCR4 mutation status can affect time to major response, depth of response, and/or progression-free survival (PFS) in patients with WM treated with cBTKi. The cBTKi zanubrutinib shows greater response activity and/or improved PFS in patients with WM with wild-type MYD88, mutated CXCR4, or altered TP53. Risks for adverse events, including atrial fibrillation, bleeding diathesis, and neutropenia can differ based on which BTKi is used in WM. Intolerance is also common with cBTKi, and dose reduction or switchover to another cBTKi can be considered. For patients with acquired resistance to cBTKis, newer options include pirtobrutinib or venetoclax. Combinations of BTKis with chemoimmunotherapy, CXCR4, and BCL2 antagonists are discussed. Algorithms for positioning BTKis in treatment naïve or previously treated patients with WM, based on genomics, disease characteristics, and comorbidities, are presented.
Introduction
Genomics of WM and BTK inhibitors
MYD88 mutations
Mutated MYD88 (MYD88Mut) is found in 95% to 97% of patients with Waldenström macroglobulinemia (WM).1-6 Nearly all cases carry the MYD88L265P variant, although other MYD88Mut variants occur.7,8 Next-generation sequencing (NGS) may miss MYD88L265P in 35% of patients with WM, particularly in patients with a bone marrow (BM) disease burden of <10%.9 Allele-specific polymerase chain reaction (AS-PCR) is therefore recommended for MYD88L265P detection.10 The signaling cascades for MYD88Mut are shown in Figure 1.11-17 Five percent of patients with WM have wild-type MYD88 (MYD88WT), many of whom carry NF-κB–activating mutations distal to Bruton tyrosine kinase (BTK) signaling.18 Patients with MYD88WT have a higher risk for disease transformation, and/or show shorter overall survival (OS).19,20 MYD88Mut status can differentially affect outcomes with BTK inhibitors (BTKis; discussed hereafter).
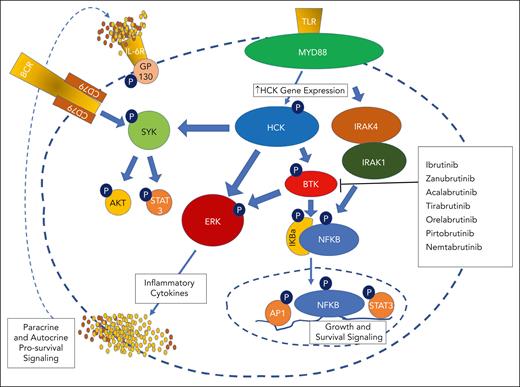
Prosurvival signaling driven by mutated MYD88 signaling. Mutated MYD88 triggers gene expression of hematopoietic cell kinase (HCK), a SRC family member through PAX5-mediated signaling. HCK is activated through interleukin-6 (IL-6)/IL-6 receptor (IL-6R)/gp130/JAK2/STAT3–mediated signaling triggered by autocrine and paracrine release from the surrounding microenvironment. HCK triggers BTK, spleen tyrosine kinase (SYK), and ERK1/2 signaling. BTK also triggers ERK1/2 as well as NF-κB p65–mediated prosurvival signaling. IRAK4/IRAK1 are also activated by mutated MYD88 through an HCK-independent pathway, and trigger along with BTK NF-κB p65–mediated prosurvival signaling.
Prosurvival signaling driven by mutated MYD88 signaling. Mutated MYD88 triggers gene expression of hematopoietic cell kinase (HCK), a SRC family member through PAX5-mediated signaling. HCK is activated through interleukin-6 (IL-6)/IL-6 receptor (IL-6R)/gp130/JAK2/STAT3–mediated signaling triggered by autocrine and paracrine release from the surrounding microenvironment. HCK triggers BTK, spleen tyrosine kinase (SYK), and ERK1/2 signaling. BTK also triggers ERK1/2 as well as NF-κB p65–mediated prosurvival signaling. IRAK4/IRAK1 are also activated by mutated MYD88 through an HCK-independent pathway, and trigger along with BTK NF-κB p65–mediated prosurvival signaling.
We therefore recommend determination of MYD88Mut status as part of the diagnostic workup by AS-PCR for the MYD88L265P variant, and, if negative, consider NGS to identify any non-MYD88L265P variants.
CXCR4 mutations
CXCR4Mut are found in 30% to 40% of patients with WM.2,19 In WM, >40 CXCR4 nonsense and frameshift variants in the C-terminal domain have been identified.2,5,21-24 Nonsense (CXCR4Mut/NS) variants, the most common being CXCR4S338X, cause truncation of the C-terminal regulatory domain.2,21,23 Frameshift (CXCR4Mut/FS) variants result from insertions or deletions. CXCR4Mut clones are usually subclonal.2,5,21 Despite heterogeneous clonality, the transcriptional profile for patients with WM with CXCR4Mut shows great uniformity.25 In response to CXCL12, CXCR4Mut triggers BTK, protein kinase B (AKT), and extracellular signal-regulated kinase (ERK) signaling, which promotes BM chemotaxis and/or ibrutinib resistance.26-28 CXCR4Mut/NS expressing WM cells exhibit more robust AKT and ERK signaling vs those expressing CXCR4WT or CXCR4Mut/FS.28 CXCR4 antagonists can sensitize CXCR4Mut-expressing WM cells to ibrutinib.26-28
Patients with CXCR4Mut/NS WM have higher BM disease burden; higher serum immunoglobulin M (sIgM) levels and incidence of symptomatic hyperviscosity; and/or shorter time to initial treatment vs those with CXCR4WT or CXCR4Mut/FS mutation status.5,22-24,29 One study also showed shorter OS in patients with CXCR4Mut/NS vs those with CXCR4WT or CXCR4Mut/FS.29 CXCR4Mut/NS may also more adversely affect treatment outcomes with BTKis vs CXCR4WT or CXCR4Mut/FS (discussed below).30 NGS may miss two-thirds of patients with CXCR4Mut, particularly those with lower BM disease burden and clonality.31 Newer NGS platforms may improve detection.29 Given the significance of CXCR4Mut/NS, AS-PCR may be used to evaluate this variant.10
We therefore recommend CXCR4Mut status as part of the diagnostic workup by NGS, and, if negative, consider AS-PCR for CXCR4S338X variants.
TP53 alterations
Alterations in TP53 (TP53Alt) occur in 5% to 10% patients with treatment-naïve WM that include TP53Mut and 17p deletions.5,32,33 The incidence of TP53Alt appears higher (25%-30%) patients with WM who had been previously treated, most of whom received alkylators (85%) and/or nucleoside analogues (22%).34,35 Patients with TP53Alt WM show shorter OS and/or progression-free survival (PFS) with BTKis.32-34
We recommend evaluating for TP53Alt at diagnosis and at relapse using NGS because their presence may guide treatment considerations (discussed in further sections). Because studies, to date, have been qualitative, no cutoffs for TP53Alt have been established.
BTK mutations
BTKCys481 is the binding site for covalent BTKis (cBTKis) including ibrutinib, zanubrutinib, acalabrutinib, and tirabrutinib. BTKCys481 variants are the most common mutations in patients with WM with acquired ibrutinib resistance.36 Importantly, multiple clones bearing different BTKCys481 mutations can occur within individual patients with WM progressing on ibrutinib.36,37 Like chronic lymphocytic leukemia, BTKCys481 mutations in patients with WM progressing on BTKis are typically subclonal.36,38 WM cells expressing the BTKCys481Ser mutation show ibrutinib resistance and reactivation of BTK-PLCγ2-ERK1/2 signaling.17 Use of ERK1/2 inhibitors triggers apoptosis in BTKCys481Ser-expressing cells, and resensitization to ibrutinib.17 Moreover, ERK1/2 reactivation is accompanied by interleukin-6 and interleukin-10 release, which protects cocultured wild-type BTKCys481 WM cells from ibrutinib, demonstrating a paracrine means for propagating cBTKi resistance.17 Deletions in chromosomes 6q or 8p, which encompass regulators of BTK, MYD88/NF-κB, and apoptotic signaling also occur in patients with WM progressing on ibrutinib.37
We continue to hold off making recommendations for routine BTKCys481 variant testing in patients with WM progressing on cBTKis because salvage treatment approaches (discussed below) did not evaluate outcomes based on BTKCys481 mutation status.
Clinical experience with BTKis in WM
Table 1 summarizes studies with BTKis in WM with regulatory approvals in any indication and jurisdiction. Clinicians should consult local guidelines on approval status and/or use of the discussed BTKis. Table 2 summarizes data for CXCR4Mut status on ibrutinib and zanubrutinib outcomes.
Summary of approved BTKis and their activity in WM
| Study . | Patient population . | Agent(s) . | n . | Time to minor/major response . | ORR (%)/MRR (%) . | ≥VGPR rate (%) . | PFS (%) . |
|---|---|---|---|---|---|---|---|
| Pivotal study39 | R/R | Ibrutinib | 63 | 0.9 mo/2.0 mo | 91/79 | 30 | 54 (60 mo) |
| INNOVATE arm C40 | R/R | Ibrutinib | 31 | 1 mo/2 mo | 87/77 | 29 | 40 (60 mo) |
| Phase 241 | TN | Ibrutinib | 30 | 0.9 mo/1.9 mo | 100/87 | 30 | 76 (48 mo) |
| INNOVATE arms A, B42 | TN, R/R | Ibrutinib Rituximab | 150 | 1 mo/3 mo | 92/76 | 31 | 68 (54 mo) |
| Phase 243 | TN, R/R | Zanubrutinib | 77 | N/A/2.8 mo | 96/82 | 45 | 76 (36 mo) |
| ASPEN cohort 1 (MYD88Mut)44 | TN, R/R | Ibrutinib | 99 | 1 mo/2.9 mo | 94/80 | 25 | 85 (42 mo) |
| TN, R/R | Zanubrutinib | 102 | 1 mo/2.8 mo | 95/81 | 36 | 88 (42 mo) | |
| ASPEN cohort 2 (MYD88WT)44 | TN, R/R | Zanubrutinib | 28 | 1 mo/3.0 mo | 78/63 | 27 | 84 (42 mo) |
| Phase 245 | TN, R/R | Acalabrutinib | 106 | 1 mo/N/A | 94/81 | 39 | 84 TN/52 R/R (66 mo) |
| Phase 246 | TN, R/R | Tirabrutinib | 27 | N/A 1.9 (TN) 2.1 (R/R) | 96/93 | 33 | 93 (24 mo) |
| Phase 247,48 | R/R | Pirtobrutinib | 80 | N/A /N/A | 81 and 67 (prior cBTKi) 88 and 88 (cBTKi naïve) | 24 (prior cBTKi) 29 (cBTKi naïve) | 57 (18 mo for prior cBTKi) N/A for cBTKi naïve |
| Study . | Patient population . | Agent(s) . | n . | Time to minor/major response . | ORR (%)/MRR (%) . | ≥VGPR rate (%) . | PFS (%) . |
|---|---|---|---|---|---|---|---|
| Pivotal study39 | R/R | Ibrutinib | 63 | 0.9 mo/2.0 mo | 91/79 | 30 | 54 (60 mo) |
| INNOVATE arm C40 | R/R | Ibrutinib | 31 | 1 mo/2 mo | 87/77 | 29 | 40 (60 mo) |
| Phase 241 | TN | Ibrutinib | 30 | 0.9 mo/1.9 mo | 100/87 | 30 | 76 (48 mo) |
| INNOVATE arms A, B42 | TN, R/R | Ibrutinib Rituximab | 150 | 1 mo/3 mo | 92/76 | 31 | 68 (54 mo) |
| Phase 243 | TN, R/R | Zanubrutinib | 77 | N/A/2.8 mo | 96/82 | 45 | 76 (36 mo) |
| ASPEN cohort 1 (MYD88Mut)44 | TN, R/R | Ibrutinib | 99 | 1 mo/2.9 mo | 94/80 | 25 | 85 (42 mo) |
| TN, R/R | Zanubrutinib | 102 | 1 mo/2.8 mo | 95/81 | 36 | 88 (42 mo) | |
| ASPEN cohort 2 (MYD88WT)44 | TN, R/R | Zanubrutinib | 28 | 1 mo/3.0 mo | 78/63 | 27 | 84 (42 mo) |
| Phase 245 | TN, R/R | Acalabrutinib | 106 | 1 mo/N/A | 94/81 | 39 | 84 TN/52 R/R (66 mo) |
| Phase 246 | TN, R/R | Tirabrutinib | 27 | N/A 1.9 (TN) 2.1 (R/R) | 96/93 | 33 | 93 (24 mo) |
| Phase 247,48 | R/R | Pirtobrutinib | 80 | N/A /N/A | 81 and 67 (prior cBTKi) 88 and 88 (cBTKi naïve) | 24 (prior cBTKi) 29 (cBTKi naïve) | 57 (18 mo for prior cBTKi) N/A for cBTKi naïve |
Listed BTKis are approved for WM and/or other indications in any jurisdiction. Clinicians should consult local regulatory approvals and guidelines for their status and use in WM.
N/A, data not available; R/R, relapsed or refractory; TN, treatment-naïve.
Impact of mutated CXCR4 on BTKi activity for ibrutinib and zanubrutinib in WM
| Study . | Patient population . | Agent(s) . | Time to major response (CXCRMut vs CXCR4WT) . | MRR (%) (CXCRMut vs CXCR4WT) . | ≥VGPR rate (%) (CXCRMut vs CXCR4WT) . | PFS (%) (CXCRMut vs CXCR4WT) . |
|---|---|---|---|---|---|---|
| Pivotal study39 | R/R | Ibrutinib | 4.7 vs 1.8 mo | 68 vs 97 | 9 vs 47 | 38 vs 70 (60 mo) |
| INNOVATE arm C40 | R/R | Ibrutinib | 3.6 vs 1.0 mo | 71 vs 88 | 14 vs 41 | 18 mo vs NR (60 mo) |
| Phase 241 | TN | Ibrutinib | 7.3 vs 1.8 mo | 78 vs 94 | 14 vs 44 | 59 vs 92 (48 mo) |
| INNOVATE arms A, B42 | TN, R/R | Ibrutinib Rituximab | 3 vs 2 mo | 77 vs 81 | 23 vs 41 | 63 vs 72 (54 mo) |
| Phase 249 | R/R | Zanubrutinib | N/A | 91 vs 87 | 27 vs 59 | ∼90 vs ∼78 (42 mo) |
| ASPEN cohort 144 | TN, R/R | Ibrutinib | 6.6 vs 2.8 mo | 65 vs 85 | 10 vs 31 | 49 vs 75 (42 mo) |
| TN, R/R | Zanubrutinib | 3.4 vs 2.8 mo | 79 vs 83 | 21 vs 45 | 73 vs 81 (42 mo) |
| Study . | Patient population . | Agent(s) . | Time to major response (CXCRMut vs CXCR4WT) . | MRR (%) (CXCRMut vs CXCR4WT) . | ≥VGPR rate (%) (CXCRMut vs CXCR4WT) . | PFS (%) (CXCRMut vs CXCR4WT) . |
|---|---|---|---|---|---|---|
| Pivotal study39 | R/R | Ibrutinib | 4.7 vs 1.8 mo | 68 vs 97 | 9 vs 47 | 38 vs 70 (60 mo) |
| INNOVATE arm C40 | R/R | Ibrutinib | 3.6 vs 1.0 mo | 71 vs 88 | 14 vs 41 | 18 mo vs NR (60 mo) |
| Phase 241 | TN | Ibrutinib | 7.3 vs 1.8 mo | 78 vs 94 | 14 vs 44 | 59 vs 92 (48 mo) |
| INNOVATE arms A, B42 | TN, R/R | Ibrutinib Rituximab | 3 vs 2 mo | 77 vs 81 | 23 vs 41 | 63 vs 72 (54 mo) |
| Phase 249 | R/R | Zanubrutinib | N/A | 91 vs 87 | 27 vs 59 | ∼90 vs ∼78 (42 mo) |
| ASPEN cohort 144 | TN, R/R | Ibrutinib | 6.6 vs 2.8 mo | 65 vs 85 | 10 vs 31 | 49 vs 75 (42 mo) |
| TN, R/R | Zanubrutinib | 3.4 vs 2.8 mo | 79 vs 83 | 21 vs 45 | 73 vs 81 (42 mo) |
Comparisons listed are for patients with MYD88 mutation.
Abbreviations are explained in Table 1.
Ibrutinib
Monotherapy
Ibrutinib was the first BTKi approved for WM after a pivotal trial in 63 patients with relapsed/refractory WM.39 The median number of therapies was 2%, and 40% of patients were refractory. With a median follow-up of 59 months, the overall response rates (ORR) and major response rates (MRR) were 90% and 79%, respectively. Thirty-percent achieved a very good partial response (VGPR). The 5-year median PFS and OS rates were 54% and 87%, respectively. In MYD88WT patients, no major responses occurred. MYD88MutCXCR4Mut patients showed longer time to major response, and lower major and VGPR rates than patients with only MYD88Mut (Table 2). The 5-year PFS rate for patients with MYD88Mut only and those with MYD88MutCXCR4Mut were 70% and 38%, respectively. For patients with MYD88WT, the median PFS was 0.4 months. Atrial fibrillation (all grades) occurred in 8 (13%) patients, although most continued ibrutinib with medical management.
A substudy of the INNOVATE trial (discussed in later sections) evaluated ibrutinib monotherapy in 31 patients with heavily pretreated rituximab-refractory WM.40 Their median prior therapies were 4%, and 40% had ≥5 prior therapies. With a median follow-up of 58 months, the ORRs and MRRs were 87% and 77%, respectively. The 5-year PFS and OS rates were 40% and 73%, respectively. Patients with MYD88MutCXCR4Mut had fewer VGPRs, and longer time to major response than patients with only MYD88Mut (Table 2). The median PFS was 18 months for patients with MYD88MutCXCR4Mut and was not reached for patients with only MYD88Mut. A single patients with MYD88WT progressed at 6 months. No atrial fibrillation events were observed.
Ibrutinib monotherapy was also evaluated in 30 patients with treatment-naive MYD88Mut WM.41 Half had a CXCR4Mut mutational status, and nearly all had CXCR4Mut/NS. With a median follow-up of 50 months, the ORRs, MRRs, and VGPR rates were 100%, 87%, and 30%, respectively. The 4-year PFS rate was 76% for all patients. MRRs and VGPR rates were lower, and time to major response longer in patients with CXCR4Mut (Table 2). The 4-year PFS was lower (59% vs 92%) in patients with CXCR4Mut. Atrial fibrillation (all grades) occurred in 20% of patients.
The importance of CXCR4Mut subtype on ibrutinib outcomes was also evaluated in 180 patients with symptomatic WM receiving ibrutinib. CXCR4Mut/NS was associated with lower MRRs and shorter PFS vs those with CXCR4Mut/FS or CXCR4WT.30
Ibrutinib in combination therapy
The phase 3 (INNOVATE) study randomized 150 patients with treatment-naïve relapsed/refractory WM to placebo/rituximab or ibrutinib/rituximab.42 With a median follow-up of 50 months, the MRR was 76% for the ibrutinib/rituximab cohort vs 31% for the placebo/rituximab cohort. Among patients treated with ibrutinib/rituximab, MRRs were similar across the genomic subtypes. Fewer VGPRs occurred in patients with MYD88MutCXCR4Mut, as well as in patients with MYD88WT on ibrutinib/rituximab vs patients with MYD88Mut/CXCR4WT. Time to MRR were 2, 3, and 7 months for patients with MYD88Mut/CXCR4WT, MYD88MutCXCR4Mut, and MYD88WT/CXCR4WT, respectively. The estimated 54-month PFS rate was 68% for the ibrutinib/rituximab cohort and 25% for the placebo/rituximab cohort. Among patients treated with ibrutinib/rituximab, the 54-month estimated PFS was 72%, 63%, and 70% for patients with MYD88Mut/CXCR4WT, MYD88MutCXCR4Mut, and MYD88WT/CXCR4WT, respectively. The high activity level for ibrutinib/rituximab in patients with MYD88WT may have reflected patients with MYD88Mut classified as having MYD88WT because NGS was used to determine MYD88Mut status. Indeed, 4-times more patients had MYD88WT in this study than findings with AS-PCR in patients with symptomatic WM.50 Atrial fibrillation (any grade) occurred in 19% of patients on ibrutinib/rituximab, and most continued protocol therapy with medical management.
Ibrutinib is active in patients with WM with symptomatic central nervous system involvement (Bing-Neel syndrome [BNS]).51,52 In a multicenter retrospective study, 85% and 60% of patients with BNS experienced symptomatic and radiological improvements, respectively, within 3 months of ibrutinib initiation.52 The 2-year event-free survival rate for patients with BNS on ibrutinib was 80%. Ibrutinib also showed activity in patients with WM with IgM-related morbidities.39,41
CXCR4 antagonists have been investigated with ibrutinib. In a phase 1 trial, ulocuplumab was combined with ibrutinib to treat patients with symptomatic MYD88MutCXCR4Mut WM.53 All achieved a major response, and 33% a VGPR. Major responses occurred at a median of 1.2 months, and the 24-month PFS rate was 90%. Reversible thrombocytopenia, and rash and skin infections were the most common adverse events. Unfortunately, ulocuplumab was discontinued by the manufacturer. A study of the oral CXCR4 antagonist mavorixafor with ibrutinib is currently underway (ClinicalTrials.gov identifier: NCT04274738).
Ibrutinib was also investigated with venetoclax in a fixed-duration study that treated 45 patients with symptomatic, treatment-naïve WM.43 All were MYD88Mut, and 17 (38%) CXCR4Mut. The ORR and MRR were 100% and 96%, respectively. Notably, 42% of patients attained a VGPR, although patients with CXCR4Mut had a lower VGPR rate (29% vs 50%). The PFS rate at 24-months was 76% and was not affected by CXCR4Mut status. The study ended early because of ventricular arrythmias that occurred in 4 (9%) patients, including 2 grade 5 events. Therefore, the combination of ibrutinib and venetoclax should be avoided.
Zanubrutinib
Monotherapy
In a phase 1/2 study, 73 patients with treatment-naïve and relapsed/refractory WM received zanubrutinib at 160 mg twice daily or 320 mg once daily.49 The median follow-up was 24 and 36 months for patients with treatment-naïve WM and patients with relapsed/refractory WM, respectively. The ORR and MRR, and VGPR rate were 96%, 78% and 45%, respectively. The MRRs were similar among patients with MYD88Mut regardless of CXCR4Mut status. VGPR/complete response (CR) attainment was lower among patients with MYD88MutCXCR4Mut (Table 2). The ORR and MRR were similar across dosing schedules, although fewer VGPR/CRs occurred in those receiving 320 mg once vs 160 mg twice daily ((32% vs 49%, respectively).
The phase 3 (ASPEN) study of zanubrutinib vs ibrutinib was recently updated.44 In cohort 1, 201 patients with treatment-naïve and relapsed/refractory MYD88Mut WM were randomized to zanubrutinib or ibrutinib. Of these patients, 20% and 32% had CXCR4Mut in the ibrutinib and zanubrutinib arms, respectively. In cohort 2, 28 patients with MYD88WT received zanubrutinib. MYD88Mut status was determined by an AS-PCR assay. The median follow-up was 44.4 and 42.9 months for cohorts 1 and 2, respectively. In cohort 1, the best ORR (94% and 95%) and MRR (80% and 81%) were similar for ibrutinib and zanubrutinib, respectively. However, more VGPRs occurred for zanubrutinib (36%) vs ibrutinib (25%). The median PFS has not been reached for either arm in cohort 1, with estimated 42-month PFS rates of 70% and 78% for ibrutinib and zanubrutinib, respectively. OS was similar at 85% and 88% for ibrutinib and zanubrutinib, respectively.
CXCR4Mut status differentially affected the 3 arms of cohort 1. The median time to major response was longer for patients with CXCR4Mut on ibrutinib vs zanubrutinib (Table 2). More VGPRs also occurred in patients with CXCR4Mut who received zanubrutinib (Table 2). At 42 months, the PFS rates for patients with CXCR4Mut were 49% and 73% for those treated with ibrutinib and zanubrutinib, respectively.
Of particular interest was the differential impact of zanubrutinib and ibrutinib in patients with TP53Alt in cohort 1. Major responses (64% vs 86%) and VGPRs (14% vs 30%) were lower among patients treated with ibrutinib with TP53Alt vs TP53WT disease, respectively.35 Among patients treated with zanubrutinib, the MRRs were similar regardless of TP53Alt status, that is, 81% and 82% for patients with TP53Alt and TP53WT, respectively. VGPRs were also higher for patients with TP53Alt disease treated with zanubrutinib (35%) vs ibrutinib (14%).35 PFS rates were adversely affected by TP53Alt status in both arms of cohort 1, although fewer progression events occurred among patients with TP53Alt on zanubrutinib vs those on ibrutinib.35
There were notable differences for adverse events in cohort 1. A higher incidence (>10% difference) of diarrhea (35% vs 23%), muscle spasms (29% vs 12%), hypertension (24% vs 12%), atrial fibrillation (21% vs 7%), and pneumonia (18% vs 5%) occurred at any grade for patients on ibrutinib vs zanubrutinib, respectively. Conversely, more patients experienced neutropenia at any grade (29% vs 16%) on zanubrutinib vs ibrutinib, respectively. Grade 3/4 hypertension (19% vs 10%) and pneumonia (10% vs 1%) were more pronounced for patients on ibrutinib, whereas neutropenia (19% vs 9%) and anemia (12% vs 6%) were more frequent for those on zanubrutinib.
In cohort 2, zanubrutinib showed marked activity in patients with MYD88WT. The ORR and MRR were 81% and 65%, respectively; VGPR was attained in 30% of patients with MYD88WT. The 42-month event-free survival rate was 54%, which greatly contrasted to the median PFS of 4 months with ibrutinib monotherapy in the pivotal trial. Efficacy data on specific morbidities in WM for zanubrutinib are limited. Response to zanubrutinib was documented in a single case report of a patients with BNS.54
Zanubrutinib in combination therapy
The combination of zanubrutinib, ixazomib, and dexamethasone was evaluated in 20 patients with symptomatic, treatment-naïve WM and showed an ORR, MRR, and VGPR attainment in 100%, 95%, and 42%, respectively.45 Therapy was well tolerated, with grade ≥3 neutropenia or rash in 2 patients (1 each).
Acalabrutinib
Monotherapy
Acalabrutinib was evaluated in a multicenter study of 106 patients with treatment-naïve and relapsed/refractory WM. An update was recently presented, with a median follow-up of 64 months.55 Treatment duration was longer for patients with relapsed/refractory (61 months) vs treatment-naïve (42 months) disease. The ORR and MRR were 94% and 83%, respectively. A VGPR was attained in 25% of patients. The 66-month PFS rate was 84% for patients with treatment-naïve disease, and 52% patients with for relapsed/refractory disease. OS rates at 66-months were 91% and 71% for patients with treatment-naïve and relapsed/refractory disease, respectively. With longer follow-up, the incidence of any grade atrial fibrillation was 11%. Grade 3/4 toxicities for all patients also included bleeding (6%), hypertension (4%), and infections (30%). Data on the impact of MYD88Mut and CXCR4Mut status on acalabrutinib response are limited.
Acalabrutinib in combination therapy
Preliminary results were recently presented for the BRAWM study that investigated fixed duration treatment with acalabrutinib, bendamustine, and rituximab (Benda-R) in patients with symptomatic, treatment-naïve WM.56 A preplanned analysis for the first 30 enrolled patient showed an MRR of 88%, and VGPR attainment for 70%, for 17 evaluable patients with WM who completed 7 cycles. Grade 3/4 toxicities included neutropenia (27%) with febrile neutropenia (6%); 1 patient had atrial fibrillation. A dedicated study of acalabrutinib with rituximab is ongoing in patients with anti-myelin-associated glycoprotein–positive demyelinating neuropathy (ClinicalTrials.gov identifier: NCT05065554).
Tirabrutinib
Tirabrutinib is approved in Japan. In a phase 2 study, 18 patients with treatment-naïve disease and 9 patients with relapsed/refractory disease received tirabrutinib.46 With a median follow-up of 25 months, the ORR and MRR were 96% and 93%, respectively. The VGPR rate was 30%. CXCR4Mut status affected time to major response and VGPR attainment, although all patients with CXCR4Mut achieved a major response. One patient with MYD88WT achieved a major response. The 24-month PFS and OS rates were 92.6% and 100%, respectively. Rash (all grades) occurred in 12 (44%) patients. Grade 3/4 neutropenia occurred in 6 (22%) patients, and 2 (7%) experienced atrial fibrillation. Tirabrutinib activity has also been reported in multiple BNS case reports.57-59
Pirtobrutinib
Pirtobrutinib is a non-cBTKi that binds to non-BTKCys481 sites and inhibits both wild-type and Cys481-mutated BTKs. Pirtobrutinib also blocks MYD88Mut-driven ERK signaling in ibrutinib-resistant BTKCys481Ser-expressing WM cells.60,61 Twenty-six patients with WM were included in a phase 1/2 (BRUIN) study.47 An expansion of the WM cohort was recently reported with 80 evaluable patients with WM.48 Sixty-three of these patients were cBTKi exposed. The MRR (66% vs 88%) was lower in patients who were cBTKi exposed vs patients naïve to cBTKis, respectively. VGPRs occurred in 24% and 29% of patients who were cBTKi exposed and naïve to c-BTKis, respectively. The median PFS for those receiving prior cBTKi therapy was 19.4 months.
Positioning of BTKis in WM
Case 1: patients with newly diagnosed WM with symptomatic hyperviscosity
A 42-year-old man presented with blurry vision and nosebleeds. Physical examination revealed retinal hemorrhages, marked axillary and inguinal adenopathy, and splenomegaly. He was pancytopenic. Serum protein electrophoresis revealed an IgMλ monoclonal protein, and sIgM of 12 400 mg/dL. Computed tomography (CT) scans showed diffuse bulky adenopathy. BM biopsy demonstrated 80% lymphoplasmacytic (LPL) cell involvement, and molecular diagnostics showed MYD88L265P and CXCR4S338X mutations.
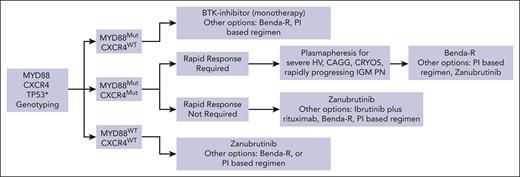
Figure 2 provides an algorithm for patients with symptomatic, treatment-naïve WM. The recommendations presented hereafter considered recent consensus panel guidance.62 For patients who are symptomatic and treatment naïve, chemoimmunotherapy with Benda-R, and dexamethasone, rituximab, and cyclophosphamide (DRC), as well as BTKis can be considered. For chemoimmunotherapy, we favor Benda-R over DRC, because the former may offer deeper responses and longer PFS.63,64 We favor using 4 cycles when possible to minimize short- and long-term adverse events, given findings from 2 retrospective studies including a dedicated study in patients with symptomatic treatment-naïve WM that showed no difference in outcomes with 4 vs 6 cycles.63,64 We do favor use of up to 6 cycles of Benda-R when treating bulky disease or symptomatic amyloidosis. As part of these recommendations, we considered a multicenter, retrospective study, wherein start dosing depended on local practice and showed a benefit in major response attainment and PFS for patients who received ≥1000 mg/m2.65 No such benefit was seen with patients with relapsed/refractory disease.
Genomic-based treatment algorithm for patients with symptomatic, treatment-naïve WM. Clinicians should consult local regulatory approvals and guidelines for BTKi status and use in WM. Algorithm represents the recommendations of the authors based on clinical trial data summarized in the text, consensus recommendations (as previously published62), and their practice experiences with patients with WM. Recommendations are intended for educational purposes. Rituximab should be held if chemoimmunotherapy is chosen until the sIgM levels are <4000 mg/dL to avoid triggering or exacerbating a hyperviscosity crisis. Benda-R can be considered for patients with bulky adenopathy or extramedullary disease. PI-based therapy or Benda-R can be considered for symptomatic amyloidosis with autologous stem cell transplantation as consolidation in select patients (as discussed elsewhere66). Rituximab alone, or with ibrutinib for MYD88Mut or Benda-R are options for patients with IgM demyelinating peripheral neuropathy depending on severity and pace of progression. Maintenance rituximab may be considered in patients aged >65 years responding to chemoimmunotherapy with rituximab (as discussed elsewhere62). Rituximab, cyclophosphamide, and dexamethasone (RCD) is an option for chemoimmunotherapy if Benda-R is not accessible (as discussed elsewhere62). ∗Zanubrutinib may also be prioritized for those with TP53 alterations (as previously reported35). Clinical trial options should always be considered. Benda, bendamustine; CAGG, cold agglutinins; CRYOS, cryoglobulinemia; HV, hyperviscosity; PN, peripheral neuropathy; R, rituximab.
Genomic-based treatment algorithm for patients with symptomatic, treatment-naïve WM. Clinicians should consult local regulatory approvals and guidelines for BTKi status and use in WM. Algorithm represents the recommendations of the authors based on clinical trial data summarized in the text, consensus recommendations (as previously published62), and their practice experiences with patients with WM. Recommendations are intended for educational purposes. Rituximab should be held if chemoimmunotherapy is chosen until the sIgM levels are <4000 mg/dL to avoid triggering or exacerbating a hyperviscosity crisis. Benda-R can be considered for patients with bulky adenopathy or extramedullary disease. PI-based therapy or Benda-R can be considered for symptomatic amyloidosis with autologous stem cell transplantation as consolidation in select patients (as discussed elsewhere66). Rituximab alone, or with ibrutinib for MYD88Mut or Benda-R are options for patients with IgM demyelinating peripheral neuropathy depending on severity and pace of progression. Maintenance rituximab may be considered in patients aged >65 years responding to chemoimmunotherapy with rituximab (as discussed elsewhere62). Rituximab, cyclophosphamide, and dexamethasone (RCD) is an option for chemoimmunotherapy if Benda-R is not accessible (as discussed elsewhere62). ∗Zanubrutinib may also be prioritized for those with TP53 alterations (as previously reported35). Clinical trial options should always be considered. Benda, bendamustine; CAGG, cold agglutinins; CRYOS, cryoglobulinemia; HV, hyperviscosity; PN, peripheral neuropathy; R, rituximab.
In patients aged >70 years or with a low estimated glomerular filtration rate, reduced-dose bendamustine should be considered. Data on the relative activity of BTKis vs chemoimmunotherapy are minimal. A multicenter, retrospective study of 246 patients with treatment-naïve MYD88Mut WM compared outcomes for Benda-R vs ibrutinib.67 Seventy-seven percent of patients received >4 cycles of Benda-R. The median follow-up was 4.2 years. Major response (92% vs 83%), VGPR (50% vs 33%), and CR (20% vs 2%) rates were greater for patients treated with Benda-R vs those treated with ibrutinib, respectively. PFS and OS were similar, supporting that BTKis offer comparable long-term disease control to Benda-R.
For patients with only MYD88Mut, we favor cBTKi to minimize risk for acquired TP53Alt. Because all cBTKis exhibit similar activity in patients with only MYD88Mut (Table 1), the choice should consider accessibility and guidance offered in Table 3. For patients with CXCR4Mut requiring a rapid response such as case 1, Benda-R or zanubrutinib may be reasonable. Plasmapheresis should be performed in those with symptomatic hyperviscosity. Rituximab should be held if Benda-R is chosen until the sIgM level is <4000 mg/dL to avoid triggering a hyperviscosity crisis.62 Zanubrutinib may also be considered in patients with CXCR4Mut needing a rapid response. The median time to a major response was 2.8 months in patients with CXCR4Mut WM receiving zanubrutinib in the ASPEN study, which is comparable with that of patients receiving Benda-R.35,68 In case 1, Benda-R is more suitable because data with BTKis in patients with bulky disease are lacking.
Guide for selecting BTKis for the treatment of WM
| . | Preference . | Alternative . |
|---|---|---|
| BTKi options for initial therapy | ||
| Convenience/compliance | Ibrutinib Zanubrutinib∗ Tirabrutinib∗ | Acalabrutinib |
| Deep IgM response needed (ie, IgM demyelinating neuropathy, cryoglobulinemia, and cold agglutinemia) | Zanubrutinib† | Ibrutinib Acalabrutinib Tirabrutinib‡ |
| BNS | Ibrutinib Tirabrutinib‡ | Zanubrutinib |
| History or predisposition to arrythmia | Zanubrutinib§ | |
| History or predisposition to bleeding | Zanubrutinib§ | |
| Neutropenic or pancytopenic | Ibrutinib | |
| MYD88WT | Zanubrutinib | |
| CXCR4Mut | Zanubrutinib | Ibrutinib plus rituximab |
| TP53 alteration | Zanubrutinib | Ibrutinib |
| BTKi options for switchover | ||
| Intolerant to ibrutinib for adverse events other than atrial fibrillation | Dose-reduction of Ibrutinib Zanubrutinib Acalabrutinib‖ | Pirtobrutinib¶ |
| Intolerant to ibrutinib due to atrial fibrillation | Zanubrutinib | Pirtobrutinib¶ |
| Acquired resistance to a cBTKi | Pirtobrutinib |
| . | Preference . | Alternative . |
|---|---|---|
| BTKi options for initial therapy | ||
| Convenience/compliance | Ibrutinib Zanubrutinib∗ Tirabrutinib∗ | Acalabrutinib |
| Deep IgM response needed (ie, IgM demyelinating neuropathy, cryoglobulinemia, and cold agglutinemia) | Zanubrutinib† | Ibrutinib Acalabrutinib Tirabrutinib‡ |
| BNS | Ibrutinib Tirabrutinib‡ | Zanubrutinib |
| History or predisposition to arrythmia | Zanubrutinib§ | |
| History or predisposition to bleeding | Zanubrutinib§ | |
| Neutropenic or pancytopenic | Ibrutinib | |
| MYD88WT | Zanubrutinib | |
| CXCR4Mut | Zanubrutinib | Ibrutinib plus rituximab |
| TP53 alteration | Zanubrutinib | Ibrutinib |
| BTKi options for switchover | ||
| Intolerant to ibrutinib for adverse events other than atrial fibrillation | Dose-reduction of Ibrutinib Zanubrutinib Acalabrutinib‖ | Pirtobrutinib¶ |
| Intolerant to ibrutinib due to atrial fibrillation | Zanubrutinib | Pirtobrutinib¶ |
| Acquired resistance to a cBTKi | Pirtobrutinib |
Preferences are based on availability and weight of clinical data supporting the use of a particular BTKi for a select patient and represent the viewpoint and experience of the authors. Supporting data for these recommendations are presented in the text. Listed BTKis are approved for WM and/or other indications in any jurisdiction. Clinicians should consult local regulatory approvals and guidelines for their status and use in WM.
Zanubrutinib is approved for use as single (320 mg) daily or twice-daily (160 mg) administration.
ASPEN data demonstrating a higher VGPR rate for zanubrutinib vs ibrutinib is based on twice-daily administration of zanubrutinib.
Tirabrutinib is only approved in Japan. No randomized data against any other cBTKi.
ASPEN data demonstrating a lower risk of atrial fibrillation and bleeding diathesis for zanubrutinib vs ibrutinib is based on twice-daily administration of zanubrutinib.
Switchover data supported by a study with patients with chronic lymphocytic leukemia (as previously reported69).
Safety data for use in WM remain limited. Alternatives to BTKis for patients who are intolerant or those with acquired resistance to BTKis are presented in the text.
Zanubrutinib can be considered in patients with CXCR4Mut not needing rapid disease control because a shorter time to major response, deeper responses, and longer PFS were observed vs ibrutinib.35,44 For patients with MYD88WT, zanubrutinib is favored for those who are symptomatic and treatment naïve because high levels of response and long-term disease control can be achieved.44 Benda-R and proteasome-inhibitor (PI)-based therapy are reasonable alternatives in patients with CXCR4Mut or MYD88WT.68,70 TP53Alt status can be considered in positioning BTKis. Zanubrutinib is our preference for patients with TP53Alt with WM, given the ASPEN study findings showing higher levels of activity and long-term disease control vs ibrutinib.35
Case 2: patients with previously treated WM with high-risk disease requiring therapy
A 42-year-old woman presented with headaches and blurry vision. Workup showed a sIgM monoclonal protein; sIgM level >10 000 mg/dL; and 85% to 90% BM LPL cell involvement. Molecular studies revealed MYD88L265P, CXCR4V340FS, and TP53Y163C mutations. Her hemoglobin was 9.9 g/dL, and her platelet count was 72 000 × 103/μL. She received bortezomib, dexamethasone, and rituximab with a partial response but progressed 1.5 years later. She then received venetoclax (on a clinical trial) with no response after 3 months of therapy.
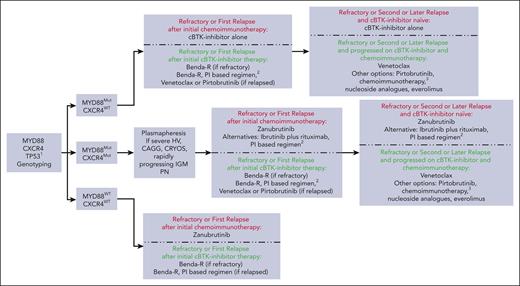
Figure 3 provides an algorithm for patients with symptomatic, previously treated WM. The recommendations considered recent consensus panel guidance.71 The panel noted that biological age, comorbidities, and fitness; nature of the relapse; patient preferences; hematopoietic reserve; and mutation status (MYD88, CXCR4, and TP53) should be considered in treatment selection. For patients with only MYD88Mut who are refractory or in first relapse after initial chemoimmunotherapy, cBTKis should be considered. Because all cBTKis exhibit similar response activity in patients with MYD88Mut (Table 1), the choice of agent should consider accessibility and guidance offered in Table 3. For patients with MYD88MutCXCR4Mut who are refractory or in first relapse after initial chemoimmunotherapy, zanubrutinib would be our preference.44 In patients with MYD88WT WM, zanubrutinib would also be our recommended treatment after initial chemoimmunotherapy.44 Zanubrutinib is also preferred for patients with TP53Alt WM such as case 2 with MYD88MutCXCR4MutTP53Alt.
Genomic-based treatment algorithm for patients with symptomatic, previously treated WM. Clinicians should consult local regulatory approvals and guidelines for BTKi status and use in WM. Algorithm represents the recommendations of the authors based on clinical trial data summarized in the text, consensus recommendations (as previously reported68), and their practice experiences with patients with WM. Recommendations are intended for educational purposes. See also notations for Figure 2. Nucleoside analogues should be avoided in younger patients, and candidates for autologous stem cell transplantation. Autologous stem cell transplantation may be considered in patients with multiple relapses and chemotherapy-sensitive disease, and those with amyloidosis for consolidation after PI or Benda-R therapy (as discussed elsewhere66). 1Zanubrutinib may also be prioritized for those with TP53 alterations (discussed elsewhere35). Clinical trial options should always be considered.
Genomic-based treatment algorithm for patients with symptomatic, previously treated WM. Clinicians should consult local regulatory approvals and guidelines for BTKi status and use in WM. Algorithm represents the recommendations of the authors based on clinical trial data summarized in the text, consensus recommendations (as previously reported68), and their practice experiences with patients with WM. Recommendations are intended for educational purposes. See also notations for Figure 2. Nucleoside analogues should be avoided in younger patients, and candidates for autologous stem cell transplantation. Autologous stem cell transplantation may be considered in patients with multiple relapses and chemotherapy-sensitive disease, and those with amyloidosis for consolidation after PI or Benda-R therapy (as discussed elsewhere66). 1Zanubrutinib may also be prioritized for those with TP53 alterations (discussed elsewhere35). Clinical trial options should always be considered.
In patients discontinuing ibrutinib, chemoimmunotherapy showed an ORR of 73%.72 Response rates were higher in patients who began chemoimmunotherapy within 2 weeks of ibrutinib discontinuation.72 Because sIgM rebound is common after cessation of BTKis, a bridging strategy for continuing BTKi until disease control with chemoimmunotherapy is achieved can be considered in patients with high sIgM levels.40,72
For patients refractory to initial cBTKi therapy, Benda-R is recommended regardless of genomic subtype. Rituximab should be held if Benda-R is chosen until the sIgM is <4000 mg/dL to avoid triggering a hyperviscosity crisis. For those progressing after initial cBTKi response, options include Benda-R, PI-based therapy, venetoclax, or pirtobrutinib. We prefer to minimize alkylator exposure whenever possible, particularly in patients aged <70 years or with TP53Alt. For these patients, venetoclax is preferable because it is highly active in patients with WM previously exposed to cBTKi or with CXCR4Mut disease.73,74 The activity of venetoclax in patients with MYD88WT or TP53Alt patients with WM remains to be clarified. Pirtobrutinib is an option post-cBTKi therapy although its activity in patients with MYD88WT or MYD88MutCXCR4Mut is not known.47,48 Benda-R or PI-based regimens can also be considered in those progressing on a cBTKi because these are active across all genomic subtypes.68,70 Additional options in second or later relapse include re-use of chemotherapy if a response lasted for >3 years, alternative chemoimmunotherapy, nucleoside analogues, or everolimus.71 Clinical trials should be prioritized for patients who are relapsing.74
For those with bulky extramedullary disease such as case 1, Benda-R is more suitable because data with BTKis in patients with bulky disease are lacking. For patients with WM with symptomatic amyloidosis, consensus recommendations favor Benda-R or PI-based therapy followed by consolidation with high-dose chemotherapy and autologous stem cell transplantation in suitable patients.66 Findings in a small number of cases treated with ibrutinib or acalabrutinib showed a short PFS as well as more pronounced bleeding and atrial fibrillation.39,75,76 BTKis, particularly ibrutinib or tirabrutinib (in Japan) can be prioritized for patients with BNS, given more extensive reports showing activity and durability for these BTKis.51,52,57-59
Management of BTKi intolerant or resistant WM disease
Case 3: patient on ibrutinib who is in a VGPR and develops atrial fibrillation
A 73-year-old man presented with fatigue and hemoglobin of 9.1 g/dL. Workup showed an sIgM monoclonal protein and an sIgM level of 2301 mg/dL. BM biopsy showed 50% LPL cell involvement, and only the MYD88L265 mutation was detected. CT scans showed diffuse adenopathy (largest node, 2.3 cm) and mild splenomegaly. He began ibrutinib and attained a VGPR. One year after starting ibrutinib, he had symptomatic atrial fibrillation with a rapid ventricular response. Cardiac workup for amyloid deposition was unremarkable. He was started on metoprolol and received anticoagulation therapy with apixaban. He was cardioverted but had multiple additional episodes of atrial fibrillation. He also had hematuria and ecchymosis on anticoagulation.
The discontinuation rate for rituximab intolerance is ∼30%.77,78 Dose reduction with ibrutinib was evaluated in 353 patients with WM followed-up for a median of 64 months.78 Ninety-six (27%) required a dose reduction because of adverse events.78 Dose reductions were more common in those aged >65 years, and in women. Following dose reduction, most patients (65%) had improvement or resolution of adverse effects. Hematologic responses were sustained or deepened in most (79%) patients. Of 10 patients with atrial fibrillation, only 1 had no recurrence of arrhythmia after dose reduction. For patients with WM such as case 3 with ibrutinib-related atrial fibrillation, switching to another BTKi is more appropriate. The use of zanubrutinib to treat patients with ibrutinib or acalabrutinib intolerance was investigated in a study that included patients with WM.69 Most intolerance events for ibrutinib (70%) and acalabrutnib (83%) did not recur after zanubrutinib switchover. Of 10 patients switched over for atrial fibrillation (all from ibrutinib), 9 had no recurrence. The long-term disease control rate was 94% after switchover to zanubrutinib. A switchover should be initiated as soon as possible to prevent an sIgM rebound.41 Data for switchover from ibrutinib to acalabrutinib are limited, with recurrence of atrial fibrillation occurring in 1 of 2 patients with chronic lymphocytic leukemia.79
Case 4: patient with heavily pretreated WM who is progressing on ibrutinib
A 55-year-old man was diagnosed 13 years earlier with WM after presenting with anemia and fatigue. At diagnosis, his hemoglobin was 9.6 g/dL, his sIgM was 2800 mg/dL, and BM biopsy showed 90% involvement. CT scans were unremarkable. He had minimal or no response to cyclophosphamide, prednisone and rituximab; Benda-R; bortezomib/dexamethasone; and everolimus. He then started ibrutinib at 420 mg/dL and attained a VGPR. Eleven years later he presented with fatigue and hemoglobin of 7.1 g/dL. BM biopsy showed 95% LPL cell involvement, and 2 distinct BTKCys481 (c.1442G>C; c1441 T>A) mutations.
In patients with BTKCys481 mutation–related acquired c-BTK-I resistance such as case 4, a switchover to another c-BTKi is not recommended. Pirtobrutinib can be considered for patients previously exposed to a cBTKi in whom a MRR of 66% and PFS of 19.4 months was reported, although specific data for patients harboring BTKCys481 mutations awaited.47,48 Venetoclax also is an option, and is highly active in patients with WM previously exposed to BTKis or with CXCR4Mut disease.73,74 Lower ORR (76% vs 93%) and MRR (76% vs 87%) were observed among patients previously exposed to cBTKi than in patient exposed to c-BTKis, although no significant difference in PFS was observed.74 As discussed earlier, chemoimmunotherapy may also be considered in those intolerant or progressing on c-BTKis.71 Additional salvage options are presented in Figure 3. Our preference in patients with WM with acquired resistance to c-BTKi is to alternate to a non-cBTKi such as pirtobrutinib, if available, then class switch to a BCL2 inhibitor such as venetoclax. Chemoimmunotherapy or clinical trial options may subsequently be considered for patients who progressed on both BTK and BCL2 inhibitors.
Considerations for the choice of BTKis in WM
In selecting BTKis, compliance, nature of disease manifestation, comorbidities, mutational status (MYD88, CXCR4, TP53, and BTKCys481), as well as tolerance and presence of acquired resistance to cBTKi should be considered. Table 3 summarizes patient and disease characteristics including genomics in the choice of BTKis for WM.
Withdrawal symptoms related to BTKis
Withdrawal symptoms can develop in 20% of patients with WM holding ibrutinib.80 Symptoms usually ensue within 2 days of ibrutinib hold and resolve rapidly after reinitiation of therapy. In two-thirds of patients experiencing withdrawal, there is no evidence of disease progression during ibrutinib hold. Withdrawal symptoms include fever, body aches, night sweats, arthralgias, chills, headaches, and fatigue. An sIgM rebound can also occur when ibrutinib is withheld.81 Reports regarding withdrawal or sIgM rebound with other BTKis are limited.
Response assessment in WM
Consensus criteria were recently updated that use a more simplified sIgM-based response assessment for most categorical responses, and help distinguish progressive disease vs sIgM rebound in patients whose BTKi is held.80
Acknowledgments
S.P.T. is supported by a National Institutes of Health SPORE grant in Multiple Myeloma (2P50CA100707-16A1), a Leukemia and Lymphoma Society Translational Research Grant (6673-24), and a Legacy Award from the International Waldenstrom's Macroglobulinemia Foundation.
Authorship
Contribution: S.P.T. wrote the first draft; all authors reviewed the first draft, and provided revisions; and the final draft was approved by all authors.
Conflict-of-interest disclosure: S.P.T. received research funding and/or consulting fees from AbbVie/Pharmacyclics Inc, Janssen Oncology Inc, BeiGene Inc, Eli Lilly Pharmaceuticals, Bristol Myers Squibb, and Ono Pharmaceuticals; and is a named inventor for MYD88 and CXCR4 testing for WM and has assigned all interests to his institution. J.J.C. received research funds from AbbVie, AstraZeneca, BeiGene, Cellectar, Loxo, Pharmacyclics, and TG Therapeutics; and reports honoraria from AbbVie, BeiGene, Cellectar, Kite, Loxo, Janssen, Pharmacyclics, and Roche Pharmaceuticals. S.S. received research funding and/or consulting fees from BeiGene, Cellectar Biosciences, and ADC Therapeutics.
Correspondence: Steven P. Treon, Bing Center for Waldenström’s Macroglobulinemia, Dana-Farber Cancer Institute, M548, 450 Brookline Ave, Boston, MA 02215; email: steven_treon@dfci.harvard.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal