Eculizumab and ravulizumab are effective, well-tolerated therapies for patients with PNH, and the risks of complications are low.
Survival is inferior to age- and sex-matched controls, but this finding is as a result of patients with significant aplasia rather than PNH.
Visual Abstract
Paroxysmal nocturnal hemoglobinuria (PNH) is an acquired clonal hematopoietic disorder that occurs on a background of bone marrow failure (BMF). In PNH, chronic intravascular hemolysis causes an increase in morbidity and mortality, mainly because of thromboses. Over the last 20 years, treatment of PNH has focused on the complement protein C5 to prevent intravascular hemolysis using the monoclonal antibody eculizumab and more recently ravulizumab. In the United Kingdom, all patients are under review at 1 of 2 reference centers. We report on all 509 UK patients with PNH treated with eculizumab and/or ravulizumab between May 2002 and July 2022. The survival of patients with eculizumab and ravulizumab was significantly lower than that of age- and sex-matched controls (P = .001). Only 4 patients died of thromboses. The survival of patients with PNH (n = 389), when those requiring treatment for BMF (clonal evolution to myelodysplastic syndrome or acute leukemia or had progressive unresponsive aplastic anemia) were excluded, was not significantly different from that of age- and sex-matched controls (P = .12). There were 11 cases of meningococcal sepsis (0.35 events per 100 patient-years). Extravascular hemolysis was evident in patients who received treatment, with 26.7% of patients requiring transfusions in the most recent 12 months on therapy. Eculizumab and ravulizumab are safe and effective therapies that reduce mortality and morbidity in PNH, but further work is needed to reduce mortality in those with concomitant BMF.
Medscape Continuing Medical Education online

In support of improving patient care, this activity has been planned and implemented by Medscape, LLC and the American Society of Hematology. Medscape, LLC is jointly accredited with commendation by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Successful completion of this CME activity, which includes participation in the evaluation component, enables the participant to earn up to 1.0 MOC points in the American Board of Internal Medicine's (ABIM) Maintenance of Certification (MOC) program. Participants will earn MOC points equivalent to the amount of CME credits claimed for the activity. It is the CME activity provider's responsibility to submit participant completion information to ACCME for the purpose of granting ABIM MOC credit.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at https://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 1199.
Disclosures
CME questions author Laurie Barclay, freelance writer and reviewer, Medscape, LLC, declares no competing financial interests.
Learning Objectives
Upon completion of this activity, participants will:
Describe clinical characteristics, presentation, and treatment of paroxysmal nocturnal hemoglobinuria (PNH), based on a follow-up study of all 509 UK patients with PNH treated with eculizumab and ravulizumab between May 2002 and July 2022
Determine survival, complications, and cause of death in patients with PNH treated with eculizumab and ravulizumab, based on a follow-up study of all 509 UK patients with PNH treated with eculizumab and ravulizumab between May 2002 and July 2022
Identify clinical implications of safety and efficacy of PNH treatment with eculizumab and ravulizumab, based on a follow-up study of all 509 UK patients with PNH treated with eculizumab and ravulizumab between May 2002 and July 2022
Release date: March 21, 2024; Expiration date: March 21, 2025
Introduction
Paroxysmal nocturnal hemoglobinuria (PNH) is a rare acquired disorder, caused by clonal expansion of hemopoietic stem cells harboring a mutation of the PIG-A gene.1-3 PNH occurs on a background of bone marrow failure (BMF), usually aplastic anemia (AA), but, in some cases, myelodysplastic syndrome (MDS).4-8 PNH cells lack a variety of surface proteins that rely on the glycophosphatidylinositol anchor for them to be expressed on the cell surface.9 These proteins play important roles in signal transduction and the immune response.9,10 Glycophosphatidylinositol anchor formation in the endoplasmic reticulum is disrupted because of these somatic mutations of the PIG-A gene.2,11 Chronic intravascular hemolysis occurs because of the lack of 2 important complement regulatory proteins, CD55 and CD59, on the surface of PNH blood cells.4,12
Clinical symptoms of PNH include fatigue, lethargy, breathlessness, hemoglobinuria, chest and abdominal pains, erectile dysfunction, renal impairment, and thromboses.12,13 Arterial or venous thrombosis, which risk serious morbidity and mortality, is the presenting feature in ∼19% of patients, and accounted for 40% to 67%% of PNH-related deaths before availability of complement inhibition.4,12,14-19
Treatment with terminal complement inhibition has been the standard of care for patients with PNH since 2007 when eculizumab was approved by both the Food and Drug Administration and the European Medicines Agency. Eculizumab is a humanized monoclonal antibody that binds to the complement protein C5 preventing its cleavage into C5a and C5b and, thereby, stopping intravascular hemolysis and its consequences,20,21 reducing the risk of thrombosis and renal damage, and decreasing transfusion requirements.22-28
Ravulizumab, a recombinant monoclonal antibody approved by the Food and Drug Administration and the European Medicines Agency for patients with PNH in 2018 and 2019, respectively, binds to C5 preventing terminal complement activation. In contrast to eculizumab, which is degraded in the endosome, ravulizumab is recycled by the FcRn-receptor, providing a half-life 4 times that of eculizumab.29 Phase-3 trials have confirmed noninferiority to eculizumab for both untreated patients with PNH and those who have changed from eculizumab to ravulizumab.29,30 Ravulizumab is now the first-line standard of care in the United Kingdom since National Institute for Health and Care Excellence approval in May 2021.
We have previously reported on survival of 79 patients with PNH on treatment with eculizumab with a median followup of 8 years.28 Here, we report on all 509 patients with PNH treated with eculizumab or ravulizumab in the United Kingdom between May 2002 and July 2022. All the data were collected as a mandated audit from our health authority as part of service provision for the national PNH service. Patients provide informed consent for data to be reviewed by clinicians and the paper fulfills the UK GDPR data requirements.
Methods
Patients and investigations
All patients treated with complement inhibition in the United Kingdom are under review at 1 of 2 nationally commissioned centers in England, Leeds Teaching Hospitals or Kings College Hospital. Five hundred and nine patients with PNH treated with the C5 inhibitor eculizumab or ravulizumab between May 2002 and July 2022 in the United Kingdom were identified via the 2 centers. This study was carried out as a service evaluation or audit in accordance with institutional guidance.
The diagnosis of PNH was established or confirmed using multicolor flow cytometry of the erythrocytes and granulocytes in all cases. Data collected for analysis included sex, age at diagnosis, symptoms at diagnosis and on commencing anti-C5 complement inhibitors (anti-C5is; eculizumab or ravulizumab), time from symptoms to diagnosis, misdiagnoses and the proportion of patients referred to nonhematological specialists, history of preceding AA or MDS, age at the start of C5i, use of medication to treat AA, occurrence of thromboses before and during C5i, hemoglobin levels after 12 and 24 months of C5i, breakthrough hemolysis episodes, clonal evolution to MDS or acute myeloid leukemia (AML), progression of AA, meningococcal infections, mortality, and cause of death.
Indications for eculizumab and ravulizumab therapy
All consecutive patients with PNH who fulfilled the agreed, nationally commissioned indications for treatment: PNH with symptomatic hemolytic anemia, a significant PNH-related complication without hemolysis (lactate dehydrogenase [LDH] <1.5× upper limit of normal) regardless of transfusion history, planned for allogeneic bone marrow transplant (allo-HSCT) and pregnancy. Treatment indications remained unchanged over this 20-year period. In addition, a small number of patients who did not fulfill these criteria were treated as exceptions because of profound symptoms after mutual agreement between the 2 PNH centers.
Eculizumab was initially delivered at the approved dosing schedule of 600-mg IV infusions each week for 4 doses followed by a 900-mg infusion after a further week. A 900-mg dose was then administered every 14 days indefinitely. After the initial doses, all therapy was administered in the patient’s home by homecare nurses under the guidance of the treating PNH center. Higher than standard doses of eculizumab were indicated for some patients identified because of inadequate concentrations of antibody to fully inhibit C5 activity.
The recurrence of a patient’s symptoms (eg, dark urine or abdominal discomfort) and increased LDH immediately before a dose of eculizumab was indicative of breakthrough from complement control. Initially we also evaluated eculizumab levels in the bloodstream to provide further assurance of pharmacokinetic failure. When this technique was no longer available a CH50 test was performed just before C5i to assess complement activation. These patients were treated with higher doses (usually 1200 mg) every 14 days.
Ravulizumab was delivered as an IV infusion according to the patient’s weight with the initial dose being at the PNH center. Subsequent doses, 2 weeks after the loading dose, then every 8 weeks, were delivered via homecare nurses, again under the guidance of the treating PNH center.
All patients were vaccinated with a tetravalent meningococcal vaccine against subgroups A, C, W, and Y, and with a separate vaccine against serogroup B. Revaccination occurred according to serological levels which are assessed annually. Antibiotic prophylaxis is recommended to all patients (penicillin V 500 mg twice a day or erythromycin 500 mg twice a day for patients who are intolerant of penicillin).
Statistical analysis
Observed survival for the PNH cohort after commencement was calculated using the Kaplan-Meier estimator, with overall survival (OS) as the outcome variable in Stata 14.2, and plotted together with expected survival estimates at yearly intervals for an age- and sex-matched cohort drawn from the population. Relative and expected survival was estimated using the Ederer II approach through the “strs” routine, also in Stata 14.2. This method uses population mortality data to estimate expected survival for patients at each point of follow up using estimates from the appropriate year. Cases were only considered at risk until they died or were censored. The population mortality data used to generate these estimates were taken from the human mortality database for the United Kingdom (1841-2020), stratified based on age and sex. Expected survival was reported at yearly intervals after diagnosis for an equivalent group matched by age and sex. Cumulative relative survival estimates were calculated at 10 and 19 years. To determine whether the outcome for the PNH cohort was significantly worse than that of the general population, the Wald test was used to test for significant differences of cumulative relative survival at these time points from that expected for the general population. Log-rank tests for overall difference in outcome between the PNH cohort and the general population were also calculated; expected failure times for the general population were estimated using cumulative monthly interval-specific expected survival, assuming identical censoring to the PNH cohort. For relative survival estimates and log-rank test, calculation survival was truncated at the end of 2020 because mortality estimates beyond this time were not available. Analyses were conducted, both including and excluding cases receiving an allogeneic bone marrow transplantation (BMT), medical therapy for AA, AA unresponsive to medical therapy, or those with clonal evolution to myeloid neoplasms.
Results
Patient characteristics
Five hundred and nine patients with PNH were treated with eculizumab and/or ravulizumab in the United Kingdom between May 2002 and July 2022. The baseline characteristics are detailed in Table 1. The mean age at diagnosis was 43 years and 11 months (standard deviation, 22 years and 5 months) and the median PNH granulocyte clone was 78.3%. A history of BMF was documented for 246 patients (48.3%), of whom 215 had AA (42.2%) and 31 (6.1%) had MDS. One hundred and thirty patients had received either horse or rabbit anti-thymocyte globulin (ATG) and cyclosporine to treat their AA, with 12 of the 130 receiving 2 courses of ATG. Forty-three patients had received cyclosporine alone.
Baseline characteristics and prior thrombosis
| Baseline characteristics . | Number (%) . |
|---|---|
| Mean age at diagnosis | 43 years and 11 months |
| Male | 237 (46.6) |
| Median granulocyte clone at diagnosis | 78.3% |
| Documented history of aplastic anemia | 215 (42.2) |
| Documented history of myelodysplastic syndrome | 31 (6.1) |
| History of thrombosis | 205 thromboses in 135 patients (26.5) |
| Thromboses before complement inhibition | |
| Budd-Chiari | 53 |
| Cerebrovascular accident and transient ischemic attack | 33 |
| Mesenteric and splenic | 27 |
| Myocardial infarction | 23 |
| Pulmonary embolus | 22 |
| Deep vein thrombosis | 20 |
| Cerebral venous thrombosis | 14 |
| Catheter related | 3 |
| Dermal | 3 |
| Limb ischemia | 3 |
| Retinal | 2 |
| Internal jugular | 1 |
| Esopageal ischemia | 1 |
| Baseline characteristics . | Number (%) . |
|---|---|
| Mean age at diagnosis | 43 years and 11 months |
| Male | 237 (46.6) |
| Median granulocyte clone at diagnosis | 78.3% |
| Documented history of aplastic anemia | 215 (42.2) |
| Documented history of myelodysplastic syndrome | 31 (6.1) |
| History of thrombosis | 205 thromboses in 135 patients (26.5) |
| Thromboses before complement inhibition | |
| Budd-Chiari | 53 |
| Cerebrovascular accident and transient ischemic attack | 33 |
| Mesenteric and splenic | 27 |
| Myocardial infarction | 23 |
| Pulmonary embolus | 22 |
| Deep vein thrombosis | 20 |
| Cerebral venous thrombosis | 14 |
| Catheter related | 3 |
| Dermal | 3 |
| Limb ischemia | 3 |
| Retinal | 2 |
| Internal jugular | 1 |
| Esopageal ischemia | 1 |
One hundred and thirty-five patients (26.5%) had experienced 205 thrombotic events before commencing C5i. Eighty (39.0%) of these were intra-abdominal with the majority being Budd-Chiari syndrome. More common sites of thromboses, deep vein thrombosis, and pulmonary embolism, occurred in 42 cases (20.5%). Fourteen patients (6.8%) experienced cerebral venous thrombosis. Arterial thrombosis was documented in 59 patients (28.8%) with the majority of these being cerebrovascular accidents (13.2%) or myocardial infarctions (11.2%). Thrombosis was a presenting feature at diagnosis in 63 cases (12.4%).
PNH presentation
The median time from developing symptoms to diagnosis of PNH was 5 months (interquartile range [IQR], 0-18; available in 454 cases). Symptoms at diagnosis were available in 494 cases and are shown in Table 2. In 172 of these cases (34.8%), the diagnosis of PNH was not made until ≥12 months from the time symptoms first occurred. The initial features experienced were those of anemia in 358 (72.5%), fatigue in 316 (64.0%), hemoglobinuria in 225 (45.5%), breathlessness in 114 (23.1%), abdominal pain in 92 (18.6%), dysphagia in 36 (7.3%), and erectile dysfunction in 32 (13.5%) of male patients.
Disease presentation
| Initial features at presentation∗ . | Number (%) . |
|---|---|
| Anemia | 358 (72.5) |
| Fatigue | 316 (64.0) |
| Hemoglobinuria | 225 (45.5) |
| Breathlessness | 114 (23.1) |
| Abdominal pain | 92 (18.6) |
| Dysphagia | 36 (7.3) |
| Erectile dysfunction | 32 (13.5)† |
| Initial referral to nonhematology/oncology specialties‡ | |
| Urology | 30 (6.1) |
| Gastroenterology | 29 (5.9) |
| Renal | 3 (0.6) |
| Cardiology | 3 (0.6) |
| Internal medicine | 2 (0.4) |
| Neurology | 2 (0.4) |
| Rheumatology | 2 (0.4) |
| Endocrinology | 1 (0.2) |
| Hepatology | 1 (0.2) |
| General surgery | 1 (0.2) |
| Vascular surgery | 1 (0.2) |
| Ear nose and throat surgery | 1 (0.2) |
| Eating disorder clinic | 1 (0.2) |
| Chronic fatigue clinic | 1 (0.2) |
| Misdiagnoses§ | |
| Genito-urinary bleeding | 17 |
| Iron deficiency anemia with cause unknown | 15 |
| Immune thrombocytopenic purpura | 10 |
| Warm autoimmune hemolytic anemia | 6 |
| Myelodysplastic syndrome | 4 |
| Vitamin B12 deficiency | 3 |
| Crohn disease | 2 |
| Adult Still disease | 1 |
| Idiopathic liver failure | 1 |
| Renal failure | 1 |
| Pyruvate kinase deficiency | 1 |
| Hereditary spherocytosis | 1 |
| Pancreatitis | 1 |
| Gallstones | 1 |
| Myalgic encephalomyelitis | 1 |
| Diverticular disease | 1 |
| Initial features at presentation∗ . | Number (%) . |
|---|---|
| Anemia | 358 (72.5) |
| Fatigue | 316 (64.0) |
| Hemoglobinuria | 225 (45.5) |
| Breathlessness | 114 (23.1) |
| Abdominal pain | 92 (18.6) |
| Dysphagia | 36 (7.3) |
| Erectile dysfunction | 32 (13.5)† |
| Initial referral to nonhematology/oncology specialties‡ | |
| Urology | 30 (6.1) |
| Gastroenterology | 29 (5.9) |
| Renal | 3 (0.6) |
| Cardiology | 3 (0.6) |
| Internal medicine | 2 (0.4) |
| Neurology | 2 (0.4) |
| Rheumatology | 2 (0.4) |
| Endocrinology | 1 (0.2) |
| Hepatology | 1 (0.2) |
| General surgery | 1 (0.2) |
| Vascular surgery | 1 (0.2) |
| Ear nose and throat surgery | 1 (0.2) |
| Eating disorder clinic | 1 (0.2) |
| Chronic fatigue clinic | 1 (0.2) |
| Misdiagnoses§ | |
| Genito-urinary bleeding | 17 |
| Iron deficiency anemia with cause unknown | 15 |
| Immune thrombocytopenic purpura | 10 |
| Warm autoimmune hemolytic anemia | 6 |
| Myelodysplastic syndrome | 4 |
| Vitamin B12 deficiency | 3 |
| Crohn disease | 2 |
| Adult Still disease | 1 |
| Idiopathic liver failure | 1 |
| Renal failure | 1 |
| Pyruvate kinase deficiency | 1 |
| Hereditary spherocytosis | 1 |
| Pancreatitis | 1 |
| Gallstones | 1 |
| Myalgic encephalomyelitis | 1 |
| Diverticular disease | 1 |
Available for 494 cases.
Of the 237 male patients.
Available for 490 cases.
Available for 489 cases.
Seventy-eight patients (15.3%) were initially referred to a nonhematology specialty for investigation of symptoms. Sixty-six patients (13.5%) were misdiagnosed with an alternative condition before the correct diagnosis of PNH was made. These are shown in Table 2. Misdiagnoses included a wide variety of conditions including iron deficiency because of menorrhagia, gastrointestinal or urological tract bleeding, immune thrombocytopenia, warm autoimmune hemolytic anemia, Crohn disease, diverticular disease, atypical Still disease, pyruvate kinase deficiency, hereditary spherocytosis, idiopathic liver failure, gallstones, renal failure, vitamin B12 deficiency, myalgic encephalomyelitis, and anorexia nervosa. Ten patients were referred to 2 different nonhematology or oncology specialties, and 1 patient to 4 specialties because of their PNH symptoms before a correct diagnosis was made.
Treatment with eculizumab and/or ravulizumab
Four hundred and seventy-four patients (93.1%) have been treated with eculizumab, with 77 (16.2%) requiring higher doses than recommended because of developing pharmacokinetic breakthrough hemolysis.
In 2021, ravulizumab became available for use in the United Kingdom and 237 patients treated with eculizumab have switched to ravulizumab at the time of analysis. Thirty-five patients who initiated treatment since 2021 have only received ravulizumab. Indication for treatment with C5i was mainly because of symptomatic hemolytic anemia (452 patients; 88.8%). However, 13 patients (2.6%) received C5i because of other PNH complications without hemolysis (LDH <1.5× upper limit of normal), these were mainly due to thrombosis (8), but included 2 patients with acute renal failure, 2 with hemoglobinuria and fatigue, and 1 with dysphagia and fatigue. Nineteen patients (3.7%) were treated owing to pregnancy. Four of these were diagnosed with hemolytic PNH while pregnant. The other 15 women had a compensated hemolysis before becoming pregnant. On becoming pregnant, they were counseled regarding the risk of maternal and fetal complications and agreed to be treated with eculizumab. After the postpartum period, 5 patients elected to remain on C5i and 10 discontinued, with half of these later restarting C5i because of hemolytic disease. Thirteen patients (2.6%) were treated because they had large PNH clones and required allo-HSCT for AA or MDS. Twelve patients (2.4%) were treated as exceptional cases. Three of these received C5i to prevent complications during surgery, and the other 9, because of extreme fatigue. These patients undertook Functional Assessment of Chronic Illness Therapy—Fatigue Scale readings before and after commencing C5i to demonstrate an improvement in symptoms. All 9 patients benefited from C5i.
The median PNH granulocyte clone size at initiation of C5i was 85.5% (IQR, 67%-95%; available in 494 cases). In the majority of cases the PNH granulocyte and monocyte clones are similar; however, in cases in which there was a discrepancy between these values, the larger clone value was used to assess the clone size. Twenty patients (3.9%) were able to discontinue C5i as both their PNH granulocyte and monocyte clone had fallen to <10%. Typically, this occurred gradually over several years. This figure is an arbitrary cutoff and is used because we would not commence C5i for patients with a granulocyte and monocyte clone <10%.
Complications
There have been 11 cases of Neisseria meningitidis septicemia in 10 individuals over 3130 treatment years of C5i, with 1 fatality. This equates to a meningococcal infection rate of 0.35 events per 100 patient-years. Thrombosis occurred in 23 patients, consistent with a thrombotic rate of 0.73 events per 100 patient-years. Clonal evolution to AML occurred in 7 cases and to MDS in 10 cases.
Blood counts
Mean hemoglobin levels 12 and 24 months after initiating C5i were 107.7 g/L (in 421 patients) and 108.6 g/L (in 374 patients), respectively. In the most recent 12 months on C5i, 123 out of 446 patients (27.6%) needed transfusions, with 94 of the 123 (76.4%) requiring ≥3 transfusions. Hemoglobin levels were in the normal range (males 135 g/L and female 115 g/L) in 20.4% cases (86 of 421) after 12 months and 20.3% cases (76 of 374) after 24 months on C5i.
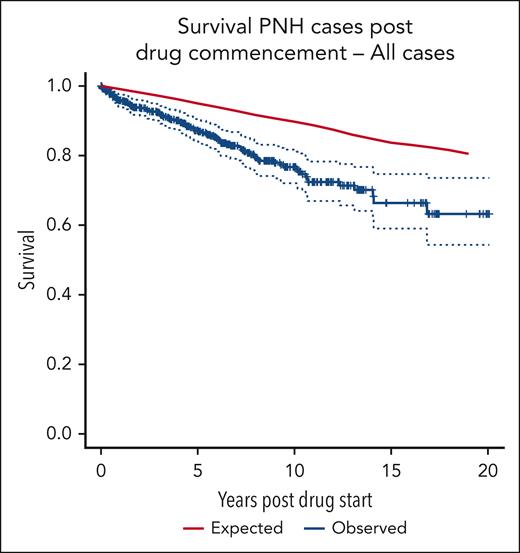
Survival
The survival curve for the patient group compared with age- and sex-matched control averages from the UK population is shown in Figure 1. There is a significant difference in OS between the 2 groups (log-rank P = .001). The 10-year cumulative relative survival is 0.8780 (95% confidence interval [CI], 0.8123-0.9311; Wald test P < .001). The 19-year cumulative relative survival is 0.7667 (95% CI, 0.5774-0.9167; Wald test P = .007).
OS of all 509 patients compared with that of age- and sex-matched controls.
OS of all 509 patients compared with that of age- and sex-matched controls.
Many patients with PNH have coexisting BMF and have required treatment specific to their BMF as well as their PNH. Table 3 shows those patients receiving these therapies either at the end of the study period or at the time they died as well as those who required allo-HSCT for AA, MDS, or AML while receiving C5i, and those with clonal evolution who did not proceed to allo-HSCT. Forty-six patients underwent allo-HSCT, 38 for AA, 7 for clonal evolution to MDS or AML, and 1 for a concurrent lymphoma. Of those who did not undergo allo-HSCT, 58 patients were receiving medical therapy for AA. Forty-two patients were treated with immunosuppressive therapy with cyclosporine or tacrolimus, 7 with the androgen oxymetholone or danazol, 7 with eltrombopag, and 2 with a combination of these therapies. Sixteen patients had either AA unresponsive to medical therapy (n = 2) or clonal evolution to MDS or AML (n = 14) but did not proceed to allo-HSCT.
Treatment with C5i
| Reason for commencing C5i . | Number (%) . |
|---|---|
| Intravascular hemolysis | 452 (88.8%) |
| Other PNH complication | 13 (2.6%) |
| Allogeneic bone marrow transplant | 13 (2.6%) |
| Pregnancy | 19 (3.7%) |
| Exceptional case | 12 (2.4%) |
| Treatment on C5i | |
| Median PNH granulocyte clone size at initiation of C5i | 85.5%∗ (range 12%-100%) |
| Median PNH erythrocyte clone size at initiation of C5i | 28.%† (range 0%-100%) |
| Death | 91 (17.9%) |
| Thromboses | 23 (4.5%) |
| Neisseria meningitidis infection | 11 (2.2%) |
| Clonal evolution to MDS or AML | 14 (2.8%) |
| Mean hemoglobin level 12 months after starting C5i‡ | 107.7g/l |
| Mean hemoglobin level 24 months after starting C5i§ | 108.6g/l |
| Requiring transfusions in the most recent 12 months on C5i‡ | 123 (26.7%) |
| Normalization of hemoglobin level at 12 months‡ | 86 (20.4%) |
| Normalization of hemoglobin level at 24 months§ | 76 (20.3%) |
| Discontinuation of C5i because of disease remission | 20 (3.9%) |
| Reason for commencing C5i . | Number (%) . |
|---|---|
| Intravascular hemolysis | 452 (88.8%) |
| Other PNH complication | 13 (2.6%) |
| Allogeneic bone marrow transplant | 13 (2.6%) |
| Pregnancy | 19 (3.7%) |
| Exceptional case | 12 (2.4%) |
| Treatment on C5i | |
| Median PNH granulocyte clone size at initiation of C5i | 85.5%∗ (range 12%-100%) |
| Median PNH erythrocyte clone size at initiation of C5i | 28.%† (range 0%-100%) |
| Death | 91 (17.9%) |
| Thromboses | 23 (4.5%) |
| Neisseria meningitidis infection | 11 (2.2%) |
| Clonal evolution to MDS or AML | 14 (2.8%) |
| Mean hemoglobin level 12 months after starting C5i‡ | 107.7g/l |
| Mean hemoglobin level 24 months after starting C5i§ | 108.6g/l |
| Requiring transfusions in the most recent 12 months on C5i‡ | 123 (26.7%) |
| Normalization of hemoglobin level at 12 months‡ | 86 (20.4%) |
| Normalization of hemoglobin level at 24 months§ | 76 (20.3%) |
| Discontinuation of C5i because of disease remission | 20 (3.9%) |
Interquartile range, 67% to 95%; available for 494 cases.
Interquartile range, 15% to 46%; available for 488 cases.
Available for 421 patients.
Available for 374 patients.
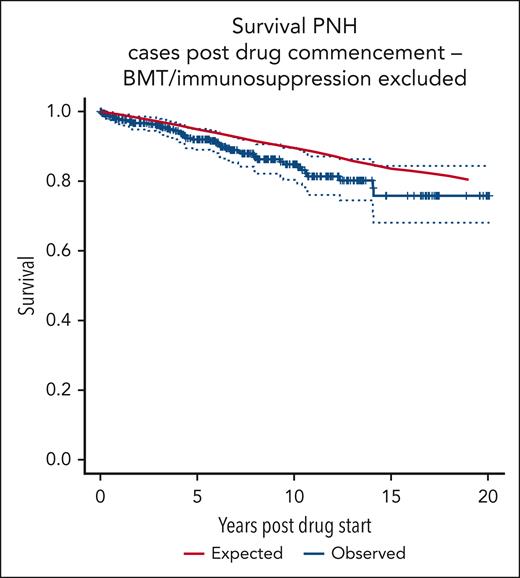
Figure 2 shows the survival curve of patients on C5i, excluding those receiving an allogeneic BMT, medical therapy for AA, AA unresponsive to medical therapy, or those with clonal evolution of their disease, compared with age- and sex-matched control averages from the UK population. There is no significant difference between the groups (log-rank P = .12). The 10-year cumulative relative survival for patients with PNH is seen to be 0.9585 (95% CI, 0.8912-1.007; Wald test P = .2). The 19-year cumulative relative survival is 0.9624 (95% CI, 0.8317-1.0562; Wald test P = .5).
OS of patients with PNH, excluding those with clonal evolution or treatment for AA.
OS of patients with PNH, excluding those with clonal evolution or treatment for AA.
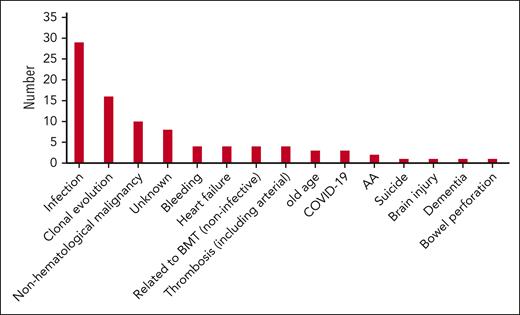
Cause of death
Ninety-one patients died during the study period. The causes of death are shown in Figure 3. Infective complications including pneumonia and septicemia occurred in 32 cases (35.2%), with 50% of these occurring in patients after allo-HSCT or on medical therapy for AA. Clonal evolution was deemed the cause of death in 16 patients (17.6%) with nonhematological malignancy, the third highest cause of death in 10 cases (11.0%). Thrombosis occurred in 4 patients (4.4%), with 2 arterial thromboses (1 myocardial infarction and 1 cerebrovascular accident) and 2 venous thromboses. Only 1 patient died secondary to meningococcal septicemia.
Discussion
To our knowledge, this retrospective study is the largest analysis of patients receiving anticomplement therapy with eculizumab and/or ravulizumab over a prolonged period of time in this ultrarare condition. In the United Kingdom, all patients are cared for via a shared care arrangement between the PNH centers (Leeds and London) and their local hematologists. The PNH center is responsible for management of the patient’s PNH and prescription of C5i. The funding of anticomplement therapy is centralized. The majority of the patients reviewed in the English PNH service are not on C5i, either because they tolerate a compensated hemolysis or have predominantly BMF with small PNH clones. These patients undergo close monitoring for signs and symptoms of PNH. For individuals with PNH, a detailed discussion with patients and their families or carers about the potential benefits and side effects of C5i is undertaken before initiating therapy.
The most common initial symptom experienced by patients with PNH is fatigue, and it is often not until hemoglobinuria or thromboses occur, in conjunction with cytopenias, that a diagnosis of PNH is made. The median time from developing symptoms to diagnosis of PNH was 5 months, but this includes patients who were diagnosed with AA with small PNH clones that then later had an expansion of their clone. In more than one-third of the cases, a diagnosis of PNH was not made for ≥12 months. The diagnosis of PNH is not always straightforward, with 13.5% of patients initially receiving an incorrect diagnosis and, in some cases, unnecessary treatments. Ten individuals were referred to multiple other specialists, with 1 patient consulting 4 different specialists before the correct diagnosis was made. Genito-urinary or gastrointestinal bleeding were the most common misdiagnoses, but there were a number of more unusual cases, with 1 patient even thought to have an eating disorder because of the severity of dysphagia he endured.
The thromboembolism rate with eculizumab treatment has previously been evaluated and compared with the pretreatment rate in the same patients.24 In that study, the thromboembolism rate with eculizumab was 1.07 events per 100 patient-years compared with a pretreatment rate of 7.37 events per 100 patient-years. We observed a similar thromboembolism rate on C5i of 0.73 events per 100 patient-years. It is important to emphasize that thromboses can still occur despite C5i with 23 patients experiencing a thrombosis and 4 patients dying. Alternative causes of thrombosis should always be considered.
In assessing patients with PNH, it is important to evaluate the severity of BMF, because patients may have symptoms because of reduced bone marrow function. Allo-HSCT is a curative therapy for adults with AA and the preferred option for younger patients with severe AA.31 However, 5-year OS in patients aged >40 years, after sibling allo-HSCT, is only ∼58%.32 In those not suitable for allo-HSCT, immunosupressive therapy is recommended.32 OS after ATG reduces with age with an estimated 5-year OS of 100% in those aged <20 years, 92% for those aged between 20 and 40 years, 71% for those between 40 and 60 years and 56% for those aged >60 years.33 In our study, we observed a documented history of BMF in 246 cases (48.3%) with the majority of these being AA. One hundred and seventy-three patients (34%) had received immunosuppressive therapy before commencing C5i. During the study period on C5i, 104 patients either underwent an allogeneic BMT or received other therapies for AA.
We previously reported improved survival in 79 consecutive patients with PNH treated with eculizumab with the survival at 8 years, no different from age- and sex-matched controls.28 Over half of these patients were treated within the eculizumab clinical trials in which severe BMF was excluded.22,23,25 In this analysis, we observed a significant difference in OS between the patients treated with C5i for PNH and age- and sex-matched controls. In order to evaluate the contribution of BMF to the difference in OS, we excluded 120 patients: those receiving an allo-HSCT, those receiving medical therapy for AA, and those with AA unresponsive to medical therapy or who had undergone clonal evolution (Table 4). In this analysis, there is no significant difference between the patients treated with C5i for PNH and age- and sex-matched controls (Figure 2).
Patients receiving allogeneic BMT on medical treatment for AA or with unresponsive AA or clonal evolution to MDS or AML
| BMT or medical treatment for AA, MDS, or AML . | Number (%) . |
|---|---|
| Allogeneic BMT | 46 |
| Reason for BMT: | |
| AA | 38 |
| MDS | 4 |
| AML | 3 |
| Lymphoma | 1 |
| Medical therapy for AA | 58 |
| Cyclosporine | 39 |
| Tacrolimus | 3 |
| Eltrombopag | 7 |
| Oxymetholone | 6 |
| Danazol | 1 |
| Cyclosporine and eltrombopag | 1 |
| Cyclosporine and danazol | 1 |
| Clonal evolution or unresponsive AA (no transplantation) | 16 |
| Unresponsive/progressive AA | 2 |
| MDS | 5 |
| AML | 9 |
| Total | 120 |
| BMT or medical treatment for AA, MDS, or AML . | Number (%) . |
|---|---|
| Allogeneic BMT | 46 |
| Reason for BMT: | |
| AA | 38 |
| MDS | 4 |
| AML | 3 |
| Lymphoma | 1 |
| Medical therapy for AA | 58 |
| Cyclosporine | 39 |
| Tacrolimus | 3 |
| Eltrombopag | 7 |
| Oxymetholone | 6 |
| Danazol | 1 |
| Cyclosporine and eltrombopag | 1 |
| Cyclosporine and danazol | 1 |
| Clonal evolution or unresponsive AA (no transplantation) | 16 |
| Unresponsive/progressive AA | 2 |
| MDS | 5 |
| AML | 9 |
| Total | 120 |
Ninety-one patients died during the study period, and Figure 3 shows the causes of death observed. Thrombosis, the most common cause of death in patients before anticomplement therapy was available, was the cause of death in 4 patients (4.4%), indicating that, for the most part, patients are no longer dying from their PNH. The main cause of death was infection (35.2%), with half of these occurring after allo-HSCT or on immunosuppressive therapy for AA. Another 4 patients died due to other complications of their allo-HSCT. Clonal evolution to AML or MDS was the cause of death in 16 patients (3.1%) which is similar to previous reported levels.15,17,18,28
The majority of patients (90.4%) treated with C5i received treatment because of intravascular hemolysis or the development of thromboses with the median PNH granulocyte clone size at initiation of C5i being 85.5% (IQR, 67%-95%; available for 494 cases). Treatment was generally well tolerated, and patients received their treatment via a nurse at their home. In order to minimize the risk of meningococcal sepsis, all patients were vaccinated against meningococcal strains A, B, C, W, and Y, with revaccination depending on serological response, and treated with antibiotic prophylaxis with either penicillin V or erythromycin. Adherence to antibiotic prophylaxis can be difficult to gauge; we have previously audited this as part of routine service evaluation with >80% stating compliance. We reported 11 cases of meningococcal sepsis in 10 patients with 1 fatality. The observed meningococcal infection rate was 0.35 events per 100 patient-years. Twenty patients were able to discontinue C5i because both their granulocyte and monocyte clone had fallen <10%, highlighting the importance of monitoring the PNH clone while on C5i.
The phenomenon of extravascular hemolysis caused by treatment with C5i, because of opsonization of PNH erythrocytes and their subsequent removal by the reticuloendothelial system has been well documented.34,35 This study shows that despite treatment with C5i, hemoglobin normalization (>135 g/L in men and >115 g/L in women) only occurred in 20.4% and 20.3% of patients, respectively, at 12 and 24 months after commencing C5i. The mean hemoglobin level at 12 and 24 months after commencing C5i was 107.7 g/L and 108.6 g/L, with more than a quarter of patients (26.7%) still requiring to undergo transfusion in the most recent 12 months on C5i. Although the cause of anemia in part may be multifactorial, the ongoing anemia experienced by the majority of patients in this study highlights an unmet need for patients and the need for PNH therapies to prevent intravascular hemolysis without causing extravascular hemolysis.
Over the 20-year period, we changed several aspects of care. We introduced prophylactic antibiotic use to prevent meningococcal infection after advice from the national meningitis service. We increased the number of sites across the country where we travel to, in order to allow easier access for patients to the PNH team as well as developed a website for both patients and health care professionals. Our laboratory team have developed a technique to evaluate the degree of C3 loading on PNH erythrocytes that helps in assessing whether extravascular hemolysis is causing anemia.
This study confirms the importance of preventing intravascular hemolysis in PNH and the safety and efficacy of eculizumab and ravulizumab. Although the OS of all the patients treated over the 20-year period was not equal to that of age- and sex-matched controls, we believe the difference in OS is due to the underlying BMF, and when patients with either progression of their BMF or those receiving treatment for their BMF are excluded, the OS is not significantly different from that of age- and sex-matched controls. The OS reported in this study is likely to be more accurate than our previous analysis of the initial 79 patients treated with eculizumab because many of those initial patients were enrolled in clinical trials of eculizumab in which significant aplasia was an exclusion criterion. Further study into patients who are symptomatic from both PNH and BMF is needed, because the major cause of mortality was related to underlying BMF and not PNH. Additionally, a large proportion of patients treated with C5i remain anemic and targeting other parts of the complement pathway to prevent intravascular hemolysis without causing extravascular hemolysis is likely to improve hemoglobin levels and the quality of life.
Acknowledgment
The authors thank all local consultant hematologists for their great cooperation in the care for these patients. The authors could not have done this study without them.
Authorship
Contribution: R.J.K., M.G., T.M., A.V., R.T., A.G.K., and S.G. designed the study; R.J.K., M.G., T.M., A.V., P.M., A.P., L.M.A., A.G.K., R.T., S.K.N., L.M., and S.G. provided the medical care for our patients; R.J.K., M.H., L.M.A., J.V., A.G.K., R.T., and S.G. collected the data; J.R.D. performed statistical analysis; R.J.K. wrote the manuscript; and all authors had significant contributions in reviewing and revising the manuscript.
Conflict-of-interest disclosure: R.J.K. received research funding from Novartis and Sobi; is a consultant at Sobi, AstraZeneca, Alexion, and Otsuka; is a member of advisory boards at Alexion, AstraZeneca, Novartis, Sobi, Roche, Jazz, and Amgen; and received honoraria from Alexion, Sobi, Biologix, and Otsuka. M.H. received research funding from Sobi. L.M.A. is a consultant at Alexion and Sobi; is an advisory board member at Alexion, AstraZeneca, Sobi; and received honoraria from Alexion and Sobi. J.L. is a consultant at Alexion, Novartis, and Sobi; is an advisory board member at Alexion, AstraZeneca, Novartis, and Sobi; and received honoraria from Alexion, Novartis, and Sobi. B.F. received honoraria from Sobi. C.B. is an advisory board member at Alexion and Sobi. M.G. serves on advisory boards for Alexion, AstraZeneca, Amgen, BioCryst, and Novartis; receives honoraria from Alexion and Sobi; and is a consultant for Regeneron and BioCryst. T.M. is a member of advisory boards for Janssen, AstraZeneca, Alexion, Sobi, Novartis, Roche, AbbVie, and Gilead; and receives honoraria from Janssen, AstraZeneca, Alexion, AbbVie, Novartis, and Roche. P.M. serves on the advisory board for Novartis and receives honoraria from Sobi. S.K.N. is on advisory boards for AstraZeneca and Novartis; and receives honoraria from Janssen and Alexion. R.T. is a consultant for Sobi and serves on the advisory board for AstraZeneca. A.G.K. receives research funding from Celgene and Novartis; has consultancy roles with Pfizer, Novo Nordisc, Achillion, Akari, Alexion, AstraZeneca, Apellis, Amgen, BioCryst, Celgene, Ra Pharma, Novartis, Sobi, and Samsung; and serves on advisory boards for AstraZeneca, Novartis, and Sobi, with honoraria from Pfizer, Novo Nordisc, Achillion, Akari, Alexion, AstraZeneca, Amgen, Apellis, BioCryst, Celgene, Ra Pharma, Novartis, Sobi, and Samsung. S.G. receives research funding from Alexion and AstraZeneca; has consultancy roles with Gilead, Sobi, Pfizer, Jazz, Celgene, Novartis, Jazz, Alexion, and AstraZeneca; serves on the advisory board for Novartis; and receives honoraria from Gilead, Sobi, Pfizer, Jazz, Celgene, Novartis, Jazz, Alexion, and AstraZeneca. The remaining authors declare no competing financial interests.
Correspondence: Richard J. Kelly, Department of Haematology, St. James’s University Hospital, Level 3 Bexley Wing, Beckett St, Leeds LS9 7TF, United Kingdom; email: richardkelly@nhs.net.
References
Author notes
Data are available on request from the corresponding author, Richard Kelly (richardkelly@nhs.net).
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal