In this issue of Blood, Dreyling et al1 reported the long-term follow-up of the ELARA trial, a phase 2 study of tisagenlecleucel (tisa-cel) in relapsed and refractory follicular lymphoma (FL). After a median follow-up of 29 months, tisa-cel continued to demonstrate a high and durable response rate without new or unexpected safety signals. These data indicated that tisa-cel may be an important treatment option in relapsed FL.
The overall prognosis of patients with FL has substantially improved over the last decades, making FL a chronic disease for the majority of patients. However, for most patients, FL is still a relapsing and remitting disease. Furthermore, response duration and survival shorten after each relapse,2 and certain subsets of patients still have a poor outcome. This includes patients with high tumor burden by total metabolic tumor volume, bulky disease, high Follicular Lymphoma International Prognostic Index score, double refractoriness (refractory to CD20 and alkylating therapy), and early relapses within 24 months from first immunochemotherapy (POD24), with POD24 as a robust indicator of poor survival.3
With the expanding knowledge of the biology and pathogenesis of B-cell malignancies, a plethora of new treatment approaches have been investigated and approved in relapsed FL, allowing movement away from chemotherapy into an era of targeted and cellular therapy. In 2019, the combination of rituximab and lenalidomide was approved by the US Food and Drug Administration (FDA) and European Medicines Agency (EMA) based on the AUGMENT trial,4 quickly acknowledged as a new standard in relapsed FL. Tazemetostat, an enhancer of zeste homolog 2 (EZH2) inhibitor, received FDA license in 2020. A significant milestone was achieved by the approval of 2 autologous anti-CD19 chimeric antigen receptor (CAR) T-cell products and a bispecific antibody in 2021/2022. Tisa-cel is available following 2 lines of therapy based on the phase 2 ELARA trial,1 and axicabtagene ciloleucel was approved after ≥3 lines of therapy based on the phase 2 ZUMA-5 trial.5 Mosunetuzumab, a CD20 × CD3 bispecific antibody, documented significant efficacy following 2 lines of therapy in a phase 2 trial.6 In this trial, the median duration of response (DOR) was 36 months, and the median progression-free survival (PFS) was 24 months. Moreover, mosunetuzumab documented high efficacy in high-risk subgroups (eg, patients with POD24). Recently, the combination of zanubrutinib and obinutuzumab achieved EMA approval for use after 2 relapses based on the ROSEWOOD data.7 There is no doubt this armamentarium of new compounds will change the treatment landscape in relapsed FL; however, the implementation of these compounds in treatment algorithms for different risk categories is still needed.
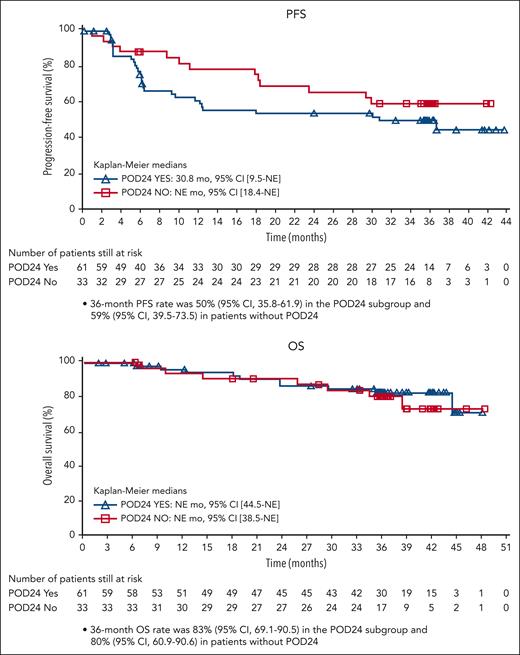
Dreyling et al analyzed a total of 97 patients receiving tisa-cel after ≥2 lines of therapy. They reported an overall response rate of 86.2% and a complete response rate of 68.1%. Estimated 24-month PFS, DOR, and overall survival (OS) were 57.4%, 66.4%, and 87.7%, respectively. Patients achieving a complete remission had a significantly longer PFS compared with the overall ELARA population. More important, tisa-cel also induced a high rate of durable responses in patients with high-risk disease characteristics, including POD24. The 3-year PFS and OS for POD24 patients compared with patients without POD24 have been presented at the recent American Society of Hematology meeting8 (see figure), showing no significant differences between both groups. Furthermore, Dreyling et al reported a correlation between the overall outcome and the levels of LAG3+CD3+ exhausted T cells in the lymphoma microenvironment and between outcome and baseline levels of naïve CD8+ cells. They also reported that patients with POD24 had a reduced CAR T-cell expansion, which, however, did not substantially affect PFS and OS. These exploratory biomarker analyses are important steps for the understanding of factors affecting the long-term prognosis of patients with FL.
The 36-month PFS and OS of patients with or without POD24; subgroup analysis of the ELARA trial presented at the American Society of Hematology meeting 20238 (with permission). CI, confidence interval; NE, not estimated; OS, overall survival; PFS, progression-free survival; POD24, progression of disease within 2 years of frontline systemic therapy.
The 36-month PFS and OS of patients with or without POD24; subgroup analysis of the ELARA trial presented at the American Society of Hematology meeting 20238 (with permission). CI, confidence interval; NE, not estimated; OS, overall survival; PFS, progression-free survival; POD24, progression of disease within 2 years of frontline systemic therapy.
There is an ongoing discussion on the use of CARs and bispecific antibodies in relapsed FL. Both treatment approaches are effective not only in the general population with FL but also in patients with high-risk features. Bispecifics are available off the shelf and may be used in the community setting and mostly outpatient setting, without the need of lymphodepleting chemotherapy. However, treatment with bispecifics requires repeated infusions over a longer period, and response is dependent on the target antigen expression. CARs require just a single infusion, response is irrespective of the target antigen expression, and data show a stable and long-term PFS for ≈60% of patients. However, the use of CARs is limited to specialized accredited centers, and they are used mostly in the inpatient setting and require a substantial upfront commitment.
The ELARA trial update clearly emphasizes the therapeutic significance of CARs in relapsed FL. The authors not only confirmed the response data following 2 relapses but also demonstrated the role of CARs in high-risk subgroups. This supports evaluation of CARs to earlier lines of treatment, with trials exploring this question already underway. Determining the optimal therapeutic sequence for the individual patient based on risk features will be a major challenge for the near future.
Conflict-of-interest disclosure: K.H. has received advisory honoraria from Roche, Bristol Myers Squibb, Incyte, AbbVie, Novartis, Gilead, and BeiGene; and research funding from Roche, Gilead, and Incyte.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal